Abstract
Ageing has been proposed to be associated with increased levels of reactive oxygen species (ROS) that scavenge nitric oxide (NO). In eight young sedentary (23 ± 1 years; Y), eight older lifelong sedentary (66 ± 2 years; OS) and eight older lifelong physically active subjects (62 ± 2 years; OA), we studied the effect of ROS on systemic and skeletal muscle NO bioavailability and leg blood flow by infusion of the antioxidant N-acetylcysteine (NAC). Infusion of NAC increased the bioavailability of NO in OS, as evidenced by an increased concentration of stable metabolites of NO (NOx) in the arterial and venous circulation and in the muscle interstitium. In OA, infusion of NAC only increased NOx concentrations in venous plasma whereas in Y, infusion of NAC did not affect NOx concentrations. Skeletal muscle protein levels of endothelial and neuronal NO synthase were 32% and 24% higher, respectively, in OA than in OS. Exercise at 12 W elicited a lower leg blood flow response that was associated with a lower leg oxygen uptake in OS than in Y. The improved bioavailability of NO in OS did not increase blood flow during exercise. These data demonstrate that NO bioavailability is compromised in the systemic circulation and in the musculature of sedentary ageing humans due to increased oxidative stress. Lifelong physical activity opposes this effect within the trained musculature and in the arterial circulation. The lower blood flow response to leg exercise in ageing humans is not associated with a reduced NO bioavailability.
Key points
Ageing has been proposed to be associated with increased levels of reactive oxygen species (ROS) that scavenge nitric oxide (NO), thereby decreasing the bioavailability of this potent vasodilator.
Here we show that NO bioavailability is compromised in the systemic circulation and in skeletal muscle of sedentary older humans as evidenced by an increase in NO metabolites after antioxidant infusion.
Lifelong physical activity opposes this effect within the trained musculature and in the arterial circulation.
The reduced blood flow to contracting leg muscles with ageing does not appear to be related to changes in NO bioavailability.
These findings expand our understanding of the mechanisms underlying the age-related changes in vascular function and highlight the beneficial effect of exercise training throughout the lifespan.
Introduction
Ageing has been proposed to be associated with increased levels of reactive oxygen species (ROS) that scavenges nitric oxide (NO) and thereby decreases the bioavailability of this vasoactive substance (Taddei et al. 2001; Eskurza et al. 2004). Evidence also suggests that exercise training reduces oxidative stress and increases NO bioavailability in the endothelium of ageing humans (Taddei et al. 2000; Eskurza et al. 2004) and animals (Spier et al. 2004; Durrant et al. 2009), but the effect of lifelong physical activity has never been investigated. The suggested increase in NO bioavailability in humans with exercise training is based on changes in flow-mediated dilatation (FMD; Eskurza et al. 2004) and changes in ACh-induced vasodilatation with and without NO synthase (NOS) inhibition (Taddei et al. 2000) in the forearm vasculature. However, direct measurements of NO bioavailability in aged individuals have not previously been conducted. Changes in NO bioavailability can be assessed by measuring the stable metabolites of NO, nitrite (NO2−) and nitrate (NO3−), either separately or combined (NOx).
Skeletal muscle blood flow and oxygen delivery are closely regulated to match the oxygen demand of the contracting muscle (Andersen & Saltin, 1985). This complex response is the result of the interplay of mechanical factors, sympathetic nervous activity, and local metabolic and endothelium-derived substances that influence vascular tone (Clifford & Hellsten, 2004). There is accumulating evidence in humans that ageing is associated with an attenuated blood flow response to exercise in both the upper (Kirby et al. 2009) and lower extremities (Wahren et al. 1974; Proctor et al. 1998; Lawrenson et al. 2003; Poole et al. 2003). The mechanisms underlying this effect of age on exercise hyperaemia is unclear, but a smaller reduction in forearm blood flow during inhibition of NO synthesis in older individuals does indicate an age-related loss of NO-mediated vasodilatation during exercise (Schrage et al. 2007). This reduced contribution from the NO system to exercise hyperaemia could be an effect of the age-related decrease in bioavailability of NO (Taddei et al. 2001; Eskurza et al. 2004). In accordance, infusion of the antioxidant ascorbic acid increases blood flow during handgrip exercise in older subjects (Kirby et al. 2009; Crecelius et al. 2010), an effect mediated primarily via an increase in the bioavailability of NO derived from the NO synthase (NOS) pathway (Crecelius et al. 2010). To what extent the changes in hyperaemia during leg exercise is an effect of an age-related decrease in bioavailability of NO is not known.
To address these issues, we examined the effect of intravenous infusion of the antioxidant N-acetylcysteine (NAC) on central and peripheral haemodynamics during resting conditions and exercise at the same absolute and relative exercise intensity in young sedentary, older lifelong sedentary and older lifelong endurance trained subjects. In addition, NO metabolites in plasma and within skeletal muscle at rest and during exercise were measured to detect acute changes in NO bioavailability. We hypothesized that infusion of NAC would increase the concentration of NO metabolites in older sedentary subjects and that lifelong physical activity attenuated this response. Increasing the bioavailability of NO would increase exercise hyperaemia in the older sedentary subjects.
Methods
Eight healthy young sedentary (less than 2 h of moderate intensity exercise per week during the last 3 years), eight healthy lifelong older sedentary (less than 2 h of moderate intensity exercise per week during the last 30 years), and eight healthy lifelong older endurance-trained (more than 5 h of high-intensity exercise per week during the last 30 years) male subjects were studied (Table 1). All subjects were non-smokers and none of the subjects had been diagnosed with cardiovascular disease, renal dysfunction, insulin resistance, diabetes, or hypercholesterolaemia. Five of the older trained subjects had extrasystoles at rest, whereas the remaining subjects had no arrhythmias at rest and none of the subjects had arrythmias during exercise (ECG).
Table 1.
Baseline characteristics
| Young sedentary (n= 8) | Older sedentary (n= 8) | Older active (n= 8) | |
|---|---|---|---|
| Age (years) | 23 ± 1 | 66 ± 2†† | 62 ± 2†† |
| Height (cm) | 183 ± 2 | 175 ± 3† | 178 ± 2 |
| Body weight (kg) | 79.4 ± 4.3 | 79.2 ± 1.8 | 75.7 ± 3.1 |
| Body fat (%) | 17.7 ± 2.6 | 26.5 ± 1.2† | 15.2 ± 1.5* |
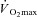 (l min−1) (l min−1) |
3.6 ± 0.1 | 2.1 ± 0.1†† | 3.7 ± 0.2** |
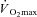 (ml min−1 kg−1) (ml min−1 kg−1) |
45.5 ± 2.3 | 25.7 ± 1.3†† | 49.2 ± 2.4** |
| 45% knee extensor Wmax (W) | 31 ± 2 | 21 ± 2† | 35 ± 2** |
| Experimental leg mass (kg) | 12.5 ± 0.7 | 11.1 ± 0.4 | 11.8 ± 0.5 |
| Experimental fat-free leg mass (kg) | 9.9 ± 0.4 | 8.5 ± 0.3† | 10.1 ± 0.4* |
| Systolic blood pressure (mmHg) | 122 ± 2 | 150 ± 5† | 152 ± 6† |
| Diastolic blood pressure (mmHg) | 66 ± 3 | 70 ± 3 | 69 ± 3 |
| Mean arterial pressure (mmHg) | 85 ± 2 | 98 ± 4 | 95 ± 3 |
| Total cholesterol (mmol l−1) | 3.8 ± 0.4 | 5.3 ± 0.4† | 4.9 ± 0.2† |
| HDL (mmol l−1) | 1.4 ± 0.1 | 1.4 ± 0.2 | 1.5 ± 0.2 |
| LDL (mmol l−1) | 1.9 ± 0.3 | 3.3 ± 0.4† | 2.9 ± 0.2 |
| Triglycerides (mmol l−1) | 0.83 ± 0.11 | 1.55 ± 0.32† | 1.00 ± 0.08 |
Values are means ± SEM. †P < 0.05; ††P < 0.001: different from young sedentary; *P < 0.05; **P < 0.001: different from older sedentary.
The study was approved by the Ethics Committee of Copenhagen and Frederiksberg communities (H-3-2009-090) and conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all subjects before enrollment into the study.
Initial testing
Before the experimental day the subjects visited the laboratory to become accustomed to the one-leg knee-extensor model (Andersen & Saltin, 1985) and to perform an incremental bicycle ergometer exercise test in which pulmonary maximal oxygen uptake (l min−1,  ) was determined (Quark CPET system, Cosmed, Rome, Italy; Table 1). An incremental test was also performed in a one-leg knee-extensor ergometer to determine maximal workload.
) was determined (Quark CPET system, Cosmed, Rome, Italy; Table 1). An incremental test was also performed in a one-leg knee-extensor ergometer to determine maximal workload.
Experimental protocol
Subjects refrained from caffeine, alcohol and exercise for 24 h before the experimental day. On the day of the experiment the subjects arrived at the laboratory after a light breakfast. After local anaesthesia, catheters were placed in the femoral artery and vein of the experimental leg and in the femoral artery of the non-experimental leg, and a muscle biopsy was obtained from m. vastus lateralis of the non-experimental leg. In addition, three microdialysis probes (CMA 63, CMA Microdialysis, Stockholm, Sweden) with a 30 mm membrane (20 kDa cut-off) were inserted into the thigh muscle (m. vastus lateralis) of the experimental leg after local anaesthesia.
Thirty minutes after insertion of the probes, the subjects performed 10 min of knee-extensor exercise (12 W) with the purpose of minimizing the tissue response to insertion trauma (Nordsborg et al. 2003). After 30–90 min of rest, ACh was infused into the femoral artery for 2.5 min at three different doses (10, 25 and 100 μg min−1 (kg of leg mass)−1). After additional 30–90 min of supine rest, the subjects completed 10 min of one-leg knee-extensor exercise at an absolute workload of 12 W and at a relative workload corresponding to 45% of the maximal workload (45%Wmax) obtained in the incremental test (separated by 10 min of rest) with infusion of saline (CON). After 70 min, intravenous infusion of NAC was started and after an additional 35 min the first of the two exercise bouts (12 W and then 45%Wmax) was performed (separated by 10 min of rest) during constant infusion of NAC.
Microdialysis
Microdialysate was collected for 10 min during resting conditions and during one-leg knee-extensor exercise. The microdialysis probes were perfused at a rate of 5 μl min−1 with Ringer acetate and to determine the relative exchange of NOx, a small amount (2.7 nm) of [2-3H]ATP (<0.1 μCi ml−1) was added to the perfusate for calculation of probe recovery. The molecular probe recovery (PR) was calculated as [PR = (dpminfusate− dpmdialysate/dpminfusate], where dpm denotes disintegrations per minute (Scheller & Kolb, 1991; Jansson et al. 1994). The [3H]ATP activity (in dpm) was measured on a liquid scintillation counter (Tri-Carb 2910 TR; Perkin Elmer) after addition of the perfusate to 3 ml of Ultima Gold scintillation liquid (Perkin Elmer). After collection of samples, the microdialysate was weighed, and the actual flow rate was calculated to estimate any loss of fluid or abnormal decrease in perfusion rate.
N-Acetylcysteine
Intravenous infusion of N-acetylcysteine (NAC) consisted of a loading dose of 125 mg kg−1 h−1 for 15 min to increase plasma [NAC], followed by a constant infusion of 25 mg kg−1 h−1 to achieve a plateau in [NAC], with exercise commencing after 20 min of constant infusion (Medved et al. 2003). NAC infusion was continued throughout exercise. Pharmacokinetics of NAC using this infusion protocol is reported elsewhere (Brown et al. 2004).
Measurements and calculations
Femoral arterial blood flow (leg blood flow, LBF) was measured with ultrasound Doppler (Logic E9, GE Healthcare, Pittsburgh, PA, USA) equipped with a linear probe operating an imaging frequency of 9 MHz and Doppler frequency of 4.2–5.0 MHz. The site of blood velocity measurements in the common femoral artery was distal to the inguinal ligament but above the bifurcation into the superficial and profound femoral branch to avoid turbulence from the bifurcation. All recordings were obtained at the lowest possible insonation angle and always below 60 deg. The sample volume was maximized according to the width of the vessel, and kept clear of the vessel walls. A low-velocity filter (velocities <1.8 m s−1) rejected noises caused by turbulence at the vascular wall. Doppler tracings and B-mode images were recorded continuously and Doppler tracings were averaged over eight heart cycles at the time of blood sampling. Vessel diameter was determined after each Doppler recording. Arterial diameter measures were assessed during the systole from arterial B-mode images with the vessel parallel to the transducer.
Intra-arterial pressure was monitored with transducers (Pressure Monitoring Kit, Baxter, Deerfield, IL, USA) positioned at the level of the heart. Blood samples were drawn after 2 min of infusion of each dose and after 2.5 and 7.5 min of exercise and blood gases, haemoglobin and lactate were measured using an ABL725 analyzer (Radiometer, Copenhagen, Denmark). Leg mass was calculated from whole-body dual-energy X-ray absorptiometry scanning (Prodigy, GE Healthcare), leg vascular conductance (LVC) was calculated as LBF/mean arterial pressure (MAP), leg lactate release was calculated as arterio-venous difference × LBF and leg NO2− and NOx uptake was calculated as arterio-venous difference × plasma flow.
Quantification of protein expression
Freeze dried tissue samples of m. vastus lateralis were homogenized in lysis buffer and Western blot analysis was performed as previously described (Bangsbo et al. 2009; Nyberg et al. 2012) with the exception that the membrane image was digitalized on a ChemiDoc MP system (Bio-Rad, Hercules, CA, USA). Equal amounts of total protein were loaded for each sample in accordance to the antibody optimization: endothelial NO synthase (eNOS; 16 μg), neuronal NOS (nNOS; 10 μg), and eNOS-PSer1177 (20 μg). All samples were run on the same gel and samples from each group were distributed evenly across the gel. Antibodies used for eNOS (1:200 dilution; 2% non-fat milk) and nNOS (1:10 000 dilution; 2% non-fat milk) detection were from BD Transduction Laboratories, San Jose, CA, USA and the antibody for eNOS-PSer1177 (1:50 dilution; 3% BSA) was from New England Biolabs, Ipswich, MA, USA. Proteins were normalized to the same human standard and expressed as arbitrary units. Total eNOS and eNOS-PSer1177 were detected on separate gels.
Analysis of nitrate, nitrite and noradrenaline
The stable metabolites of NO, NO2− and NO3− (NOx), were measured using fluorometric assay kit (Cayman Chemical Co., Ann Harbor, MI, USA). NO2− was analysed using a modification of the chemiluminescence technique as previously reported (Bailey et al. 2010a). Plasma noradrenaline (NA) concentrations were determined with a radioimmunoassay (LDN, Nordhorn, Germany).
Statistical analysis
The number of subjects (n= 8 in each group) in the current study was selected on the basis of detecting differences in leg blood flow with age and increases in exercise hyperaemia, NOx and NO2− within each group with infusion of NAC, as these variables were the main outcomes of the study. A two-way repeated measures ANOVA was used to test significance within and between CON and NAC and a two-way ANOVA was used to test significance between the young sedentary, older sedentary and older active subjects within trials. Differences in baseline characteristics and protein expression were assessed with a one-way ANOVA. After a significant F test, pairwise differences were identified using Tukey's honestly significant difference post hoc procedure. A probability value less than 0.05 was accepted as statistically significant and data are presented as mean ± SEM.
Results
Interstitial NOx and plasma nitrate and nitrite at rest and during one-leg knee-extensor exercise performed during control conditions and with infusion of N-acetylcysteine
Infusion of NAC increased (P < 0.05) muscle interstitial NOx at rest and during both exercise intensities in the older sedentary group (OS), whereas no effect of NAC was detected in the young sedentary (Y) and older active (OA) subjects (Fig. 1). During control conditions, muscle interstitial NOx at rest was lower (P < 0.05) in OS when compared to Y. In Y, exercise at 12 W and 45%Wmax decreased (P < 0.05) interstitial NOx during control conditions and during infusion of NAC. Infusion of NAC increased (P < 0.05) arterial and venous plasma NO2− and NOx at rest in OS and venous plasma NO2− and NOx at rest in OA (Fig. 2 and Supplemental Table 1 and 2). When accounting for arterio-venous differences and plasma flow, leg NO2− uptake decreased (P < 0.05) in OA with infusion of NAC. The r2 value for the correlation between the change in plasma NO2− and NOx with infusion of NAC in the older subjects was 0.383 (P= 0.011).
Figure 1. Muscle interstitial NOx at rest and during 12 W and 45%Wmax without and with infusion of NAC.
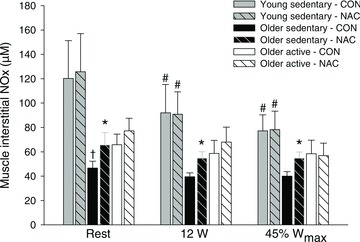
Exercise was performed at the same absolute workload of 12 W and at a relative workload corresponding to 45%Wmax without (CON) or with (NAC) infusion of N-acetylcysteine in young sedentary (n= 8), older sedentary (n= 8) and older active (n= 8) subjects. Values are means ± SEM. †Significantly different from young sedentary within same condition, P < 0.05; *significantly different from control conditions, P < 0.05; #significantly different from rest within same condition, P < 0.05.
Figure 2. Plasma NO2− and NOx at rest without and with infusion of NAC.
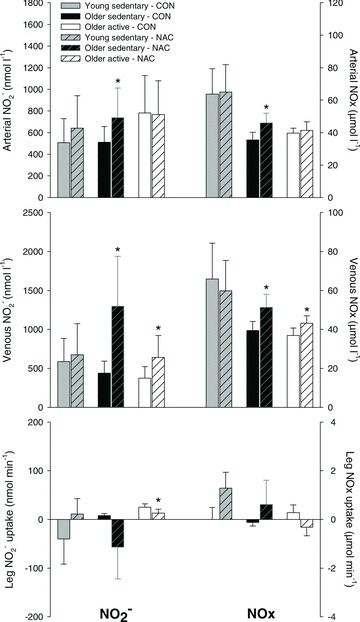
Femoral arterial and venous blood samples were collected during resting conditions without (CON) or with (NAC) infusion of N-acetylcysteine in young sedentary (n= 6–8), older sedentary (n= 6–8) and older active (n= 6–8) subjects. Values are means ± SEM. *Significantly different from control conditions, P < 0.05.
Leg and systemic variables at rest and during one-leg knee-extensor exercise performed during control conditions and with infusion of N-acetylcysteine
LBF (1.75 ± 0.05 versus 2.12 ± 0.15 l min−1) and leg oxygen delivery (342 ± 14 versus 425 ± 31 ml min−1) during 12 W exercise was lower (P < 0.05) in OS when compared to Y (Fig. 3). This attenuated blood flow response was paralleled by a lower (P < 0.05) leg oxygen uptake (211 ± 5 versus 271 ± 23 ml min−1) and increase (P < 0.05) in leg lactate release in OS. Lactate release at 12 W was higher (P < 0.05) in OS when compared to OA. Infusion of NAC did not affect LBF, leg oxygen delivery, leg oxygen uptake or lactate release in any of the groups. MAP was higher (P < 0.05) during seated rest and exercise at 12 W in OS when compared to Y. Infusion of NAC lowered (P < 0.05) MAP at rest and during 12 W exercise in OS and during 12 W and 45%Wmax exercise in OA. LVC was lower (P < 0.05) in OS and OA when compared to Y during control conditions and NAC infusion. Infusion of NAC did not affect LVC in any of the three groups. Blood variables are presented in Supplemental Tables 3 and 4.
Figure 3. Systemic and leg haemodynamics at rest and during 12 W and 45%Wmax.

Exercise was performed at the same absolute workload of 12 W and at a relative workload corresponding to 45%Wmax without (CON) or with (NAC) infusion of N-acetylcysteine in young sedentary (n= 8), older sedentary (n= 8) and older active (n= 8) subjects. Values are means ± SEM. †Significantly different from young sedentary within same condition, P < 0.05; *significantly different from control conditions, P < 0.05; #significantly different from rest, P < 0.05, ‡significantly different from older sedentary within same condition, P < 0.05.
Protein expression
Skeletal muscle eNOS and nNOS content was 32% and 24% lower (P < 0.05), respectively, in OS when compared to OA (Fig. 4). eNOS content tended (P= 0.091) to be lower in Y when compared OA. There was no difference in eNOS-PSer1177 between any of the groups, but the ratio between eNOS-PSer1177 and total eNOS was higher (P < 0.05) in OS when compared to OA. Representative blots are presented in Supplemental Fig. 1.
Figure 4. Protein expression of eNOS, Ser1177-phosphorylated eNOS and nNOS in vastus lateralis muscle.
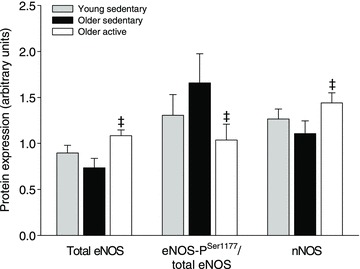
Protein expression in young (n= 7), older sedentary (n= 6) and older active subjects (n= 8). ‡Significantly different from older sedentary, P < 0.05.
Haemodynamic responses to acetylcholine infusion
Baseline LBF and LVC was lower in both of the older groups compared to the young subjects (P < 0.05). ACh infusion increased LBF and LVC to 4.0 ± 0.6 l min−1 and 50 ± 8 ml min−1 mmHg−1, respectively, at the highest infusion dose, but it was lower in both the older sedentary (0.8 ± 0.6 l min−1 and 9 ± 2 ml min−1 mmHg−1, respectively) and older active (2.8 ± 0.5 l min−1 and 31 ± 5 ml min−1 mmHg−1, respectively) subjects. The older active subjects had a higher vasodilator response to ACh compared to the sedentary elderly (P < 0.05). These data are presented elsewhere (Mortensen et al. 2012).
Discussion
The major findings from the current study are that NO bioavailability is clearly compromised in the systemic circulation and in skeletal muscle of sedentary older humans as evidenced by an increase in NO metabolites after antioxidant infusion. However, lifelong physical activity opposes this effect within the trained musculature and in the arterial circulation. The higher protein expression of eNOS and bioavailability of NO in the physically active subjects are likely to explain the higher vascular response to the endothelium-dependent vasodilator ACh. In addition, the attenuated blood flow response to leg exercise in ageing humans does not appear to be an effect of reduced NO bioavailability.
Lifelong physical activity prevents an oxidative stress-induced decrease in NO bioavailability in human skeletal muscle and arterial circulation
ROS have been described to be more abundant in aged human skeletal muscle (Bailey et al. 2010b) and in arteries of aged rodents (Hamilton et al. 2001; Durrant et al. 2009). Based on changes in haemodynamics during infusion of ascorbic acid with a combination of pharmacological interventions (Taddei et al. 2001) or FMD (Eskurza et al. 2004), it has been suggested that this increase in ROS decreases NO bioavailability with age in the human forearm vasculature. The results from the current study provide direct evidence for a decreased NO bioavailability with age and inactivity, by determination of NO metabolites in the systemic circulation and skeletal muscle interstitium. The systemic effect of antioxidants on NO bioavailability is supported by the decrease in blood pressure at rest and during exercise as NO is known to be important for whole body pressure regulation in humans (Joyner & Casey, 2009).
In contrast to the older sedentary group, the active older group did not show an increase in bioavailability of NO in the interstitium of the trained muscle or in the arterial circulation with NAC infusion. This finding, in combination with the higher eNOS protein level in the older active than the older sedentary individuals agrees well with the higher vasodilator response to arterial ACh infusion in the active older individuals. This effect of training is in congruence with observations in the forearm vasculature of older trained individuals demonstrating no effect of antioxidants on the NO component of the ACh-induced vascular response (Taddei et al. 2000) and magnitude of FMD (Eskurza et al. 2004), which is mainly dependent on NO formation (Joannides et al. 1995). The present study extends these findings by demonstrating the effect of exercise training on NO bioavailability in the trained musculature and arterial circulation in humans. The unaffected NO bioavailability in the active subjects could be linked to a suppression of ROS levels by improved antioxidant defence and/or reduced ROS production (Durrant et al. 2009).
In the active older group we did detect a small increase in resting venous NO2− and NOx and a reduction in blood pressure during both exercise intensities with antioxidant infusion. This observation suggests that oxidative stress does affect venous NO bioavailability and blood pressure regulation irrespective of physical activity.
Protein expression and phosphorylation of endothelial and neuronal nitric oxide synthase
Skeletal muscle protein expression of eNOS or nNOS was not different between the young sedentary and older sedentary subjects. However, the ratio between ser1177 phosphorylated eNOS and total eNOS was higher in the older sedentary group when compared to the older active subjects, suggesting higher enzyme activity in the older sedentary subjects. The eNOS content and phosphorylation status found in the current study are in congruence with observations on vascular endothelial cells collected from the brachial artery and peripheral vein of young and older sedentary humans (Donato et al. 2009) and the higher activation status is likely to be a compensatory mechanism elicited by increased scavenging of NO by ROS.
Increased NO bioavailability does not improve exercise hyperaemia in ageing humans
In the sedentary older group, exercise at 12 W elicited a lower blood flow response that was associated with a lower leg oxygen uptake when compared to the young subjects and an increase in lactate release. Mitochondrial respiratory capacity has been shown to be similar in young and older subjects (Rasmussen et al. 2003; Larsen et al. 2012), suggesting that the reduced aerobic metabolism found in the current study is associated with an impaired skeletal muscle O2 delivery and/or alterations in blood-myocyte oxygen exchange. In laboratory animals, microvascular oxygen delivery during contractions is severely compromised in aged muscles (Hammer & Boegehold, 2005; Copp et al. 2009). Despite this potential limitation in oxygen delivery and microvascular oxygen tension and blood-myocyte oxygen transfer (Poole & Ferreira, 2007), restoration of NO bioavailability with antioxidant infusion did not increase exercise hyperaemia in the older sedentary subjects. This finding is in contrast to observations in the human forearm (Kirby et al. 2009; Crecelius et al. 2010) where infusion of the antioxidant ascorbic acid increased blood flow via an increase in the bioavailability of NO derived from the NOS pathway (Crecelius et al. 2010). Given that human limbs are exposed to differing homeostatic challenges and uses, this discrepancy could reflect limb-specific differences in vascular regulatory mechanisms (Newcomer et al. 2004). Accordingly, in young subjects NO has been shown to be essential for exercise hyperaemia in the human forearm (Schrage et al. 2004, 2007), but not leg (Bradley et al. 1999; Rådegran & Saltin 1999; Frandsen et al. 2000). The lack of increase in exercise hyperaemia with increased NO bioavailability in the older sedentary subjects appear to support these findings and suggest that NO is not essential for leg blood flow regulation during exercise. These results also suggest that the reduced exercise hyperaemia with ageing is not related to changes in NO bioavailability.
NO2− and NOx as biomarkers of NO bioavailability
Plasma NO2− has been suggested to reflect constitutive NOS activity in mammals (Kleinbongard et al. 2003) and regional NOS activity (Lauer et al. 2001), suggesting that NO2− is a more sensitive approach. In the current study, we detected similar relative changes in NO2− and NOx levels with infusion of NAC, demonstrating that within the present conditions both biomarkers can be used to detect acute changes in NO bioavailability in plasma.
Conclusion
The current study provides evidence for that NO bioavailability is compromised in the systemic circulation and in the musculature of sedentary ageing humans due to increased oxidative stress. Lifelong physical activity opposes this reduction in NO bioavailability in the arterial circulation and skeletal muscle. Furthermore, the reduced blood flow to contracting muscle with ageing does not appear to be related to changes in NO bioavailability.
Acknowledgments
This work was supported by a grant from the Danish Medical Research Council and the Lundbeck Foundation. M.N. was supported by a grant from the Lundbeck foundation. S.P.M. was supported by a grant from the Danish Council for Independent Research – Medical Sciences.
Glossary
- FMD
flow-mediated dilatation
- LBF
leg blood flow
- LVC
leg vascular conductance
- NA
noradrenaline
- NAC
N-acetylcysteine
- NO
nitric oxide
- NOx
nitrite and nitrate
- NO2−
nitrite
- OA
older active
- OS
older sedentary
- ROS
reactive oxygen species
- Y
young sedentary
Author contributions
The experiments were conducted at the Copenhagen Muscle Research Centre, Rigshospitalet, Denmark. The contributions of the authors were as follows: conception and design of the study: M.N., Y.H. and S.P.M; collection, analysis and interpretation of data: M.N., J.R.B., R.D., A.M.J., Y.H. and S.P.M.; drafting the article or revising it critically for important intellectual content: M.N., Y.H. and S.P.M. All authors approved the final version.
Supplementary material
Supplemental Table 1 and 2
Supplemental Tables 3 and 4
Supplemental Fig. 1
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor in humans. J Appl Physiol. 2010a;109:135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- Bailey DM, McEneny J, Mathieu-Costello O, Henry RR, James PE, McCord JM, Pietri IS, Richardsson RS. Sedentary ageing increases resting and exercise-induced intramuscular free radical formation. J Appl Physiol. 2010b;109:449–456. doi: 10.1152/japplphysiol.00354.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Gunnarson TP, Wendell J, Nybo L, Thomassen M. Reduced volume and increased training intensity elevate muscle Na+-K+ pump α-2-subunit expression as well as short- and long-term work capacity in humans. J Appl Physiol. 2009;107:1771–1780. doi: 10.1152/japplphysiol.00358.2009. [DOI] [PubMed] [Google Scholar]
- Brown M, Bjorksten A, Medved I, McKenna M. Pharmacokinetics of intravenous N-acetylcysteine in men at rest and during exercise. Eur J Clin Pharmacol. 2004;60:717–723. doi: 10.1007/s00228-004-0862-9. [DOI] [PubMed] [Google Scholar]
- Bradley SJ, Kingwell BA, McConell GK. Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes. 1999;48:1815–1821. doi: 10.2337/diabetes.48.9.1815. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Copp SW, Ferreira LF, Herspring KF, Musch TI, Poole DC. The effects of ageing on capillary hemodynamics in contracting rat spinotrapezius muscle. Mircovasc Res. 2009;77:113–119. doi: 10.1016/j.mvr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperaemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol. 2010;299:1633–1641. doi: 10.1152/ajpheart.00614.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelail dysfunction with ageing: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–H432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen U, Bangsbo J, Langberg H, Saltin B, Hellsten Y. Inhibition of nitric oxide synthesis by systemic NG-monomethyl-L-arginine administration in humans: Effects of interstitial adenosine, prostacyclin and potassium concentrations in resting and contracting skeletal muscle. J Vasc Res. 2000;37:297–302. doi: 10.1159/000025743. [DOI] [PubMed] [Google Scholar]
- Hammer LW, Boegehold MA. Functional hyperaemia is reduced in skeletal muscle of aged rats. Microcirculation. 2005;12:517–526. doi: 10.1080/10739680591003396. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and ageing: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- Jansson PA, Veneman T, Nurjhan N, Gerich J. An improved method to calculate adipose tissue interstitial substrate recovery for microdialysis studies. Life Sci. 1994;54:1621–1624. doi: 10.1016/0024-3205(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Casey DP. The catacholamines strike back – what NO does not. Circ J. 2009;73:1783–1792. doi: 10.1253/circj.cj-09-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol. 2009;587:1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Gödecke A, Schrader J, Schulz R, Heusch G, Schaub G, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- Larsen S, Hey-Mogensen M, Rabol R, Stride N, Helge JW, Dela F. The influence of age and aerobic fitness: effects on mitochondrial respiration in skeletal muscle. Acta Physiol. 2012;205:423–432. doi: 10.1111/j.1748-1716.2012.02408.x. [DOI] [PubMed] [Google Scholar]
- Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285:1023–1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Medved I, Brown MJ, Bjorksten AR, Leppik JA, Sostaric S, McKenna MJ. N-Acetylcysteine infusion alters blood redox status but not time to fatigue during intense exercise in humans. J Appl Physiol. 2003;94:1572–1582. doi: 10.1152/japplphysiol.00884.2002. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Winding K, Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signaling in the human leg. J Physiol. 2012 doi: 10.1113/jphysiol.2012.240093. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol. 2004;556:1001–1011. doi: 10.1113/jphysiol.2003.059717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordsborg N, Mohr M, Pedersen LD, Nielsen JJ, Langberg H, Bangsbo J. Muscle interstitial potassium kinetics during intense exhaustive exercise: effect of previous arm exercise. Am J Physiol Regul Integr Comp Physiol. 2003;285:R143–R148. doi: 10.1152/ajpregu.00029.2003. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Jensen LG, Thaning P, Hellsten Y, Mortensen SP. Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: effect of high-intensity aerobic training. J Physiol. 2012;590:1481–1494. doi: 10.1113/jphysiol.2011.225136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol. 2003;284:1251–1259. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- Poole DC, Ferreira LF. Oxygen exchange in muscle of young and old rats: muscle-vascular-pulmonary coupling. Exp Physiol. 2007;92:341–346. doi: 10.1113/expphysiol.2006.036764. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Experimental evidence against the mitochondrial theory of ageing. A study of isolated human skeletal muscle mitochondria. Exp Gerontol. 2003;38:877–886. doi: 10.1016/s0531-5565(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Rådegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol. 1999;276:H1951–1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and calculating tissue concentration from dialysate samples. J Neurosci Methods. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol. 2004;557:599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol. 2007;579:227–236. doi: 10.1113/jphysiol.2006.124313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availibilty and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Wahren J, Saltin B, Jorfeldt L, Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest. 1974;33:79–86. doi: 10.3109/00365517409114201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


