Abstract
In vitro studies using rat and fetal sheep cardiomyocytes indicate that, in addition to its role as a clearance receptor, the insulin-like growth factor 2 receptor (IGF-2R) can induce cardiomyocyte hypertrophy. In the present study, we have determined the effect of specific activation of the IGF-2R in the heart of the late gestation fetus on cardiomyocyte development. Leu27IGF-2, an IGF-2R agonist, was infused into the fetal left circumflex coronary artery for 4 days beginning at 128.1 ± 0.4 days gestation. Ewes were humanely killed at 132.2 ± 1.2 days gestation (term, 150 days). Fetuses were delivered and hearts dissected to isolate the cardiomyocytes and to collect and snap-freeze tissue. Leu27IGF-2 infusion into the left circumflex coronary artery of fetal sheep increased the area of binucleated cardiomyocytes in the left, but not the right, ventricle. However, this infusion of Leu27IGF-2 did not change fetal weight, heart weight, blood pressure, blood gases or cardiomyocyte proliferation/binucleation. The increase in cardiomyocyte size in the Leu27IGF-2-infused group was associated with increased expression of proteins in the Gαs, but not the Gαq, signalling pathway. We concluded that infusion of Leu27IGF-2 into the left circumflex coronary artery causes cardiac IGF-2R activation in the left ventricle of the heart, and this stimulates cardiomyocyte hypertrophy in a Gαs-dependent manner.
Key points
This study investigates the impact that insulin-like growth factor 2 receptor (IGF-2R) activation has on the fetal heart, by infusing Leu27IGF-2 into the left circumflex coronary artery of the sheep fetus, to specifically activate IGF-2R and it's downstream signalling pathway.
Activation of cardiac IGF-2R resulted in cardiomyocyte hypertrophy, but with no changes in heart weight, cardiomyocyte proliferation, binucleation or apoptosis. This hypertrophy was mediated via protein kinase A activation.
Infusion of Leu27IGF-2 increases atrial natriuretic peptide abundance, a marker of cardiac pathological hypertrophy.
Cardiac IGF-2R activation may alter important regulators of cardiac contractility and relaxation by decreasing sarcoplasmic reticulum Ca2+-ATPase and phospho-troponin I abundance.
This study places the interaction between the IGF-2R and Gαs signalling pathway as a potential mechanism that can contribute to cardiomyocyte growth in fetal life, but which may result in pathological cardiac hypertrophy in postnatal life.
Introduction
Pathological cardiac hypertrophy is associated with reduced left ventricular function and often leads to heart failure and death (Kannel et al. 1987; Levy et al. 1990). Pathological cardiac hypertrophy can be caused by increased pressure overload due to increased blood pressure or myocardial injury (Oakley, 1971). In the absence of pressure overload, cardiac hypertrophy can be mediated by the activation of specific receptor signalling pathways (Botting et al. 2011).
The insulin-like growth factor (IGF) system plays an important role in physiological cardiac hypertrophy, as well as the maturation of cardiomyocytes in late gestation. The IGF-1 receptor (IGF-1R) can be activated by either IGF-1 or IGF-2, and in the heart signalling from this receptor has been associated with normal growth and physiological hypertrophy. IGF-2 can also bind directly to the IGF-2R, but this has previously been thought to act as a clearance mechanism (Kornfeld, 1992), due to this receptor's association with the endosome–lysosome system, which has a function in degradation. The degradation of IGF-2 limits its interaction with the IGF-1R (Kornfeld, 1992), and in fetal life this appears to be an important regulatory system for cardiac growth (Powell et al. 2006; Wang et al. 2011). However, in vitro studies in rat cardiomyocytes have shown that IGF-2 can play a role in cardiomyocyte proliferation (Reini et al. 2009) and has the ability to induce cardiac hypertrophy in fetal (Wang et al. 2012) and adult (Chu et al. 2008) cardiomyocytes. This hypertrophic response is thought to occur through the activation of an IGF-2R signalling pathway (Huang et al. 2002; Chu et al. 2008).
The IGF-2R has been shown to activate phospholipase C-β via a heterotrimeric G protein-coupled receptor, involving αq subunits (Gαq). This Gαq signalling mechanism activates protein kinase C-α (PKC-α), Ca2+–calmodulin-dependent protein kinase II (CaMKII) and p44/42 MAP kinase (ERK), all of which appear to have a role in cardiac hypertrophy (Chu et al. 2008; Wang et al. 2012). In contrast, in vitro activation of the IGF-2R has been shown to induce cardiomyocyte apoptosis via the Gαq–calcineurin pathway (Chen et al. 2009; Chu et al. 2009b). This can involve a reduction in Gαs binding and decreased protein kinase A (PKA) phosphorylation (Chu et al. 2009a), changes that have been implicated in regulating cardiac contractility (Noland et al. 1995). Thus, there is now little doubt that the function of the IGF-2R is not limited to that of IGF-2 clearance (Kornfeld, 1992), but that this receptor also has critical involvement in signalling processes, which may contribute to adult cardiac pathogenesis.
Interestingly, we have shown that when growth is reduced in utero, the IGF-2R plays a role in signalling, which results in cardiac hypertrophy and this response extends at least into neonatal life (Wang et al. 2011, 2012). Altered signalling from the IGF-2R in cardiac development may provide an explanation for the link between suboptimal growth in fetal life and increased risk of heart disease in adulthood (Barker, 1995; Rich-Edwards et al. 1997). Therefore the aim of this study was to investigate the impact that IGF-2R activation has on the fetal heart, by selectively infusing Leu27IGF-2 into the left circumflex coronary artery in the sheep fetus, to specifically interrogate IGF-2R signalling. We hypothesized that activation of cardiac IGF-2R would not induce cardiomyocyte proliferation, binucleation or apoptosis, but would cause cardiomyocyte hypertrophy.
Methods
Animals and surgery
All procedures were approved by The University of South Australia Animal Ethics Committee. The authors have read, and the experiments comply with, the policies and regulations of The Journal of Physiology given by Drummond (2009). Vascular surgery was performed in 25 fetal sheep at 125.2 ± 0.3 days gestation (term, 150 ± 3 days), under aseptic conditions. Anaesthesia was induced with intravenous diazepam–ketamine (0.3 mg kg−1–5 mg kg−1) and maintained with 1–2.5% isoflurane in oxygen. Catheters (Critchley Electrical Products, Siverwater, NSW, Australia) were inserted in the maternal jugular vein, the fetal left circumflex coronary and carotid arteries, jugular vein, trachea and the amniotic cavity, as previously described (Edwards et al. 1999; Morrison et al. 2001; Danielson et al. 2005). At surgery, antibiotics were administered to the ewe (3.5 ml, 150 mg ml−1 procaine penicillin; 112.5 mg ml−1 benzathine penicillin; 2 ml, 250 g ml−1 dihydrostreptomycin, Lyppard, Keysborough, VIC, Australia) and fetus (1 ml, 150 mg ml−1 procaine penicillin; 112.5 mg ml−1 benzathine penicillin; 1 ml, 250 g ml−1 dihydrostreptomycin, Lyppard) and then post-operatively to ewes intramuscularly for 3 days and to fetuses intraamniotically for 4 days (500 mg ampicillin, Lyppard).
Experimental protocol
After 3 days recovery from surgery, fetuses were randomly assigned to different treatment groups: vehicle (100 mm of acetic acid (Sigma-Aldrich, Australia; n = 4) or Leu27IGF-2 (an analogue of IGF-2 that binds to the IGF-2R but not to the IGF-1R (Sakano et al. 1991; Oh et al. 1993); Novozymes GroPep, Adelaide, SA, Australia; 0.4 μg h−1, n = 8; 0.8 μg h−1, n = 2) and infused via the left circumflex coronary artery for 4 days. This effect was expected to be specific to the left ventricle because the left circumflex coronary artery directly perfuses the left ventricle and a posterior portion of the interventricular septum, but not the right ventricle (Rudolph et al. 1999). Furthermore, this approach was designed to specifically activate the IGF-2R in myocardium without promoting haemodynamic effects that may occur with a systemic infusion (Bauman et al. 1987). Some fetuses underwent the surgical procedure, but the coronary artery catheter was not patent (sham; n = 10). There were no differences between the sham and vehicle infused fetuses (Giraud et al. 2006); and there were no differences between the fetuses that received different doses of Leu27IGF-2; for any of the physiological or cardiomyocyte measurements. Therefore the data presented was for Control and Leu27IGF-2 groups.
Fetal arterial blood gas measurements
Fetal arterial blood samples (1 ml) were collected daily to measure 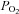 ,
, 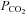 , pH, oxygen saturation (
, pH, oxygen saturation (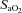 ) and haemoglobin (Hb) using an ABL CO-OX 80 FLEX analyser (Radiometer Pacific Pty Ltd, Mount Waverley, Australia) and temperature corrected to 39°C.
) and haemoglobin (Hb) using an ABL CO-OX 80 FLEX analyser (Radiometer Pacific Pty Ltd, Mount Waverley, Australia) and temperature corrected to 39°C.
Fetal blood pressure and heart rate measurements
Fetal carotid artery, jugular vein and amniotic catheters were connected to displacement transducers and a quad-bridge amplifier (ADInstruments, Australia, Bella Vista, NSW) to record fetal arterial blood pressure and amniotic fluid pressure, for 1 day prior to infusion and during the 4 day infusion period. Each day, blood pressure was measured in the left circumflex coronary artery for 10 min to ensure patency of the catheter. All data were digitized and recorded at a rate of 1000 Hz using LabChart 7 (ADInstruments). Blood pressure data were selected over 20 min each morning and averaged for analysis. Fetal arterial blood pressure was calculated by subtracting amniotic fluid pressure from fetal resting arterial blood pressure. Fetal systolic (SBP) and diastolic (DBP) blood pressures were calculated as the mean maximum and minimum pressures, respectively. Mean arterial pressure (MAP) was calculated using the formula MAP = DBP + 0.4(SBP − DBP) (Morrison et al. 1997; Danielson et al. 2005). Heart rate (HR) was derived from the blood pressure signal using the beats per minute function in LabChart 7 (ADInstruments, Australia). Rate pressure product ((RPP) = SBP × HR) was used as an indirect measure of myocardial mechanical work (Hawkins et al. 2000).
Postmortem and tissue collection
All animals were humanely killed with an overdose of sodium pentobarbitone (Lethobarb, Lyppard) at 132.2 ± 0.2 days gestation. Fetuses were delivered by hysterotomy and the fetal and heart weights recorded. Before dissection of the heart, the placement of the catheter tip in the left circumflex coronary artery was confirmed by injecting methylene blue dye. A sample of the left ventricle that had been perfused by the left coronary circumflex artery was removed and snap frozen. The remainder of the heart was then dissociated to isolate cardiomyocytes as previously described (Sundgren et al. 2003; Morrison et al. 2007; Wang et al. 2011).
Determination of proportion of mononucleated cardiomyocytes, their proliferation rate and the size of binucleated cardiomyocytes
The relative proportion of mononucleated and binucleated cardiomyocytes was determined for a total of 300 cardiomyocytes (Morrison et al. 2007). The length, width and area of binucleated cardiomyocytes were determined as previously described (Wang et al. 2011). Immunohistochemistry was performed with a Ki67 antibody (Dianova, Hamburg, Germany) on fixed cells and analysed as previously described (Giraud et al. 2006; Morrison et al. 2007).
Protein extraction and Western blotting
Left and right ventricle tissue (∼50 mg) was sonicated separately (John Morris Scientific, Australia) in 500 μl of sonication buffer (MilliQ water, 1 mm Tris HCl pH 8, 5 m NaCl, 1% NP-40, 1 mm sodium orthovanadate, 30 mm NaF, 10 mm sodium tetrapyrophosphate, 10 mm EDTA, a protease inhibitor tablet). The suspensions were centrifuged (Eppendorf Centrifuge 5415, Crown Scientific, Kingston, Australia) for 14 min at 14,300 g and 4°C. Protein content of extracts was determined using a Micro BCA Protein Assay Kit (Pierce, Thermo Fisher Scientific Inc., USA) as previously described (Wang et al. 2011).
The abundance of the proteins present in samples between each well and the linearity of the density measurements were tested as previously described (Muhlhausler et al. 2009). Protein was transferred onto a PolyScreen polyvinylidene difluoride hybridization transfer membrane (PerkinElmer, USA). The membranes were blocked with 5% BSA in Tris-buffered saline with 1% Tween (TBS-T) for 1 h at room temperature. The membranes were then washed 3 times for 5 min with TBS-T and then incubated with the respective primary antibody. These included proliferation markers: proliferating cell nuclear antigen (PCNA, Cell Signaling Technology, Inc., Danvers, MA, USA), cyclin-dependent kinase inhibitor 21 (p21, Cell Signalling Technology), p27 (Cell Signaling Technology); apoptosis markers: calcineurin A (Abcam, Cambridge, UK), nuclear factor of activated T-cells (NFATc3, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), phospho-NFATc3 (Ser265; Santa Cruz Biotechnology), cleaved-caspase 3 (Cell Signaling Technology); proteins in the Gαq signalling pathways: CaMKII (Cell Signaling Technology), phospho-CaMKII (Thr286; Santa Cruz Biotechnology), PKC-α (Cell Signaling Technology), phospho-PKC-α (Thr638; Santa Cruz Biotechnology), histone deacetylases (HDAC) 4 (Cell Signaling Technology), HDAC 5 (Cell Signaling Technology), phospho-HDAC 4(Ser632)/HDAC 5 (Ser498; Cell Signaling Technology), downstream proteins of both the IGF-1R (Lavandero et al. 1998) and Gαq (Yue et al. 2000) pathway: Erk1/Erk2 (Cell Signaling Technology) and phospho-Erk1/2 (Thr202/Tyr204; Cell Signaling Technology); cardiomyocyte hypertrophy markers: atrial natriuretic peptide (ANP; Abcam); contractility markers: sarcoplasmic reticulum Ca2+-ATPase (SERCA; Pierce, Thermo Fisher Scientific), phospho-troponin I (cardiac; Ser23/24; Cell Signaling Technology); proteins in the Gαs signalling pathways: PKA α/β/γ catalytic subunits (Santa Cruz Biotechnology), phospho-PKA α/β/γ catalytic subunits (Thr198; Santa Cruz Biotechnology), phospho-PKAII α regulatory subunit (Ser96; Santa Cruz Biotechnology), cAMP response element-binding (CREB; Cell Signaling Technology) and phospho-CREB (Ser133; Cell Signalling Technology) overnight with agitation at 4°C. These membranes were washed 3 times for 5 min in TBS-T. Membranes were then incubated with their respective horse radish peroxidase labelled secondary IgG antibodies for 1 h. The membranes were washed with TBS-T 3 times for 5 min and the antigen–antibody complexes were detected by enhanced chemiluminescence. Protein abundance was quantified by densitometry using software AlphaEaseFC 4.0 (Cell Biosciences/Protein Simple, Santa Clara, CA, USA). Protein abundance was not measured in all animals for all proteins because background interference on some blots prevented analysis of some samples.
Cell culture protocol
Cardiomyocytes from the left and right ventricle of hearts (n = 5) from a separate group of fetuses that did not undergo surgery were isolated at 132.2 ± 0.2 days gestation and cultured in serum-free culture medium with 4 × 105 cells per coverslip in a final volume of 2 ml per culture plate well and incubated for 24 h before drug treatment commenced as previously described (Sundgren et al. 2003; Wang et al. 2012).
PKA involvement in Leu27IGF-2 induced hypertrophy
Serum-free medium was replaced with either 2 ml of fresh serum-free medium, or 2 ml of medium with serum (to increase cardiomyocyte size), or 2 ml of serum-free medium containing an analogue of IGF-2 which binds to the IGF-2R but not IGF-1R (1 nm Leu27IGF-2; Novozymes, GroPep) (Sakano et al. 1991; Oh et al. 1993) and/or a PKA inhibitor (2.5 μm H-89; Cell Signaling Technology) (Chijiwa et al. 1990; Zou et al. 1999). For cardiomyocytes that were exposed to both inhibitor and agonist, the inhibitor was added 30 min prior to the addition of the agonist. After 48 h incubation, cardiomyocytes were fixed and stained and analysed as previously described (Sundgren et al. 2003; Wang et al. 2012).
Statistical analysis
Basal blood pressure was calculated for each fetus by averaging every 30 s of a 20 min recording period between 09.00 and 10.00 h. The effects of treatment on blood pressure and blood gases were compared using multifactorial ANOVA with repeated measures (STATA 11, StataCorpLP, College Station, TX, USA). All cultured cardiomyocyte cross-sectional area measurements were expressed relative to the serum-free culture medium control for each animal. Comparisons were tested with a one way ANOVA (factor = treatment) followed by Duncan's post hoc test using SPSS 18 for Windows. For all cardiomyocyte size and protein expression measures, multiple comparisons were tested with ANOVA using SPSS 18 for Windows. Data were presented as the mean ± SEM. A probability of less than 5% (P < 0.05) was considered statistically significant.
Results
Leu27IGF-2 infusion into the left circumflex coronary artery did not change fetal blood gases, blood pressure or organ/body weight of fetal lambs
The infusion of Leu27IGF-2 into the left circumflex coronary artery of fetal sheep did not cause any significant change in arterial blood gases (Table 1), blood pressure, heart rate or rate pressure product (Table 2), either before or during the infusion period, when compared to the Control group. There were also no changes in fetal body weight, absolute organ weight or relative organ weight, following Leu27IGF-2 infusion, when compared to the Control group (Table 3).
Table 1.
Daily arterial blood gas values for control and Leu27IGF-2-treated fetuses before and during the infusion period
| Day of infusion | |||||||
|---|---|---|---|---|---|---|---|
| –1 day | 0 day (prior to infusion) | +1 day | +2 days | +3 days | +4 days | ||
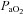 (mmHg) (mmHg) |
Control | 18.8 ± 1.3 (15) | 20.1 ± 1.2 (14) | 19.0 ± 1.2 (15) | 19.3 ± 1.2 (14) | 18.8 ± 1.2 (14) | 19.1 ± 1.5 (10) |
| Leu27IGF-2 | 18.7 ± 1.1 (10) | 18.6 ± 1.3 (10) | 19.8 ± 0.9 (10) | 19.5 ± 1.2 (10) | 19.7 ± 0.9 (10) | 20.5 ± 0.9 (10) | |
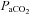 (mmHg) (mmHg) |
Control | 44.9 ± 1.1 (15) | 48.3 ± 0.8 (14) | 49.6 ± 1.1 (15) | 50.9 ± 1.1 (14) | 52.0 ± 1.3 (14) | 53.2 ± 1.8 (10) |
| Leu27IGF-2 | 45.9 ± 1.4 (10) | 47.0 ± 1.4 (10) | 47.7 ± 1.6 (10) | 49.6 ± 1.1 (10) | 49.5 ± 1.3 (10) | 48.5 ± 1.2 (10) | |
| pH | Control | 7.380 ± 0.006 (15) | 7.385 ± 0.008 (14) | 7.379 ± 0.004 (15) | 7.383 ± 0.013 (14) | 7.366 ± 0.004 (14) | 7.360 ± 0.007 (10) |
| Leu27IGF-2 | 7.366 ± 0.015 (10) | 7.393 ± 0.008 (10) | 7.392 ± 0.005 (10) | 7.385 ± 0.003 (10) | 7.379 ± 0.003 (10) | 7.375 ± 0.005 (10) | |
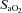 (%) (%) |
Control | 59.7 ± 4.0 (15) | 63.3 ± 3.4 (14) | 57.5 ± 3.4 (15) | 57.5 ± 3.7 (14) | 56.5 ± 3.4 (14) | 51.8 ± 4.8 (10) |
| Leu27IGF-2 | 64.2 ± 3.7 (10) | 65.43 ± 3.8 (10) | 67.5 ± 3.3 (10) | 63.8 ± 3.6 (10) | 64.5 ± 3.2 (10) | 66.0 ± 2.8 (10) | |
| Hb (g l−1) | Control | 92.0 ± 3.4 (15) | 92.1 ± 2.7 (13) | 91.6 ± 3.2 (15) | 91.5 ± 3.2 (13) | 91.5 ± 3.1 (14) | 91.1 ± 3.0 (9) |
| Leu27IGF-2 | 74.4 ± 6.4 (10) | 73.5 ± 6.5 (10) | 72.8 ± 6.3 (10) | 74.4 ± 5.2 (10) | 76.5 ± 4.9 (10) | 79.0 ± 4.5 (10) | |
Values are mean ± SEM (n). 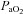 , fetal arterial partial pressure of oxygen;
, fetal arterial partial pressure of oxygen; 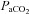 , fetal arterial partial pressure of carbon dioxide;
, fetal arterial partial pressure of carbon dioxide;  , oxygen saturation; Hb, haemoglobin.
, oxygen saturation; Hb, haemoglobin.
Table 2.
Mean arterial blood pressure, heart rate and rate pressure product over a 20 min period on each day of the experimental protocol in control and Leu27IGF-2-infused sheep fetuses
| Day of infusion | |||||||
|---|---|---|---|---|---|---|---|
| –1 day | 0 day (prior to infusion) | +1 days | +2 days | +3 days | +4 days | ||
| SBP (mmHg) | Control | 44.7 ± 4.7 (8) | 42.5 ± 2.6 (11) | 41.7 ± 3.9 (12) | 38.9 ± 2.8 (12) | 39.2 ± 2.5 (13) | 39.8 ± 3.3 (9) |
| Leu27IGF-2 | 44.9 ± 4.3 (8) | 47.0 ± 3.6 (9) | 49.6 ± 3.2 (8) | 49.5 ± 2.9 (8) | 50.9 ± 3.7 (8) | 48.9 ± 3.3 (9) | |
| DBP (mmHg) | Control | 30.0 ± 2.7 (8) | 29.8 ± 1.6 (11) | 29.2 ± 2.2 (12) | 28.7 ± 2.0 (12) | 29.8 ± 1.7 (13) | 32.2 ± 3.0 (9) |
| Leu27IGF-2 | 31.6 ± 3.6 (8) | 32.5 ± 3.3 (9) | 34.8 ± 3.4 (8) | 36.5 ± 3.2 (8) | 38.0 ± 3.6 (8) | 36.7 ± 2.9 (9) | |
| MAP (mmHg) | Control | 35.9 ± 3.4 (8) | 34.8 ± 1.9 (11) | 34.2 ± 2.9 (12) | 32.8 ± 2.2 (12) | 33.6 ± 1.9 (13) | 35.3 ± 3.1 (9) |
| Leu27IGF-2 | 36.9 ± 3.8 (8) | 36.7 ± 3.7 (9) | 40.7 ± 3.2 (8) | 41.7 ± 3.0 (8) | 43.2 ± 3.2 (8) | 41.6 ± 3.0 (9) | |
| HR (beats min−1) | Control | 168.3 ± 3.8 (8) | 172.8 ± 4.6 (11) | 171.2 ± 4.0 (12) | 171.8 ± 3.9 (12) | 172.5 ± 2.6 (13) | 167.4 ± 4.0 (9) |
| Leu27IGF-2 | 179.5 ± 6.7 (8) | 180.5 ± 4.4 (9) | 176.7 ± 3.9 (8) | 174.9 ± 4.0 (8) | 170.3 ± 3.5 (8) | 159.9 ± 2.2 (9) | |
| RPP (mmHg× | Control | 7504.6 ± 775.5 (8) | 7302.6 ± 435.4 (11) | 7126.7 ± 664.1 (12) | 6651.2 ± 468.5 (12) | 6756.5 ± 427.5 (13) | 6706.6 ± 670.9 (9) |
| beats min−1) | Leu27IGF-2 | 8149.4 ± 921.7 (8) | 8493.7 ± 680.7 (9) | 8774.1 ± 605.1 (8) | 8698.4 ± 626.2 (8) | 8668.3 ± 638.2 (8) | 7826.6 ± 558.3 (9) |
Values are mean ± SEM (n). SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; RPP, rate pressure product.
Table 3.
Fetal body, absolute and relative organ weights at 130–134 days gestation
| Control (n = 15) | Leu27IGF-2 (n = 10) | |
|---|---|---|
| Gestational age (days) | 131.9 ± 0.3 | 132.7 ± 0.4 |
| Body weight (kg) | 3.5 ± 0.2 | 3.8 ± 0.1 |
| Absolute organ weight (g) | ||
| Heart | 25.9 ± 1.2 | 28.3 ± 1.2 |
| Kidney | 21.2 ± 1.2 | 21.6 ± 1.5 |
| Liver | 94.9 ± 6.2 | 109.2 ± 7.4 |
| Pericardial fat | 4.2 ± 0.3 | 5.0 ± 0.3 |
| Organ weight relative to body weight (g kg−1) | ||
| Heart | 7.3 ± 0.2 | 7.4 ± 0.3 |
| Kidney | 6.0 ± 0.2 | 5.6 ± 0.3 |
| Liver | 26.7 ± 1.1 | 28.3 ± 1.5 |
| Pericardial fat | 1.2 ± 0.1 | 1.3 ± 0.1 |
Values are mean ± SEM.
Leu27IGF-2 infusion into the left circumflex coronary artery of fetal sheep did not alter the amount of cardiomyocyte proliferation, apoptosis or binucleation in the left ventricle
Leu27IGF-2 infusion did not alter the proliferation of cardiomyocytes in the left ventricle, when compared to the Control group, as defined by the proliferation markers Ki67 (a nuclear protein, which is associated with ribosomal RNA transcription; Fig. 1A), PCNA (a marker of cells in S-phase; Fig. 1B), p21 (an inhibitor of cell cycle progression; Fig. 1C) and p27 (an inhibitor of cyclin dependent kinase; Fig. 1D). Leu27IGF-2 infusion did not cause any change in the amount of apoptosis in the left ventricle, when compared to the Control group, as defined by calcineurin A (Fig. 2A), its NFATc3 signalling pathway (Fig. 2B and C) or caspase 3 cleavage (Fig. 2D). There was also no change in the percentage mononucleated cardiomyocytes in the left ventricle (Fig. 3A), septum (Fig. 3B) or right ventricle (Fig. 3C) following Leu27IGF-2 infusion, when compared with the Control fetuses.
Figure 1. Activation of cardiac IGF-2R via Leu27IGF-2 infusion into the coronary artery did not increase cardiomyocyte proliferation.
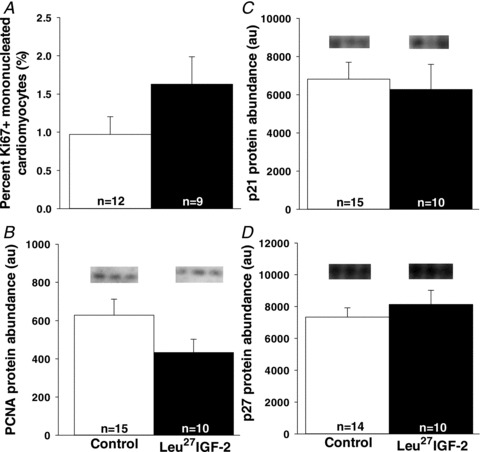
There was no change in markers of proliferation such as the percentage of Ki67+ mononucleated cardiomyocytes (A) or PCNA (B) and regulators of cell cycle at G1 phase such as p21 (C) or p27 (D) protein abundance in the left ventricle of control compared with Leu27IGF-2-infused fetuses. Representative blots from 3 animals in each group for each protein. Sample size for each group is indicated in the bar.
Figure 2. Activation of cardiac IGF-2R via Leu27IGF-2 infusion into the coronary artery did not induce cardiomyocyte apoptosis.
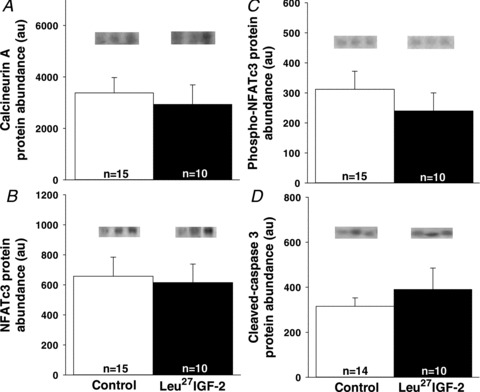
There were no changes in the protein abundance of calcineurin A (A), NFATc3 (B), phospho-NFATc3 (C) and cleaved-caspase 3 (D) in the left ventricle of control compared with Leu27IGF-2-infused fetuses. Representative blots from 3 animals in each group for each protein. Sample size for each group is indicated in the bar.
Figure 3. Activation of cardiac IGF-2R via Leu27IGF-2 infusion into the coronary artery did not change the percentage of mononucleated cardiomyocytes but did increase the area of binucleated cardiomyocytes in only the left ventricle.
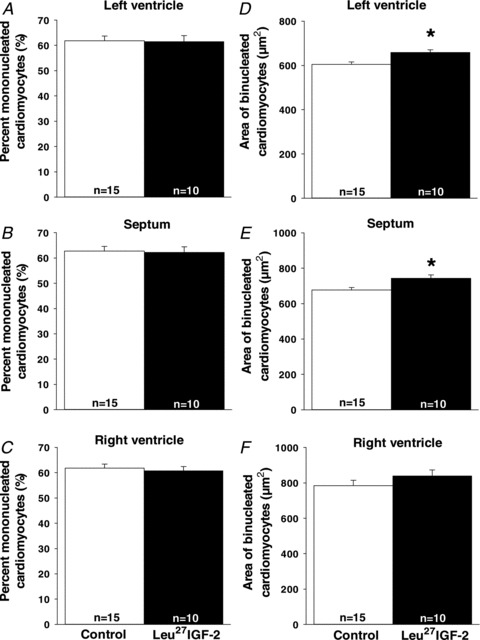
There were no changes in the proportion of mononucleated cardiomyocytes in the left ventricle (A), septum (B) or right ventricle (C) in control and Leu27IGF-2-infused fetuses. There was an increase in the area of binucleated cardiomyocytes in the left ventricle (D) and septum (E), areas perfused by the left circumflex coronary artery, but not the right ventricle (F) in control and Leu27IGF-2-infused fetuses. Sample size for each group is indicated in the bar. *Significantly different from control fetuses (P < 0.05).
Leu27IGF-2 infusion into the left circumflex coronary artery in fetal sheep caused an increase in the cross-sectional area of cardiomyocytes in the left ventricle
The infusion of Leu27IGF-2 resulted in increased cross-sectional area of binucleated cardiomyocytes in both the left ventricle (Fig. 3D) and the septum (Fig. 3E), when compared with control fetuses. There was, however, no change in the area of binucleated cardiomyocytes in the right ventricle, following the infusion of Leu27IGF-2 (Fig. 3F).
The increased left ventricular cardiomyocyte hypertrophy in response to Leu27IGF-2 infusion was not associated with Gαq signalling
There were no differences in CaMKII (Fig. 4A), phospho-CaMKII (Fig. 4B), HDAC 4 (Fig. 4C), phospho-HDAC 4 (Fig. 4D), PKC-α (Fig. 4E), phospho-PKC-α (Fig. 4F), HDAC 5 (Fig. 4G) or phospho-HDAC 5 (Fig. 4H) protein detected in Control and Leu27IGF-2 infused fetuses in either the left or the right ventricle. There were also no differences in the amount of ERK (Control, 53107 ± 2534 au; Leu27IGF-2 infused, 58068 ± 3731 au) and phospho-ERK (Control, 2180 ± 519 au; Leu27IGF-2 infused, 2098 ± 667 au) in the left ventricle (or the right ventricle, data not shown) for Control and Leu27IGF-2 infused fetuses.
Figure 4. The increase in the area of binucleated cardiomyocytes in the left ventricle was not stimulated through activation of the Gαq signalling pathway.
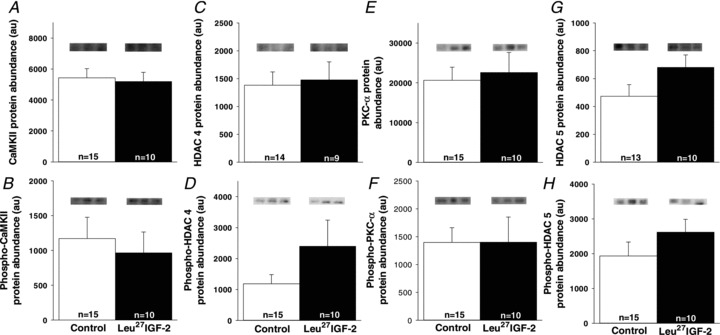
There were no differences in the amounts of CaMKII (A), phospho-CaMKII (B), HDAC 4 (C), phospho-HDAC 4 (D), PKC-α (E), phospho-PKC-α (F), HDAC 5 (G) and phospho-HDAC 5 (H) proteins in control compared with Leu27IGF-2-infused fetuses. Representative Western blots from 3 animals in each group for each protein. Sample size for each group is indicated in the bar.
Leu27IGF-2 infusion into the left circumflex coronary artery of fetal sheep induced the expression of markers of cardiomyocyte hypertrophy
The increased area of cardiomyocytes in the left ventricle of Leu27IGF-2 infused fetuses was associated with an increase in the amount of ANP (Fig. 5A), and a concomitant decrease in the protein expression of SERCA (Fig. 5C) and phospho-troponin I (Fig. 5E), when compared to the control fetuses.
Figure 5. Activation of cardiac IGF-2R via Leu27IGF-2 infusion into the coronary artery causes significant changes in the protein expression of markers of cardiac hypertrophy.
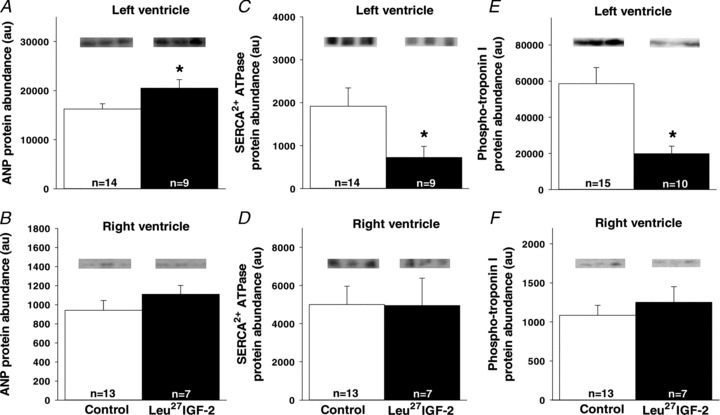
The amount of ANP protein (A) was significantly increased in the left ventricle of Leu27IGF-2-infused fetuses, while SERCA (C) and phospho-troponin I (E) were decreased. There were no changes in the amount of ANP (B), SERCA (D) and phospho-troponin I (F) proteins in the right ventricle of hearts from control compared with Leu27IGF-2-infused fetuses. Representative Western blots from 3 animals in each group for each protein. Sample size for each group is indicated in the bar. *Significantly different from control fetuses (P < 0.05).
The increased left ventricular cardiomyocyte hypertrophy in response to Leu27IGF-2 infusion was associated with Gαs signalling
Although there was no difference in the amount of PKA α/β/γ catalytic subunits (Fig. 6A), there was a significant increase in the amount of phospho-PKA α/β/γ catalytic subunits (Fig. 6B) in the left, but not right, ventricle (data not shown for right ventricle) of Leu27IGF-2 infused compared with control fetuses. There was no difference in the amount of phospho-PKAII α regulatory subunit for control (749 ± 62 au) and Leu27IGF-2 infused (738 ± 28 au) fetuses. There were significant increases in the amount of CREB (Fig. 6C) and phospho-CREB (Fig. 6D) in the left ventricle, but not the right ventricle (data not shown), for Leu27IGF-2 infused compared to control fetuses. There was no change in the size of mononucleated cardiomyocyte after treatment with Leu27IGF-2 (data not shown). Leu27IGF-2 treatment of binucleated cardiomyocytes significantly increased the cell cross-sectional area relative to cardiomyocytes that had been cultured in serum-free medium. The PKA inhibitor H-89 (Chijiwa et al. 1990; Zou et al. 1999) prevented the increase in cardiomyocyte area caused by Leu27IGF-2 (Fig. 7).
Figure 6. Cardiac activation of IGF-2R induced cardiomyocyte hypertrophy is mediated by the Gαs signalling pathway in normally grown fetuses.
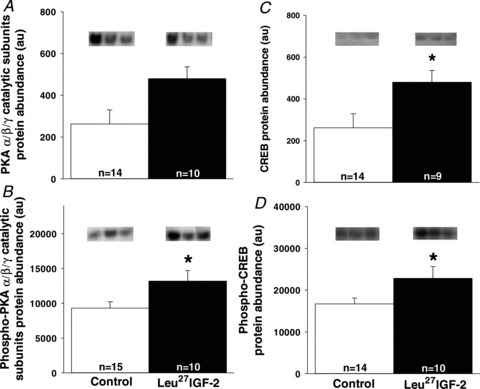
No change in the amount of PKAα/β/γ catalytic subunits (A) was observed in control and Leu27IGF-2-infused fetuses. There were increased amounts of left ventricle phospho-PKAα/β/γ catalytic subunits (B), CREB (C) and phospho-CREB (D) in Leu27IGF-2-infused fetuses. Representative blots from 3 animals in each group for each protein. Sample size for each group is indicated in the bar. *Significantly different from control fetuses (P < 0.05).
Figure 7. Activation of IGF-2R-induced cardiomyocyte hypertrophy in binucleated cardiomyocytes that is PKA dependent.
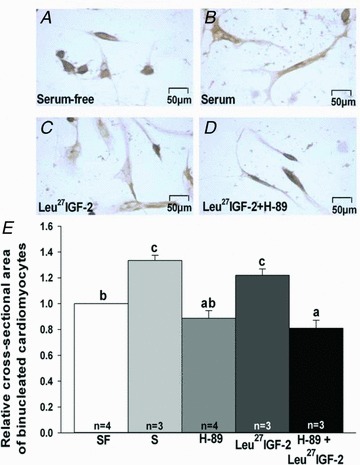
Images of fetal sheep cardiomyocytes treated with serum-free (A), serum (B), Leu27IGF-2 (C) and Leu27IGF-2 + H-89, a specific inhibitor of PKA (Chijiwa et al. 1990; Zou et al. 1999) (D) (scale bar for all panels, 50 μm). Results (E) were expressed as the mean ± SEM. Different letters denote significant differences between treatment groups (P < 0.05). SF, serum-free; S, serum.
Discussion
The role of the IGF-2R and the signalling pathways that it activates during cardiac development are not well established. This is the first study to specifically activate the cardiac IGF-2R signalling pathways during fetal life, in an animal model where cardiomyocyte maturation occurs in late gestation, as in the human (Woodcock & Matkovich, 2005). Our study demonstrated that cardiac IGF-2R activation resulted in hypertrophy via PKA activation that was independent of CaMKII, PKC or ERK signalling. Cardiac IGF-2R activation, however, did not result in cardiomyocyte proliferation, binucleation or apoptosis. This study places the IGF-2R-Gαs interaction as a potential contributor to cardiomyocyte hypertrophy.
The size of cardiomyocytes can be affected by changes in mechanical load (Jonker et al. 2007a); for example, right ventricular overload results in an increase in the percentage and size of binucleated cardiomyocytes (Barbera et al. 2000). The increase in cardiomyocyte size observed in this study was not due to changes in mechanical load because the intra-cardiac infusion of Leu27IGF-2 did not change after load, heart rate or cardiac work load. Thus, the infusion of Leu27IGF-2 into the left circumflex coronary artery to activate cardiac IGF-2R signalling demonstrated a specific effect on left ventricular cardiomyocyte hypertrophy, without any apparent systemic effects.
The increase in cardiomyocyte size in the absence of an increase in heart weight is consistent with our findings in the intrauterine growth restriction (IUGR) fetus (Morrison et al. 2007) where there is an increase in cardiac IGF-2R gene expression (Wang et al. 2011). In both cases, this may be due to a decrease in cardiomyocyte number. We did not observe a change in proliferation or apoptosis but it should be noted that the Leu27IGF-2 treatment was specific to the left ventricle and septum. We were not able to weigh the ventricle and although there was no change in relative heart weight (right ventricle, left ventricle, septum, atria and some vessels), it is not known if there was an increase in relative left ventricular weight. Furthermore, to determine cardiomyocyte number, the weight of the left ventricle would be required (Brüel et al. 2005; Bensley et al. 2010); however, we do not have this information because we isolated the cardiomyocytes and thus did not dissect the left ventricle prior to isolating the cardiomyocytes. In addition, it was suggested that hypertrophic growth of binucleated cardiomyocytes plays a minor role in increasing heart weight during late gestation of fetal sheep (Jonker et al. 2007b), which may explain the absence of increase heart weight.
Pathological cardiac hypertrophy can be induced through the activation of IGF-2R signalling, via phospho-PKC-α and/or CaMKII, which are downstream of Gαq (Chu et al. 2008). While IGF-2R signalling can induce apoptosis in cultured neonatal rat ventricular myocytes via Gαq and calcineurin signalling (Chu et al. 2009b), there was no upregulation of proteins involved in Gαq or of apoptosis signalling pathways, following Leu27IGF-2 infusion. There were, however, changes in proteins that are markers of cardiac pathological hypertrophy, including ANP, SERCA and phospho-troponin I. The increase in ANP and decrease in SERCA and phospho-troponin I suggested that an alternative signalling pathway may be involved. All of these markers can be affected by PKA, a protein that responds to Gαs (Skalhegg & Tasken, 2000). Gαs signalling has been linked to cardiac hypertrophy through its interaction with the β-adrenergic receptor (Barki-Harrington et al. 2004).
Gαs signals through the activation of adenylyl cyclase, resulting in the accumulation of cAMP, in turn resulting in activation of PKA (Skalhegg & Tasken, 2000); the PKA substrate then mediates a range of gene expression changes in response to cAMP, which can play an important regulatory role in cardiac function (Gonzalez et al. 1989). The IGF-2R has previously been linked to the Gi2 (Nishimoto, 1993) and Gαq (Chu et al. 2008) subunit signalling, where both were associated with cardiac hypertrophy (Böhm et al. 1994; D’Angelo et al. 1997). In an in vitro cardiomyocyte study of apoptosis, IGF-2R activation resulted in decreased PKA protein expression (Chu et al. 2009a), but no previous studies have shown a link between the IGF-2R and cardiomyocyte hypertrophy involving Gαs signalling. Therefore our finding that IGF-2R activation can cause cardiomyocyte hypertrophy in vivo, in a Gαs signalling-dependent manner, is novel and this signalling could have potential implications for cardiac pathogenesis. Together, these findings suggest that IGF-2R activation can induce cardiomyocyte hypertrophy either through the Gi2, Gαq or Gαs signalling pathways. Further studies investigating the trigger (e.g. glucose, oxygen, calcium levels) that regulates the specific activation of these different IGF-2R downstream signalling pathways are required.
An increase in cAMP and the phosphorylation of PKA leads to an increased amount of phospho-troponin I and SERCA and is suggestive of enhanced cardiac contractile ability (Noland et al. 1995). We have, however, observed a discrepancy between PKA and phospho-troponin I protein expression. A similar finding was also reported in 12- to 13-week-old spontaneously hypertensive rats, possibly due to local regulation of PKA activity or the existence of multiple subcellular pools of cAMP (Bokník et al. 2001). The discrepancy between phospho-troponin I and cAMP may be due to the dissociation of the total cellular cAMP levels and downstream activation of PKA-dependent substrate phosphorylation (McConnell et al. 1998). In addition, many studies have suggested that compartmentalization of cAMP or PKA in cardiomyocytes may account for the lack of correspondence between increased total cellular cAMP and phospho-PKA substrate (Buxton & Brunton, 1983). Overall, our findings suggest that cardiac IGF-2R activation may alter important regulators of cardiac contractility and relaxation. It is possible that these changes precede the onset of chronic heart failure and this also requires further investigation, for example investigating cardiac Ca2+ sensitivity for the contractile apparatus and maximum Ca2+-activated force (Posterino et al. 2011).
In summary, Leu27IGF-2 infusion into the left circumflex coronary artery activated cardiac IGF-2R and induced Gαs signalling that was associated with cardiomyocyte hypertrophy (Fig. 8). This is significant as it places the interaction between the IGF-2R and the Gαs signalling pathway as a potential mechanism that can contribute to cardiomyocyte growth in fetal life, but which may result in pathological cardiomyocyte hypertrophy in postnatal life. This link may be important to understand why individuals who are born with low birth weight have an increased vulnerability to cardiovascular disease in adult life (Barker, 1995; Rich-Edwards et al. 1997).
Figure 8. Infusion of Leu27IGF-2 into the left circumflex coronary artery leads to cardiomyocyte hypertrophy in the normally grown fetus in late gestation.
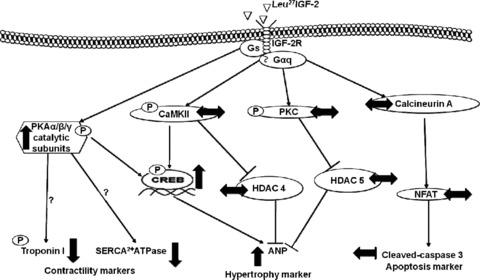
Activation of the IGF-2R with Leu27IGF2 in vivo did not change the amount of downstream proteins in the Gαq signalling pathway. However, there was an upregulation of the Gαs pathway as indicated by increased amounts of phospho-PKA α/β/γ catalytic subunits. ↑, increased protein expression compared with control fetuses; ↓, decreased protein expression compared with control fetuses; ↔, no changes in protein expression compared with control fetuses; p, protein phosphorylation.
Acknowledgments
We acknowledge Ms Stacey Dunn and Dr Kirsty Warnes who assisted during the surgical procedures, Ms Allison Martinez and Mr Rajan Poudel who provided expert post-surgical care of the ewe and fetus as well as members of the Early Origins of Adult Health Research Group for their expert assistance in sheep surgery and postmortems in this study. This work and J.L.M. were supported by a South Australian Cardiovascular Research Network Fellowship (CR10A4988) from the Heart Foundation of Australia. D.A.B. was supported by a Senior Research Fellowship from the National Health and Medical Research Council of Australia (NHMRC; 349405). The authors have no conflict of interest to declare.
Glossary
- ANP
atrial natriuretic peptide
- CaMKII
Ca2+–calmodulin-dependent protein kinase II
- CREB
cAMP response element-binding
- DBP
diastolic blood pressure
- Erk1/2
p44/42 MAP kinase
- Gαq
G protein-coupled αq
- Hb
haemoglobin
- HDAC
histone deacetylase
- HR
heart rate
- IGF
insulin-like growth factor
- IGF-1R
insulin-like growth factor-1 receptor
- MAP
mean arterial pressure
- NFATc3
nuclear factor of activated T-cells
- p21
cyclin-dependent kinase inhibitor 21
- PCNA
proliferating cell nuclear antigen
- PKA
protein kinase A
- PKC-α
protein kinase C-α
- RPP
rate pressure product
- SBP
systolic blood pressure
- SERCA
sarcoplasmic reticulum Ca2+-ATPase
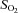
oxygen saturation
Author contributions
K.W.C.W., D.A.B. and J.L.M. were responsible for the conception and design of the experiments. K.W.C.W., K.L.T. and J.L.M. were each involved in data acquisition. K.W.C.W., D.A.B. and J.L.M. were involved in analysis and interpretation of the data. K.W.C.W., D.A.B. and J.L.M. drafted the article and all authors contributed to the final version.
References
- Barbera A, Giraud GD, Reller MD, Maylie J, Morton MJ, Thornburg KL. Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1157–R1164. doi: 10.1152/ajpregu.2000.279.4.R1157. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barki-Harrington L, Perrino C, Rockman HA. Network integration of the adrenergic system in cardiac hypertrophy. Cardiovasc Res. 2004;63:391–402. doi: 10.1016/j.cardiores.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Bauman RP, Rembert JC, Himmelstein SI, Klotman PE, Greenfield JC., Jr Effect of atrial natriuretic factor on transmural myocardial blood flow distribution in the dog. Circulation. 1987;76:705–709. doi: 10.1161/01.cir.76.3.705. [DOI] [PubMed] [Google Scholar]
- Bensley JG, Stacy VK, De Matteo R, Harding R, Black MJ. Cardiac remodelling as a result of pre-term birth: implications for future cardiovascular disease. Eur Heart J. 2010;31:2058–2066. doi: 10.1093/eurheartj/ehq104. [DOI] [PubMed] [Google Scholar]
- Böhm M, Moll M, Schmid B, Paul M, Ganten D, Castellano M, Erdmann E. β-Adrenergic neuroeffector mechanisms in cardiac hypertrophy of renin transgenic rats. Hypertension. 1994;24:653–662. doi: 10.1161/01.hyp.24.6.653. [DOI] [PubMed] [Google Scholar]
- Bokník P, Heinroth-Hoffmanb I, Kirchhefer U, Knapp J, Linck B, Lüss H, Müller T, Schmitz W, Brodde OE, Neumann J. Enhanced protein phosphorylation in hypertensive hypertrophy. Cardiovasc Res. 2001;51:717–728. doi: 10.1016/s0008-6363(01)00346-7. [DOI] [PubMed] [Google Scholar]
- Botting KJ, Wang KC, Padhee M, McMillen IC, Summers-Pearce B, Rattanatray L, Cutri N, Posterino GS, Brooks DA, Morrison JL. Early origins of heart disease: low birth weight and determinants of cardiomyocyte endowment. Clin Exp Pharmacol Physiol. 2011;39:814–823. doi: 10.1111/j.1440-1681.2011.05649.x. [DOI] [PubMed] [Google Scholar]
- Brüel A, Oxlund H, Nyengaard JR. The total length of myocytes and capillaries, and total number of myocyte nuclei in the rat heart are time-dependently increased by growth hormone. Growth Horm IGF Res. 2005;15:256–264. doi: 10.1016/j.ghir.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Buxton IL, Brunton LL. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem. 1983;258:10233–10239. [PubMed] [Google Scholar]
- Chen RJ, Wu HC, Chang MH, Lai CH, Tien YC, Hwang JM, Kuo WH, Tsai FJ, Tsai CH, Chen LM, Huang CY, Chu CH. Leu27IGF2 plays an opposite role to IGF1 to induce H9c2 cardiomyoblast cell apoptosis via Gaq signaling. J Mol Endocrinol. 2009;43:221–230. doi: 10.1677/JME-08-0121. [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- Chu CH, Huang CY, Lu MC, Lin JA, Tsai FJ, Tsai CH, Chu CY, Kuo WH, Chen LM, Chen LY. Enhancement of AG1024-induced H9c2 cardiomyoblast cell apoptosis via the interaction of IGF2R with Gα proteins and its downstream PKA and PLC-β modulators by IGF-II. Chin J Physiol. 2009a;52:31–37. doi: 10.4077/cjp.2009.amh004. [DOI] [PubMed] [Google Scholar]
- Chu CH, Tzang BS, Chen LM, Kuo CH, Cheng YC, Chen LY, Tsai FJ, Tsai CH, Kuo WW, Huang CY. IGF-II/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/BNP expression via Gαq interaction and protein kinase C-α/CaMKII activation in H9c2 cardiomyoblast cells. J Endocrinol. 2008;197:381–390. doi: 10.1677/JOE-07-0619. [DOI] [PubMed] [Google Scholar]
- Chu CH, Tzang BS, Chen LM, Liu CJ, Tsai FJ, Tsai CH, Lin JA, Kuo WW, Bau DT, Yao CH, Huang CY. Activation of insulin-like growth factor II receptor induces mitochondrial-dependent apoptosis through Gaq and downstream calcineurin signaling in myocardial cells. Endocrinology. 2009b;150:2723–2731. doi: 10.1210/en.2008-0975. [DOI] [PubMed] [Google Scholar]
- D’Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW., II Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson L, McMillen IC, Dyer JL, Morrison JL. Restriction of placental growth results in greater hypotensive response toa-adrenergic blockade in fetal sheep during late gestation. J Physiol. 2005;563:611–620. doi: 10.1113/jphysiol.2004.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LJ, Simonetta G, Owens JA, Robinson JS, McMillen IC. Restriction of placental and fetal growth in sheep alters fetal blood pressure responses to angiotensin II and captopril. J Physiol. 1999;515:897–904. doi: 10.1111/j.1469-7793.1999.897ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud GD, Louey S, Jonker SS, Schultz J, Thornburg KL. Cortisol stimulates cell cycle activity in the cardiomyocyte of the sheep fetus. Endocrinology. 2006;147:3643–3649. doi: 10.1210/en.2006-0061. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Yamamoto KK, Fischer WH, Karr D, Menzel P, Biggs W, 3rd, Vale WW, Montminy MR. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989;337:749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Hawkins P, Steyn C, Ozaki T, Saito T, Noakes DE, Hanson MA. Effect of maternal undernutrition in early gestation on ovine fetal blood pressure and cardiovascular reflexes. Am J Physiol Regul Integr Comp Physiol. 2000;279:R340–R348. doi: 10.1152/ajpregu.2000.279.1.R340. [DOI] [PubMed] [Google Scholar]
- Huang CY, Hao LY, Buetow DE. Hypertrophy of cultured adult rat ventricular cardiomyocytes induced by antibodies against the insulin-like growth factor (IGF)-I or the IGF-I receptor is IGF-II-dependent. Mol Cell Biochem. 2002;233:65–72. doi: 10.1023/a:1015514324328. [DOI] [PubMed] [Google Scholar]
- Jonker SS, Faber JJ, Anderson DF, Thornburg KL, Louey S, Giraud GD. Sequential growth of fetal sheep cardiac myocytes in response to simultaneous arterial and venous hypertension. Am J Physiol Regul Integr Comp Physiol. 2007a;292:R913–R919. doi: 10.1152/ajpregu.00484.2006. [DOI] [PubMed] [Google Scholar]
- Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol. 2007b;102:1130–1142. doi: 10.1152/japplphysiol.00937.2006. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Levy D, Cupples LA. Left ventricular hypertrophy and risk of cardiac failure: insights from the Framingham Study. J Cardiovasc Pharmacol. 1987;10:S135–140. [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Lavandero S, Foncea R, Pérez V, Sapag-Hagar M. Effect of inhibitors of signal transduction on IGF-1-induced protein synthesis associated with hypertrophy in cultured neonatal rat ventricular myocytes. FEBS Lett. 1998;422:193–196. doi: 10.1016/s0014-5793(98)00008-8. [DOI] [PubMed] [Google Scholar]
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- McConnell BK, Moravec CS, Bond M. Troponin I phosphorylation and myofilament calcium sensitivity during decompensated cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 1998;274:H385–H396. doi: 10.1152/ajpheart.1998.274.2.H385. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R306–R313. doi: 10.1152/ajpregu.00798.2006. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Carmichael L, Homan J, Richardson BS. The effects of ‘sleep promoting agents’ on behavioural state in the ovine fetus. Brain Res Dev Brain Res. 1997;103:1–8. doi: 10.1016/s0165-3806(97)00093-x. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Chien C, Gruber N, Rurak D, Riggs W. Fetal behavioural state changes following maternal fluoxetine infusion in sheep. Brain Res Dev Brain Res. 2001;131:47–56. doi: 10.1016/s0165-3806(01)00255-3. [DOI] [PubMed] [Google Scholar]
- Muhlhausler BS, Duffield JA, Ozanne SE, Pilgrim C, Turner N, Morrison JL, McMillen IC. The transition from fetal growth restriction to accelerated postnatal growth: a potential role for insulin signalling in skeletal muscle. J Physiol. 2009;587:4199–4211. doi: 10.1113/jphysiol.2009.173161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto I. The IGF-II receptor system: a G protein-linked mechanism. Mol Reprod Dev. 1993;35:398–406. doi: 10.1002/mrd.1080350414. [DOI] [PubMed] [Google Scholar]
- Noland TA, Jr, Guo X, Raynor RL, Jideama NM, Averyhart-Fullard V, Solaro RJ, Kuo JF. Cardiac troponin I mutants. Phosphorylation by protein kinases C and A and regulation of Ca2+-stimulated MgATPase of reconstituted actomyosin S-1. J Biol Chem. 1995;270:25445–25454. doi: 10.1074/jbc.270.43.25445. [DOI] [PubMed] [Google Scholar]
- Oakley C. Ventricular hypertrophy in cardiomyopathy. Br Heart J. 1971;33:179–186. doi: 10.1136/hrt.33.suppl.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Müller HL, Zhang H, Ling N, Rosenfeld RG. Synthesis and characterization of IGF-II analogs: Applications in the evaluation of IGF receptor function and IGF-independent actions of IGFBPs. Adv Exp Med Biol. 1993;343:41–54. doi: 10.1007/978-1-4615-2988-0_5. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Dunn SL, Botting KJ, Wang W, Gentili S, Morrison JL. Changes in cardiac troponins with gestational age explain changes in cardiac muscle contractility in the sheep fetus. J Appl Physiol. 2011;111:236–243. doi: 10.1152/japplphysiol.00067.2011. [DOI] [PubMed] [Google Scholar]
- Powell K, Rooke JA, McEvoy TG, Ashworth CJ, Robinson JJ, Wilmut I, Young LE, Sinclair KD. Zygote donor nitrogen metabolism and in vitro embryo culture perturbs in utero development and IGF2R expression in ovine fetal tissues. Theriogenology. 2006;66:1901–1912. doi: 10.1016/j.theriogenology.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Reini SA, Wood CE, Keller-Wood M. The ontogeny of genes related to ovine fetal cardiac growth. Gene Expr Patterns. 2009;9:122–128. doi: 10.1016/j.gep.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards J, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, Colditz GA, Willett WC, Hennekens CH. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ. 1997;315:396–400. doi: 10.1136/bmj.315.7105.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph AM, Roman C, Gournay V. Perinatal myocardial DNA and protein changes in the lamb: effect of cortisol in the fetus. Pediatr Res. 1999;46:141–146. doi: 10.1203/00006450-199908000-00002. [DOI] [PubMed] [Google Scholar]
- Sakano K, Enjoh T, Numata F, Fujiwarat H, Marumoto Y, Higashihashi N, Sato Y, Perduell JF, Fujita-Yamaguchi Y. The design, expression, and characterization of human insulin-like growth factor II (IGF-II) mutants specific for either the IGF-II/cation-independent mannose 6-phosphate receptor or IGF-I receptor. J Biol Chem. 1991;266:20626–20635. [PubMed] [Google Scholar]
- Skalhegg BS, Tasken K. Specificity in the cAMP/PKA signaling pathway. Differential expression,regulation, and subcellular localization of subunits of PKA. Front Biosci. 2000;5:D678–693. doi: 10.2741/skalhegg. [DOI] [PubMed] [Google Scholar]
- Sundgren NC, Giraud GD, Schultz JM, Lasarev MR, Stork PJ, Thornburg KL. Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1481–R1489. doi: 10.1152/ajpregu.00232.2003. [DOI] [PubMed] [Google Scholar]
- Wang KC, Brooks DA, Botting KJ, Morrison JL. IGF-2R-mediated signaling results in hypertrophy of cultured cardiomyocytes from fetal sheep. Biol Reprod. 2012;86:183. doi: 10.1095/biolreprod.112.100388. [DOI] [PubMed] [Google Scholar]
- Wang KC, Zhang L, McMillen IC, Botting KJ, Duffield JA, Zhang S, Suter CM, Brooks DA, Morrison JL. Fetal growth restriction and the programming of heart growth and cardiac insulin-like growth factor 2 expression in the lamb. J Physiol. 2011;589:4709–4722. doi: 10.1113/jphysiol.2011.211185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Matkovich SJ. Cardiomyocytes structure, function and associated pathologies. Int J Biochem Cell Biol. 2005;37:1746–1751. doi: 10.1016/j.biocel.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Yue TL, Gu JL, Wang C, Reith AD, Lee JC, Mirabilei RC, Kreutz R, Wang Y, Maleeffi B, Parsons AA, Ohlstein EH. Extracellular signal-regulated kinase plays an essential role in hypertrophic agonists, endothelin-1 and phenylephrine-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275:37895–37901. doi: 10.1074/jbc.M007037200. [DOI] [PubMed] [Google Scholar]
- Zou Y, Komuro I, Yamazaki T, Kudoh S, Uozumi H, Kadowaki T, Yazaki Y. Both Gs and Gi proteins are critically involved in isoproterenol-induced cardiomyocyte hypertrophy. J Biol Chem. 1999;274:9760–9770. doi: 10.1074/jbc.274.14.9760. [DOI] [PubMed] [Google Scholar]


