Abstract
Energy transfer between mitochondrial and cytosolic compartments is predominantly achieved by creatine-dependent phosphate shuttling (PCr/Cr) involving mitochondrial creatine kinase (miCK). However, ADP/ATP diffusion through adenine nucleotide translocase (ANT) and voltage-dependent anion carriers (VDACs) is also involved in this process. To determine if exercise alters the regulation of this system, ADP-stimulated mitochondrial respiratory kinetics were assessed in permeabilized muscle fibre bundles (PmFBs) taken from biopsies before and after 2 h of cycling exercise (60%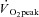 ) in nine lean males. Concentrations of creatine (Cr) and phosphocreatine (PCr) as well as the contractile state of PmFBs were manipulated in situ. In the absence of contractile signals (relaxed PmFBs) and miCK activity (no Cr), post-exercise respiratory sensitivity to ADP was reduced in situ (up to 126% higher apparent Km to ADP) suggesting inhibition of ADP/ATP diffusion between matrix and cytosolic compartments (possibly ANT and VDACs). However this effect was masked in the presence of saturating Cr (no effect of exercise on ADP sensitivity). Given that the role of ANT is thought to be independent of Cr, these findings suggest ADP/ATP, but not PCr/Cr, cycling through the outer mitochondrial membrane (VDACs) may be attenuated in resting muscle after exercise. In contrast, in contracted PmFBs, post-exercise respiratory sensitivity to ADP increased with miCK activation (saturating Cr; 33% lower apparent Km to ADP), suggesting prior exercise increases miCK sensitivity in situ. These observations demonstrate that exercise increases miCK-dependent respiratory sensitivity to ADP, promoting mitochondrial–cytosolic energy exchange via PCr/Cr cycling, possibly through VDACs. This effect may mask an underlying inhibition of Cr-independent ADP/ATP diffusion. This enhanced regulation of miCK-dependent phosphate shuttling may improve energy homeostasis through more efficient coupling of oxidative phosphorylation to perturbations in cellular energy charge during subsequent bouts of contraction.
) in nine lean males. Concentrations of creatine (Cr) and phosphocreatine (PCr) as well as the contractile state of PmFBs were manipulated in situ. In the absence of contractile signals (relaxed PmFBs) and miCK activity (no Cr), post-exercise respiratory sensitivity to ADP was reduced in situ (up to 126% higher apparent Km to ADP) suggesting inhibition of ADP/ATP diffusion between matrix and cytosolic compartments (possibly ANT and VDACs). However this effect was masked in the presence of saturating Cr (no effect of exercise on ADP sensitivity). Given that the role of ANT is thought to be independent of Cr, these findings suggest ADP/ATP, but not PCr/Cr, cycling through the outer mitochondrial membrane (VDACs) may be attenuated in resting muscle after exercise. In contrast, in contracted PmFBs, post-exercise respiratory sensitivity to ADP increased with miCK activation (saturating Cr; 33% lower apparent Km to ADP), suggesting prior exercise increases miCK sensitivity in situ. These observations demonstrate that exercise increases miCK-dependent respiratory sensitivity to ADP, promoting mitochondrial–cytosolic energy exchange via PCr/Cr cycling, possibly through VDACs. This effect may mask an underlying inhibition of Cr-independent ADP/ATP diffusion. This enhanced regulation of miCK-dependent phosphate shuttling may improve energy homeostasis through more efficient coupling of oxidative phosphorylation to perturbations in cellular energy charge during subsequent bouts of contraction.
Key point
ATP transfer from mitochondria to the cytoplasm occurs mainly through phosphate transfer to creatine by mitochondrial creatine kinase (miCK) but also by transport and/or diffusion of ADP and ATP through specific mitochondrial transport protein complexes.
Determining the effect of exercise on phosphate shuttling may require contractile signals in situ and varying creatine concentrations to alter miCK activity.
Mitochondrial respiratory sensitivity to ADP was assessed in permeabilized muscle fibre bundles (PmFBs) before and after 2 h cycling exercise in human skeletal muscle.
In relaxed PmFBs, ADP sensitivity decreased post-exercise when miCK phosphate shuttling was low (no creatine) with no change in net ADP sensitivity in the presence of creatine, whereas in contracting fibres post-exercise ADP sensitivity was higher with creatine.
This shows miCK activity is increased post-exercise, especially during contraction in PmFBs, and suggests exercise regulates phosphate shuttling, which would improve maintenance of energy homeostasis during contraction.
Introduction
Muscle contraction imposes an enormous challenge on cellular energy homeostasis with an increase in aerobic ATP turnover by up to 100-fold (Hochachka & McClelland, 1997). While it is understood that the considerable rise in free ADP (ADPf) relative to ATP during contraction drives oxidative phosphorylation, very little is known about the mechanism by which ADPf and ATP are transported across the mitochondria through specific protein complexes. A previous report in permeabilized muscle fibre bundles (PmFBs) demonstrated increased muscle mitochondrial respiration in response to a single submaximal ADP concentration in the presence of creatine following acute exercise (Tonkonogi et al. 1998). Interestingly, this effect was not observed in the absence of creatine suggesting altered regulation of mitochondrial creatine kinase (miCK) had occurred (Tonkonogi et al. 1998; Walsh et al. 2001a). In stark contrast, chronic exercise has the opposite effect whereby respiratory sensitivity to ADP in PmFBs is decreased in situ (Mettauer et al. 2001; Walsh et al. 2001b; Zoll et al. 2002, 2003a,b; Guerrero et al. 2005). A lower sensitivity following chronic exercise is particularly perplexing as this would seem counter-productive to efficiently increasing oxidative phosphorylation in response to a perturbation in energy charge. Collectively the disparate findings across these studies highlight our limited understanding with respect to exercise effects on mitochondrial ADP-stimulated respiratory kinetics.
While the reason for the paradoxical apparent difference between acute and chronic exercise is unclear, the lone observation of a miCK-dependent enhancement in respiratory sensitivity to ADP following acute exercise (Tonkonogi et al. 1998) warrants further analyses into how exercise or contraction signals impact ADP sensitivity. The importance of this re-evaluation is underscored by the recent development of methods permitting control of PmFB contractile state in situ during assessments of mitochondrial respiratory kinetics. PmFBs appear to undergo Ca2+-independent ADP-induced contraction during in situ assessments of ADP-stimulated respiration (Perry et al. 2011). This effect has previously been unrecognized in permeabilized skeletal muscle despite indications in permeabilized cardiomyocytes (Ventura-Clapier & Vassort, 1985; Anmann et al. 2005, 2006; Kuznetsov et al. 2012). PmFB contraction increases respiratory sensitivity to ADP in the presence of Cr suggesting miCK activity is coupled to the contractile state of skeletal muscle. Importantly, PmFB spontaneous contraction is considerably attenuated by the myosin-ATPase inhibitor blebbistatin (BLEB), thereby providing the ability to manipulate the contractile state during assessments of mitochondrial respiration (Perry et al. 2011). Thus, as an extension of previous findings (Tonkonogi et al. 1998; Walsh et al. 2001a), mitochondrial respiratory control by ADP in response to acute exercise and during recovery can, for the first time, be performed by manipulating the contractile state of the muscle itself in situ. This approach may reveal whether the effect of exercise on respiratory sensitivity to ADP depends on the presence, or absence, of contraction-generated signals in addition to experimental manipulation of miCK activity.
The purpose of this study was to determine if exercise acutely increases miCK-dependent and -independent mitochondrial respiratory sensitivity to ADP in human skeletal muscle when assessed post-exercise in PmFBs. We hypothesized that the effect of exercise may depend on whether PmFBs contract or remain relaxed during in situ assessments of mitochondrial respiratory function. Therefore, the kinetic properties of ADP stimulated mitochondrial respiration were determined in PmFBs from muscle biopsies taken before and after cycling exercise in the presence (relaxation) or absence (contraction) of a myosin ATPase inhibitor (BLEB). The contribution of miCK to regulating mitochondrial respiratory sensitivity to ADP via the phosphate shuttle model for energy transfer (Veksler et al. 1995; Kuznetsov et al. 1996; Tonkonogi et al. 1998; Walsh et al. 2001c; Guzun et al. 2012) was manipulated by varying assay medium creatine (Cr) and phosphocreatine (PCr) concentrations. Our results demonstrate that exercise alters post-exercise mitochondrial respiratory control in a highly specific, and divergent, manner depending on the contractile state of PmFBs, revealing a complex and dynamic interplay between contraction stimuli and phosphate shuttling.
Methods
All experimental procedures with humans and rodents were approved by the Research Ethics Boards at the University of Guelph in Guelph, ON and McMaster University in Hamilton, ON AND the Institutional Review Board at East Carolina University, NC. The human experiments conformed to the Declaration of Helsinki.
Human subjects, exercise testing and muscle biopsies
Nine healthy, recreationally active men (Group I) were recruited to participate in this investigation. Their mean ± SEM age, height, weight, BMI and 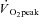 were 21.4 ± 0.6 years, 1.78 ± 0.03 m, 74.1 ± 3.7 kg, 23.4 ± 0.9 kg m−2 and 53.5 ± 3.5 ml kg−1 min−1, respectively. Two additional men (Group II: sedentary, 25.4 ± 0.1 years, 1.80 ± 0.01 m, 84.5 ± 3.0 kg, 26.2 ± 0.7 kg m−2) were recruited for separate validation experiments as described below. All participants were non-smokers, free of disease and not taking prescription medications or supplements. Subjects were given both oral and written information about the experimental procedures before giving their informed consent.
were 21.4 ± 0.6 years, 1.78 ± 0.03 m, 74.1 ± 3.7 kg, 23.4 ± 0.9 kg m−2 and 53.5 ± 3.5 ml kg−1 min−1, respectively. Two additional men (Group II: sedentary, 25.4 ± 0.1 years, 1.80 ± 0.01 m, 84.5 ± 3.0 kg, 26.2 ± 0.7 kg m−2) were recruited for separate validation experiments as described below. All participants were non-smokers, free of disease and not taking prescription medications or supplements. Subjects were given both oral and written information about the experimental procedures before giving their informed consent.
Subjects initially completed a standardized graded 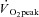 test on a cycle ergometer. After at least 72 h, subjects returned to the laboratory and completed a practice 2 h cycling session at 60%
test on a cycle ergometer. After at least 72 h, subjects returned to the laboratory and completed a practice 2 h cycling session at 60%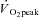 . Subjects then reported to the clinical laboratory at least 1 week later for the actual experiment. Group II subjects arrived after an overnight fast. With the subject lying supine on a bed, a single skeletal muscle sample was obtained from the lateral aspect of vastus lateralis by percutaneous needle biopsy technique under local subcutaneous anaesthesia (2% lidocaine without norepinephrine). Group I subjects then moved to the cycle ergometer and cycled at the pre-determined intensity matching 60%
. Subjects then reported to the clinical laboratory at least 1 week later for the actual experiment. Group II subjects arrived after an overnight fast. With the subject lying supine on a bed, a single skeletal muscle sample was obtained from the lateral aspect of vastus lateralis by percutaneous needle biopsy technique under local subcutaneous anaesthesia (2% lidocaine without norepinephrine). Group I subjects then moved to the cycle ergometer and cycled at the pre-determined intensity matching 60%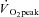 for 2 h. Immediately following exercise, the subject moved back to the bed and a second biopsy was taken in the opposite leg as the pre-exercise biopsy. Subjects remained at rest in the sitting position for 3 h after which a third and final biopsy was taken on the initial leg sampled with the subject lying supine on a bed. Group II subjects received only one biopsy and did not complete the exercise protocol. A portion of each biopsy sample was immediately placed into ice-cold BIOPS (described below) and used to prepare permeabilized fibre bundles (PmFBs) within an hour.
for 2 h. Immediately following exercise, the subject moved back to the bed and a second biopsy was taken in the opposite leg as the pre-exercise biopsy. Subjects remained at rest in the sitting position for 3 h after which a third and final biopsy was taken on the initial leg sampled with the subject lying supine on a bed. Group II subjects received only one biopsy and did not complete the exercise protocol. A portion of each biopsy sample was immediately placed into ice-cold BIOPS (described below) and used to prepare permeabilized fibre bundles (PmFBs) within an hour.
Rodents and microscopic imaging of PmFB conformation
Male Sprague–Dawley rats (Charles River Laboratories) were housed in a temperature- (22°C) and light-controlled room and given free access to food and water. Rats were 8–12 weeks old and weighed 350–375 g at the time of the experiments. Red gastrocnemius was obtained from anaesthetized animals (100 mg kg−1 i.p. ketamine–xylazine or 6 mg kg−1 sodium pentobarbital). After surgery animals were killed by cervical dislocation while anaesthetized. Videos of red gastrocnemius PmFB conformation were taken (Infinity 2 attached to Zeiss Stemi 2000, Oberkochen, Germany) following exposure to MiR05 at 37°C with or without protease inhibitors to determine if proteolysis is a cause of PmFB contraction (see Results). MiR05 temperature was maintained by a temperature-controlled base (pre-heated metal block).
Preparation of permeabilized muscle fibres
The technique is partially adapted from previous methods (Kuznetsov et al. 1996; Tonkonogi et al. 2003) and has been described previously (Anderson et al. 2007; Perry et al. 2011; Smith et al. 2011). Briefly, small portions (∼25 mg) of muscle were dissected from each biopsy and placed in ice-cold BIOPS, containing (in mm): 50 MES, 7.23 K2EGTA, 2.77 CaK2EGTA, 20 imidazole, 0.5 dithiothreitol (DTT), 20 taurine, 5.77 ATP, 15 PCr, and 6.56 MgCl2·6 H2O (pH 7.1). The muscle was trimmed of connective tissue and fat and divided into several small muscle bundles (∼2–7 mm, 1.0–2.5 mg wet weight). Each bundle was gently separated along their longitudinal axis with a pair of anti-magnetic needle-tipped forceps under magnification (Zeiss Stemi 2000). Bundles from human vastus lateralis were then treated with 30 μg ml−1 saponin in BIOPS and incubated on a rotor for 30 min at 4°C. Saponin at 30 μg ml−1 has previously been shown to optimize respiration in human skeletal muscle (Kane et al. 2011). Bundles from rat red gastrocnemius were permeabilized with 40μg ml−1 saponin, which optimizes state III respiratory kinetics in rodent muscle (authors’ unpublished observations). Saponin is a mild, cholesterol-specific detergent that selectively permeabilizes the sarcolemmal membranes while keeping mitochondrial membranes, which contain little cholesterol, intact (Veksler et al. 1987; Kuznetsov et al. 2008). Following permeabilization, the PmFBs were placed in MiR05 containing (in mm): 0.5 EGTA, 10 KH2PO4, 3 MgCl2.6 H2O, 60 potassium lactobionate, 20 Hepes, 110 sucrose and 1 mg ml−1 fatty acid free BSA (pH 7.1). PmFBs were washed at 4°C (<30 min) in MiR05 until the respiratory measurements were initiated.
Mitochondrial respiration in permeabilized fibres
High-resolution O2 consumption measurements were conducted in 2 ml of respiration medium (MiR05) using the Oroboros Oxygraph-2k (Oroboros Instruments, Corp., Innsbruck, Austria) with stirring at 750 rpm. Respiration medium contained various concentrations of creatine hydrate (0, 12 and 20 mm) or phosphocreatine (0 or 24 mm) to modify kinetics of creatine kinase, which facilitates mitochondrial ADP transport (Saks et al. 1991, 1994, 1995; Walsh et al. 2001c; Anmann et al. 2005). For ADP-stimulated respiratory kinetics, 5 mm pyruvate and 5 mm malate were added as complex I substrates (via generation of NADH to saturate electron entry into complex I) followed by ADP titrations in step-wise increments. Pyruvate or glutamate was titrated under maximal State 3 conditions (5 mm ADP and 5 mm malate, 20 mm creatine) in separate experiments. All experiments were completed before the oxygraph chamber [O2] reached 150 μm. PmFBs spontaneously contract in assay medium, a phenomenon which can be prevented by the myosin II-specific inhibitor (BLEB) (Perry et al. 2011). BLEB at 25 μm, dissolved in DMSO (5 mm stock), was used to prevent spontaneous contraction in some PmFB experiments for comparison with contracted (–BLEB) respiratory kinetics before and after exercise. Polarographic oxygen measurements were acquired in 2 s intervals, with the rate of respiration derived from 40 data points, and expressed as pmol s−1 (mg dry weight)−1. Dry bundle weights were consistently between ∼0.2 and 0.6 mg to avoid normalization errors with excessively low or high weights (unpublished observations). Cytochrome c was added to test for mitochondrial membrane integrity, with all experiments demonstrating <10% increase in respiration. At the conclusion of each experiment, PmFBs were dried via lyophilization.
The apparent Km for ADP was determined through the Michaelis–Menten enzyme kinetics – fitting model (Y=Vmax×X/(Km+X)), where X is [free ADP] (ADPf) and Y is 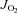 at [ADPf]– using Prism (GraphPad Software, Inc., La Jolla, CA, USA), as published previously (Perry et al. 2011). Similar analyses were performed for pyruvate and glutamate using their respective titration kinetics.
at [ADPf]– using Prism (GraphPad Software, Inc., La Jolla, CA, USA), as published previously (Perry et al. 2011). Similar analyses were performed for pyruvate and glutamate using their respective titration kinetics.
Statistics
Data are presented as means ± SEM. One-way ANOVA with repeated measures Student–Newman–Keuls method and Student's paired t test were used where appropriate to determine significance. The α level of significance was set at P < 0.05.
Results
Verification of ADP-induced contraction in skeletal muscle PmFBs
Several validation experiments were performed to further characterize the PmFB spontaneous contraction phenomenon reported previously (Perry et al. 2011). It has recently been proposed that PmFB contraction is mediated via proteolysis following sarcolemmal permeabilization (Kuznetsov et al. 2012). Following recommendations for including protease inhibitors in PmFBs (Kuznetsov et al. 2012), we first verified previous reports (Perry et al. 2011) of ADP-induced contraction in PmFB (Supplementary video 1). ATP transiently reverses ADP-induced contraction consistent with this phenomenon being contraction per se in skeletal muscle PmFBs as opposed to the permeabilization-induced proteolytic cell shortening reported to occur in cardiomyocytes (Kuznetsov et al. 2012). As demonstrated in Supplementary videos 2–3, the addition of protease inhibitors to permeabilization medium (Supplementary video 2) or both permeabilization and assay media (Supplementary video 3) does not prevent ADP-triggered PmFB contraction at 37°C. Furthermore, inclusion of protease inhibitors during permeabilization, wash and assay medium did not prevent contraction-related reductions in apparent Km for ADP in human skeletal muscle PmFBs when assessed with MiR05, Buffer Z (Perry et al. 2011) or Mitomed (Kuznetsov et al. 2012) (data not shown). These data provide evidence that ADP triggers contraction per se in skeletal muscle PmFBs independent of the proteolysis reported for cardiomyocytes (Kuznetsov et al. 2012). This contraction is especially apparent given that the effect is prevented by myosin inhibition (present study and Perry et al. 2011). Altogether these data suggest skeletal muscle PmFB contraction in situ represents a tool to examine the effects of contraction on the regulation of Cr-dependent and -independent respiratory sensitivity to ADP and control of energy homeostasis.
Verification of stable [ADP] during respiratory kinetic assessments
We also determined if [ADP] remained stable during ADP titrations in PmFBs, particularly in the absence of creatine whereby miCK inactivation would impair ATP–ADP recycling. Clamping [ADP] at steady state with a hexokinase–glucose ADP regenerating system (da-Silva et al. 2004; Anderson et al. 2009) did not change the apparent Km to ADP (863 μm vs. 819 μm with clamp, P= 0.20). This suggests endogenous ATPases may not alter the effective [ADP] or assessments of apparent Km for ADP in the absence of creatine.
Relaxed PmFBs: effects of exercise on miCK-independent and -dependent ADP sensitivity
Having confirmed PmFB contraction occurs in response to ADP, we then determined the effect of exercise on mitochondrial respiratory sensitivity to ADP in relaxed PmFBs. ADP-stimulated respiration exhibited typical Michaelis–Menten kinetics before and after exercise (Figs 1–2). In relaxed PmFBs (+BLEB) and miCK inactivation (no Cr), exercise decreased respiratory sensitivity to ADP (126% higher apparent Km; Fig. 1A and D) in both post-exercise biopsies. This effect was attenuated with typical resting muscle Cr and PCr concentrations that provide mild miCK activation (20% higher apparent Km; Fig. 1B and E). In contrast, in the relaxed state no effect of exercise was observed in the presence of 20 mm Cr (Fig. 1C and F). These observations were sustained 3 h into recovery from exercise. These results translated into a ∼100% higher creatine kinase efficiency (apparent Km without Cr/apparent Km with Cr) in the presence of BLEB (Pre, 1.7 ± 0.4; Post, 3.3 ± 0.6; 3 h Post, 3.5 ± 0.9)
Figure 1. The effect of exercise on mitochondrial respiratory sensitivity to ADP in relaxed (+BLEB) human skeletal muscle.
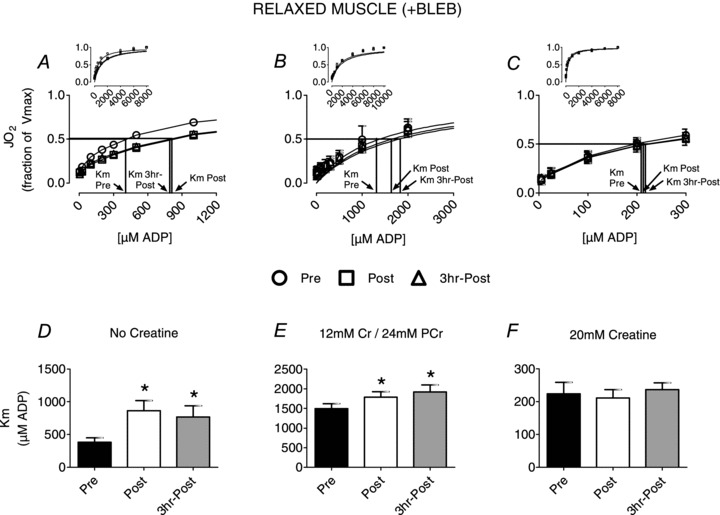
The Km to ADP following exercise varied in the absence of creatine (A and D), the presence of 12 mm creatine and 24 mm phosphocreatine (B and E) or 20 mm creatine (C and F). Results represent means ± SEM; n= 8–9; *P < 0.05 compared to Pre.
Contracted PmFB: effects of exercise on miCK-dependent ADP sensitivity
It was then determined whether the effect of exercise on respiratory apparent Km to ADP was altered by the presence of contraction in situ (–BLEB). We have verified PmFB contraction increases respiratory sensitivity to ADP (Fig. 2A and B) in the presence of 20 mm creatine (compared to Fig. 1C and F, +BLEB), as has been reported previously (Perry et al. 2011). Contrary to the relaxed state (Fig. 1C and F), we observed a greater respiratory sensitivity to ADP in contracting PmFBs post-exercise in the presence of 20 mm Cr (33% lower apparent Km relative to Pre; Fig. 2). The greater sensitivity to ADP post-exercise in contracted PmFB was transient, as apparent Km returned to Pre levels by 3 h recovery (Fig. 2).
Figure 2. The effect of exercise on mitochondrial respiratory sensitivity to ADP in spontaneously contracted (–BLEB) human skeletal muscle.
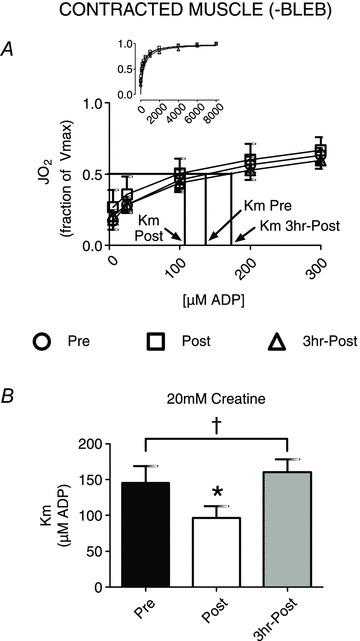
The Km to ADP was determined in the presence of 20 mm creatine (A and B). Results represent means ± SEM; n= 8; *P < 0.05 compared to Pre; †P < 0.05 compared to relaxed muscle Km with 20 mm creatine (Fig. 1C).
Effect of exercise on sensitivity to complex I-linked substrates and state IV respiration
We also determined whether the respiratory sensitivities to pyruvate and glutamate are subject to regulation in response to exercise. Exercise increased the respiratory sensitivity to both pyruvate and glutamate (28% and 26% lower apparent Km respectively; Fig. 3A and B), which generates NADH entry into complex I, in the presence of 20 mm Cr in relaxed PmFBs. This is in contrast to the lack of effect of exercise on apparent Km to ADP in the presence of 20 mm Cr (Fig. 1C and F) indicating changes in pyruvate or glutamate sensitivities may not explain the observed alterations in ADP sensitivity.
Figure 3. Mitochondrial respiratory sensitivity to pyruvate and glutamate is altered by exercise in relaxed (+BLEB) human skeletal muscle.
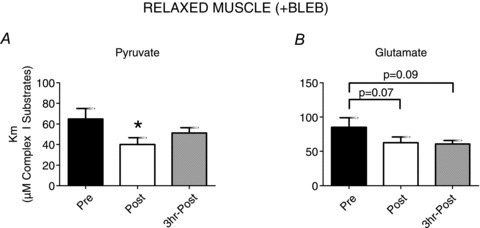
The Km to pyruvate (A) and glutamate (B) was derived in the presence of 20 mm creatine with BLEB. Results represent means ± SEM; n= 5; *P < 0.05 compare to Pre.
No change in Vmax occurred in any protocols following exercise (data not shown). Respiratory control ratio (RCR) did not change with exercise regardless of Cr and PCr concentrations (Pre, 11.1 ± 0.9; Post, 9.8 ± 1.0; 3 h Post, 9.1 ± 0.9 for all ADP titrations) although there was a significant increase (∼55%, P < 0.05) in state IV respiration supported by pyruvate and malate after exercise, suggesting a greater degree of mitochondrial uncoupling (data not shown).
Discussion
This study investigated the role of miCK in mediating exercise-induced changes in skeletal muscle respiratory sensitivity to ADP. The findings demonstrate that post-exercise ADP sensitivity increased only when contractile signals were present (contracting PmFBs) and when miCK activity was maximized (20 mm Cr). This suggests that during exercise, when creatine is elevated, mitochondrial respiratory sensitivity to ADP is also increased. Hence, this study provides compelling support that the sensitivity of phosphate shuttling is highly regulated during exercise in relation to both contractile state and creatine balance. In addition, exercise increased respiratory sensitivity to pyruvate and glutamate titrations suggesting glutamate catabolism (possibly glutamate dehydrogenase (GDH) or glutamic oxalaloacetic transaminase (GOT)) and pyruvate dehydrogenase, or alternatively complex I sensitivity to NADH, may be regulated by exercise.
PmFB contraction reveals miCK-sensitive increases in respiratory sensitivity to ADP post-exercise
The phosphate shuttling mechanism for energy transfer between matrix and cytosolic compartments includes three major protein complexes (Fig. 4): voltage-dependent anion channel (VDAC) on the outer mitochondrial membrane, miCK in the inter membrane space (IMS) and adenine nucleotide translocase (ANT) on the inner mitochondrial membrane (Saks et al. 2004; Aliev et al. 2011; Guzun et al. 2012). A leading model describing energy transfer from the matrix to cytosolic compartments proposes ATP produced within the matrix is transported to the IMS via ANT where phosphate transfer to Cr occurs through miCK. The PCr product is then transported through VDAC to the cytosol for ADP/ATP cycling via CK associated with cytosolic ATPases. While this model proposes that ATP/ADP freely diffuse across concentration gradients through VDAC and ANT (see Fig. 4), it is estimated that as much as 80% of the energy transfer from the matrix to cytoplasm occurs through miCK-dependent phosphate shuttling in cardiac muscle (Aliev et al. 2011; Guzun et al. 2012). Evidence for a similar model of energy transfer has also been reported in oxidative skeletal muscle (Seppet et al. 2001).
Figure 4. A schematic representation of phosphate shuttling mechanisms of energy transfer between mitochondrial and cytosolic compartments.
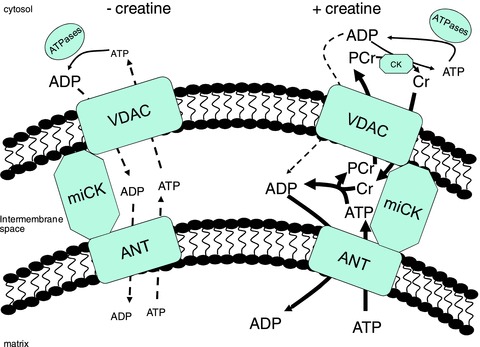
The leading model for energy transfer (see Guzun et al. 2012) proposes a mitochondrial creatine kinase (miCK)-dependent (+creatine) and -independent (–creatine) energy transfer system. In the absence of creatine, adenine nucleotide transfer is believed to occur by diffusion through a voltage-dependent anion channel (VDAC) on the outer mitochondrial membrane and an adenine nucleotide translocase (ANT) on the inner mitochondrial membrane with no contribution from miCK. In the presence of creatine, energy transfer between compartments is achieved via miCK whereby phosphate from ATP is moved to creatine (Cr) in the inner membrane space with the phosphocreatine (PCr) product being exported via VDAC for recylcling at ATPases throughout the cytosol. It is estimated that ∼80% of the energy transfer from matrix to cytosol occurs through miCK-dependent phosphate shuttling with ∼20% through diffusion of adenine nucleotides (Aliev et al. 2011). This model is consistent with increased respiration in the presence of Cr (discussed in text). Figure adapted from Alieve et al. 2011, Guzun et al. 2012 and Wallimann et al. 2011.
Therefore to comprehensively examine the potential for miCK-dependent (primarily PCr/Cr cycling via miCK-VDAC) vs. miCK-independent (primarily direct ATP/ADP cycling via ANT-VDAC) energy exchange to be regulated by exercise, we examined ADP respiratory sensitivity in the presence and absence of Cr and PCr. In these experiments, saturating Cr (20 mm) maximizes miCK contribution to phosphate shuttling. Conversely, the absence of Cr or presence of PCr minimizes miCK contribution to energy transfer between matrix and cytosol potentially placing a greater reliance on ADP/ATP diffusion through VDAC and ANT (Guzun et al. 2012). Hence, manipulating [Cr] and [PCr]in situ provides insight into the potential for miCK vs. miCK-independent (possibly ANT–VDAC) energy transfer to be altered with exercise.
Using this approach Cr was added to contracting PmFBs to further reflect miCK activation during exercise. With maximal miCK activation a higher respiratory sensitivity to ADP was observed but only during PmFB contraction. This demonstrates miCK sensitivity to creatine is increased post-exercise in the presence of contractile signals in situ. Exercise may therefore prime miCK to be more responsive to subsequent bouts of contraction, thereby improving the regulation of energy homeostasis during later challenges. These observations therefore implicate miCK as a critical mediator of exercise-induced increases in respiratory sensitivity during subsequent contraction that is partially dependent on the presence of other unknown contractile signals. This notion is supported by recent findings demonstrating contraction of skinned fibres accelerates activation of oxidative phosphorylation during subsequent contraction in skinned fibres (Gandra et al. 2012).
PmFB relaxation reveals decreased miCK-independent respiratory sensitivity to ADP post-exercise
We also tested ADP sensitivity in relaxed PmFBs at a range of miCK activities. The findings demonstrate a lower respiratory sensitivity to ADP when miCK activity is minimal (no creatine or presence of PCr). This implicates miCK-independent ADP/ATP diffusion through VDAC and/or ANT (Fig. 4) may be inhibited post-exercise (Guzun et al. 2012) in the absence of contractile signals (relaxed PmFBs). However, exercise did not change the apparent Km when miCK was saturated (20 mm Cr) in relaxed PmFBs (Fig. 1). Saturating miCK therefore seemingly masks or eliminates this inhibition of miCK-independent energy exchange. Given that miCK activation is proposed to switch energy transfer from cycling of ATP/ADP to PCr/Cr cycling via VDAC these findings suggest VDAC becomes inhibited specifically in to adenine nucleotides but not PCr/Cr when miCK is active. Furthermore, given that ANT inhibition should, in theory, also limit creatine-stimulated (miCK-dependent) respiration, the normalization of ADP apparent Km by creatine strongly suggests ANT is unaffected by exercise. Overall, combined with the evidence that ANT is not inhibited in contracting muscle, the data suggest exercise promotes energy transfer through the more efficient PCr/Cr energy transfer shuttle by increasing miCK sensitivity to Cr and contractile signals.
Implications for in vivo regulation of energy homeostasis during contraction
Previous work has reported a greater respiratory rate in response to 100 mm[ADP] (∼apparent Km) after exercise in human PmFBs with 20 mm Cr (Tonkonogi et al. 1998). This is in agreement with the present findings of greater sensitivity post-exercise during contraction but opposite to that observed in relaxed PmFBs where no effect of exercise was observed with 20 mm Cr. Whether PmFBs in these previous studies were contracted is impossible to determine but the very recent findings of ADP-induced contraction in PmFBs (Perry et al. 2011) and present study) suggests this is a possibility. These pioneering experiments (Tonkonogi et al. 1998) were performed long before powerful myosin-specific inhibitors were created or the recognition of ADP-induced contraction of skeletal muscle PmFBs was first reported (Perry et al. 2011). Hence, it is possible the PmFBs in these important earlier experiments underwent ADP-induced contraction, which would agree with the present results of enhanced respiratory sensitivity in contracting PmFBs postexercise.
Several studies have reported a reduced respiratory sensitivity to ADP measured in PmFBs in situ cross-sectionally between trained and untrained skeletal muscle (Mettauer et al. 2001; Zoll et al. 2002). While this may be related to a greater content of mitochondrial enzymes (Mettauer et al. 2001; Zoll et al. 2002; Guerrero et al. 2005) and lower respiratory sensitivity to ADPf in Type I fibres (Kuznetsov et al. 1996), this finding remains inconsistent with the notion of improved respiratory control following training. Specifically, exercise training attenuates the rise in ADPf/ATP and level of substrate phosphorylation during exercise (Karlsson et al. 1972; Dudley et al. 1987; Phillips et al. 1996; Leblanc et al. 2004; Perry et al. 2008). This improvement in energy homeostasis following training has been attributed, in part, to increased mitochondrial respiratory sensitivity to ADPf afforded by a greater mitochondrial content (Holloszy & Booth, 1976). The present study demonstrates that greater respiratory sensitivity occurs after a single exercise session which must be independent of greater mitochondrial content or fibre type transformation given the focus on acute exercise. While the present study does not address the paradox of ADP sensitivity in trained muscle (Mettauer et al. 2001; Walsh et al. 2001b; Zoll et al. 2002; Zoll et al. 2003a; Guerrero et al. 2005), a re-examination of respiratory control in response to exercise training with manipulation of PmFB contractile state may provide critical insight into the manner by which trained muscle regulates energy homeostasis (Holloszy & Booth, 1976).
A major implication of these findings is that exercise improves the ability of skeletal muscle to respond to a perturbation in energy charge during a subsequent period of contraction. The specific contractile signals generated during in situ contraction regulating miCK-dependent ADP sensitivity remain to be determined. Nevertheless, these findings imply an initial bout of exercise may optimize the regulation of energy homeostasis during subsequent exercise. As an example, these findings may provide a basis for the general belief in beneficial effects of an initial ‘warm-up’ to improve energy homeostasis during subsequent exercise, and may be consistent with known accelerations in 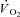 on-kinetics with repeated exercise (Gurd et al. 2005). Alternatively, when viewing exercise as a series of repeated contractions, it may be possible that the first contraction or series of contractions leads to a rapid ‘priming’ of phosphate shuttling in order to respond to and minimize substantial elevations in ADPf/ATP for the remainder of exercise. If true, these data suggest fundamental control of mitochondrial respiratory on-kinetics during contraction are highly regulated through mechanisms that have yet to be established.
on-kinetics with repeated exercise (Gurd et al. 2005). Alternatively, when viewing exercise as a series of repeated contractions, it may be possible that the first contraction or series of contractions leads to a rapid ‘priming’ of phosphate shuttling in order to respond to and minimize substantial elevations in ADPf/ATP for the remainder of exercise. If true, these data suggest fundamental control of mitochondrial respiratory on-kinetics during contraction are highly regulated through mechanisms that have yet to be established.
While the mechanism by which phosphate shuttling (miCK, VDAC and/or ANT) may be modified by exercise is at present unknown, it is clear that the modifications are robust enough to sustain PmFB preparation and assessments of respiratory kinetics. It would seem that classic allosteric regulators may not be the primary mechanism, as such modifications may regress during the time required to prepare PmFBs or are removed during saponin permeabilization and subsequent washing. While speculative, physical changes in protein conformation are likely to represent a modification that is retained post-exercise with this methodology, and therefore future research should determine the potential for covalent regulation (e.g. phosphorylation, acetylation). This may especially be true for miCK activity which is sensitive to conformational state (Kaldis & Wallimann, 1995). Nevertheless, it is now clear that mitochondrial phosphate shuttling is regulated in response to acute exercise, and may represent a critical control point for responding to a metabolic challenge.
Potential regulation of complex I or dehydrogenases during exercise
Given that pyruvate and glutamate oxidation generates NADH, but not FADH2 (Gnaiger, 2009), in the presence of malate and ADP, the increased sensitivity to both substrate titrations suggests complex I sensitivity to NADH may be increased post-exercise. Alternatively, the lower apparent Km to both substrates could be explained by the well known increase in pyruvate dehydrogenase activity with exercise, but also a previously unrecognized activation of pyruvate and glutamate transport or glutamate metabolism via GDH and/or GOT. Indeed, evidence suggests several mitochondrial dehydrogenases are regulated by calcium during exercise in human skeletal muscle (Crabtree & Newsholme, 1970; Vaughan & Newsholme, 1970a,b) which would suggest GDH is also activated. However, while GDH is allosterically regulated by adenine nucleotides, NAD and Ca2+ in fungi (LeJohn, 1968a,b; LeJohn & Jackson, 1968), little is known regarding its regulation in human skeletal muscle. Given the ability of glutamate to influence tricarboxylic acid cycle flux through anapleurosis (Gibala et al. 1997; Mourtzakis et al. 2008), these findings warrant future investigation into the regulation of GDH and GOT during exercise.
Speculations on PmFB contraction in situ
Finally, the mechanism of spontaneous contraction in PmFB remains to be determined. While evidence suggests the phenomenon may be calcium independent in skeletal muscle (Perry et al. 2011) other investigations have suggested that permeabilization induces proteolysis in cardiomyocytes (Anmann et al. 2005, 2006; Kuznetsov et al. 2012). To test this possibility in skeletal muscle, we added a variety of protease inhibitors to the permeabilization medium as suggested previously (Kuznetsov et al. 2012) as well as the assay medium (MiR05) during assessments of ADP-stimulated respiration. Protease inhibition during permeabilization and/or during respiratory assessments had no effect on the apparent Km to ADP (data not shown) nor on the apparent rate of contraction of PmFBs (Supplemental videos 1–3). These experiments clearly demonstrate that proteolysis does not explain spontaneous contraction in skeletal muscle PmFBs. In addition to the fact that this phenomenon is prevented by the addition of BLEB (Perry et al. 2011), and that permeabilization with saponin does not appear to disrupt the normal arrangement of the contractile apparatus in skeletal muscle (Kuznetsov et al. 2012), these findings indicate that apparent skeletal muscle PmFB contraction is actually contraction per se, as reported in skinned cardiac fibres (Ventura-Clapier & Vassort, 1985), and not due to cell ‘shrinking’, which may occur in cardiomyocytes with permeabilization (Anmann et al. 2005, 2006; Kuznetsov et al. 2012). These experiments strengthen the applicability of our in situ findings for understanding regulation of mitochondrial respiration by contraction in skeletal muscle.
Conclusions
In the current study, controlling the contractile state of PmFBs provides compelling evidence that the phosphate shuttling system for energy exchange is regulated in response to exercise. This is an extension of the prevailing dogma that the predominant mechanisms for increasing oxidative phosphorylation in response to a given rate of ATP hydrolysis are via activation of key enzymatic control points regulating substrate catabolism upstream from electron entry and adenine nucleotide cycling in the mitochondria. The phosphate shuttling mechanism itself (VDAC–miCK–ANT axis) appears the most likely candidate site for regulation by exercise, with miCK apparently activated and sensitized to Cr and subsequent contraction in situ. These findings also highlight the importance of controlling the contractile state of PmFBs in situ (Perry et al. 2011) during future examinations of mitochondrial respiratory kinetics for developing additional insight into the regulation of energy homeostasis in human muscle. While the regulatory mechanisms of phosphate shuttling activity during exercise remain to be determined, the enhanced respiratory sensitivity to ADP during contraction post-exercise indicates energy exchange between mitochondrial and cytosolic compartments is highly regulated in human skeletal muscle following exercise. This sensitization would seemingly tighten the coupling of oxidative phosphorylation to perturbations in the energy charge of the cell and improve metabolic control during successive periods of contraction.
Acknowledgments
We thank the study participants for their efforts and dedication. Funding was provided by NSERC, CFI and NIH R01 DK074825.
Glossary
- ADPf
free adenosine diphosphate
- ANT
adenine nucleotide translocase
- BLEB
blebbistatin
- Cr
creatine
- ETS
electron transport system
- IMS
inter membrane space
- miCK
mitochondrial creatine kinase
- PCr
phosphocreatine
- PmFB
permeabilized muscle fibre bundle
- VDAC
voltage-dependent anion channel
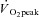
peak volume of oxygen consumption
Author contributions
All clinical biopsy trials were performed in the laboratory of G.J.F.H at McMaster University, while all experiments were conducted in the laboratories of G.P.H., D.C.W and P.D.N. C.G.R.P., D.A.K., P.D.N. and G.P.H. contributed to study design. C.G.R.P., D.A.K., E.A.H., G.J.F.H., L.L.S. and G.P.H. conducted clinical trials and experiments while C.G.R.P., D.A.K., P.D.N. and G.P.H. analysed and interpreted the data. C.G.R.P., D.C.W. and G.P.H. were the primary writers of the manuscript while all authors contributed to critical interpretation and manuscript preparation. All authors approved the final manuscript.
Supplementary material
Supplementary video 1
Supplementary videos 2--3
References
- Aliev M, Guzun R, Karu-Varikmaa M, Kaambre T, Wallimann T, Saks V. Molecular system bioenergics of the heart: experimental studies of metabolic compartmentation and energy fluxes versus computer modeling. Int J Mol Sci. 2011;12:9296–9331. doi: 10.3390/ijms12129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54:1891–1898. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem. 2007;282:31257–31266. doi: 10.1074/jbc.M706129200. [DOI] [PubMed] [Google Scholar]
- Anmann T, Eimre M, Kuznetsov AV, Andrienko T, Kaambre T, Sikk P, Seppet E, Tiivel T, Vendelin M, Seppet E, Saks VA. Calcium-induced contraction of sarcomeres changes the regulation of mitochondrial respiration in permeabilized cardiac cells. FEBS J. 2005;272:3145–3161. doi: 10.1111/j.1742-4658.2005.04734.x. [DOI] [PubMed] [Google Scholar]
- Anmann T, Guzun R, Beraud N, Pelloux S, Kuznetsov AV, Kogerman L, Kaambre T, Sikk P, Paju K, Peet N, Seppet E, Ojeda C, Tourneur Y, Saks V. Different kinetics of the regulation of respiration in permeabilized cardiomyocytes and in HL-1 cardiac cells. Importance of cell structure/organization for respiration regulation. Biochim Biophys Acta. 2006;1757:1597–1606. doi: 10.1016/j.bbabio.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Crabtree B, Newsholme EA. The activities of nicotinamide-adenine dinucleotide- and nicotinamide-adenine dinucleotide phosphate-linked isocitrate dehydrogenase in insect and vertebrate muscles. Biochem J. 1970;116:22P–23P. doi: 10.1042/bj1160022p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da-Silva WS, Gomez-Puyou A, de Gomez-Puyou MT, Moreno-Sanchez R, De Felice FG, de Meis L, Oliveira MF, Galina A. Mitochondrial bound hexokinase activity as a preventive antioxidant defense: steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J Biol Chem. 2004;279:39846–39855. doi: 10.1074/jbc.M403835200. [DOI] [PubMed] [Google Scholar]
- Dudley GA, Tullson PC, Terjung RL. Influence of mitochondrial content on the sensitivity of respiratory control. J Biol Chem. 1987;262:9109–9114. [PubMed] [Google Scholar]
- Gandra PG, Nogueira L, Hogan MC. Mitochondrial activation at the onset of contractions in isolated myofibres during successive contractile periods. J Physiol. 2012;590:3597–3609. doi: 10.1113/jphysiol.2012.232405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, MacLean DA, Graham TE, Saltin B. Anaplerotic processes in human skeletal muscle during brief dynamic exercise. J Physiol. 1997;502:703–713. doi: 10.1111/j.1469-7793.1997.703bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnaiger E. Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol. 2009;41:1837–1845. doi: 10.1016/j.biocel.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Guerrero K, Wuyam B, Mezin P, Vivodtzev I, Vendelin M, Borel JC, Hacini R, Chavanon O, Imbeaud S, Saks V, Pison C. Functional coupling of adenine nucleotide translocase and mitochondrial creatine kinase is enhanced after exercise training in lung transplant skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1144–1154. doi: 10.1152/ajpregu.00229.2005. [DOI] [PubMed] [Google Scholar]
- Gurd BJ, Scheuermann BW, Paterson DH, Kowalchuk JM. Prior heavy-intensity exercise speeds VO2 kinetics during moderate-intensity exercise in young adults. J Appl Physiol. 2005;98:1371–1378. doi: 10.1152/japplphysiol.01028.2004. [DOI] [PubMed] [Google Scholar]
- Guzun R, Gonzalez-Granillo M, Karu-Varikmaa M, Grichine A, Usson Y, Kaambre T, Guerrero-Roesch K, Kuznetsov A, Schlattner U, Saks V. Regulation of respiration in muscle cells in vivo by VDAC through interaction with the cytoskeleton and MtCK within mitochondrial interactosome. Biochim Biophys Acta. 2012;1818:1545–1554. doi: 10.1016/j.bbamem.2011.12.034. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, McClelland GB. Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. J Exp Biol. 1997;200:381–386. doi: 10.1242/jeb.200.2.381. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- Kaldis P, Wallimann T. Functional differences between dimeric and octameric mitochondrial creatine kinase. Biochem J. 1995;308:623–627. doi: 10.1042/bj3080623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane DA, Lin CT, Anderson EJ, Kwak HB, Cox JH, Brophy PM, Hickner RC, Neufer PD, Cortright RN. Progesterone increases skeletal muscle mitochondrial H2O2 emission in non-menopausal women. Am J Physiol Endocrinol Metab. 2011;300:E528–E535. doi: 10.1152/ajpendo.00389.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J, Nordesjo LO, Jorfeldt L, Saltin B. Muscle lactate, ATP, and CP levels during exercise after physical training in man. J Appl Physiol. 1972;33:199–203. doi: 10.1152/jappl.1972.33.2.199. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Guzun R, Boucher F, Bagur R, Kaambre T, Saks VA. Mysterious Ca2+- independent muscular contraction: deja vu. Biochem J. 2012;445:333–336. doi: 10.1042/BJ20120439. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Tiivel T, Sikk P, Kaambre T, Kay L, Daneshrad Z, Rossi A, Kadaja L, Peet N, Seppet E, Saks VA. Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. Eur J Biochem. 1996;241:909–915. doi: 10.1111/j.1432-1033.1996.00909.x. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- Leblanc PJ, Howarth KR, Gibala MJ, Heigenhauser GJ. Effects of 7 wk of endurance training on human skeletal muscle metabolism during submaximal exercise. J Appl Physiol. 2004;97:2148–2153. doi: 10.1152/japplphysiol.00517.2004. [DOI] [PubMed] [Google Scholar]
- LeJohn HB. On the involvement of Ca2+ and Mn2+ in the regulation of mitochondrial glutamic dehydrogenase from Blastocladiella. Biochem Biophys Res Commun. 1968a;32:278–283. doi: 10.1016/0006-291x(68)90381-1. [DOI] [PubMed] [Google Scholar]
- LeJohn HB. Unidirectional inhibition of glutamate dehydrogenase by metabolites. A possible regulatory mechanism. J Biol Chem. 1968b;243:5126–5131. [PubMed] [Google Scholar]
- LeJohn HB, Jackson S. Allosteric interactions of a regulatory nicotinamide adenine dinucleotide-specific glutamate dehydrogenase from Blastocladiella. A molecular model for the enzyme. J Biol Chem. 1968;243:3447–3457. [PubMed] [Google Scholar]
- Mettauer B, Zoll J, Sanchez H, Lampert E, Ribera F, Veksler V, Bigard X, Mateo P, Epailly E, Lonsdorfer J, Ventura-Clapier R. Oxidative capacity of skeletal muscle in heart failure patients versus sedentary or active control subjects. J Am Coll Cardiol. 2001;38:947–954. doi: 10.1016/s0735-1097(01)01460-7. [DOI] [PubMed] [Google Scholar]
- Mourtzakis M, Graham TE, Gonzalez-Alonso J, Saltin B. Glutamate availability is important in intramuscular amino acid metabolism and TCA cycle intermediates but does not affect peak oxidative metabolism. J Appl Physiol. 2008;105:547–554. doi: 10.1152/japplphysiol.90394.2008. [DOI] [PubMed] [Google Scholar]
- Perry CG, Heigenhauser GJ, Bonen A, Spriet LL. High-intensity aerobic interval training increases fat and carbohydrate metabolic capacities in human skeletal muscle. Appl Physiol Nutr Metab. 2008;33:1112–1123. doi: 10.1139/H08-097. [DOI] [PubMed] [Google Scholar]
- Perry CG, Kane DA, Lin CT, Kozy R, Cathey BL, Lark DS, Kane CL, Brophy PM, Gavin TP, Anderson EJ, Neufer PD. Inhibiting myosin-ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem J. 2011;437:215–222. doi: 10.1042/BJ20110366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJ, Grant SM. Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Am J Physiol Endocrinol Metab. 1996;270:E265–272. doi: 10.1152/ajpendo.1996.270.2.E265. [DOI] [PubMed] [Google Scholar]
- Saks VA, Belikova YO, Kuznetsov AV, Khuchua ZA, Branishte TH, Semenovsky ML, Naumov VG. Phosphocreatine pathway for energy transport: ADP diffusion and cardiomyopathy. Am J Physiol. 1991;261(4 Suppl):30–38. doi: 10.1152/ajplung.1991.261.4.L30. [DOI] [PubMed] [Google Scholar]
- Saks VA, Khuchua ZA, Vasilyeva EV, Belikova O, Kuznetsov AV. Metabolic compartmentation and substrate channelling in muscle cells. Role of coupled creatine kinases in in vivo regulation of cellular respiration – a synthesis. Mol Cell Biochem. 1994;133–134:155–192. doi: 10.1007/BF01267954. [DOI] [PubMed] [Google Scholar]
- Saks VA, Kuznetsov AV, Khuchua ZA, Vasilyeva EV, Belikova JO, Kesvatera T, Tiivel T. Control of cellular respiration in vivo by mitochondrial outer membrane and by creatine kinase. A new speculative hypothesis: possible involvement of mitochondrial-cytoskeleton interactions. J Mol Cell Cardiol. 1995;27:625–645. doi: 10.1016/s0022-2828(08)80056-9. [DOI] [PubMed] [Google Scholar]
- Saks VA, Kuznetsov AV, Vendelin M, Guerrero K, Kay L, Seppet EK. Functional coupling as a basic mechanism of feedback regulation of cardiac energy metabolism. Mol Cell Biochem. 2004;256–257:185–199. doi: 10.1023/b:mcbi.0000009868.92189.fb. [DOI] [PubMed] [Google Scholar]
- Seppet EK, Kaambre T, Sikk P, Tiivel T, Vija H, Tonkonogi M, Sahlin K, Kay L, Appaix F, Braun U, Eimre M, Saks VA. Functional complexes of mitochondria with Ca,MgATPases of myofibrils and sarcoplasmic reticulum in muscle cells. Biochim Biophys Acta. 2001;1504:379–395. doi: 10.1016/s0005-2728(00)00269-3. [DOI] [PubMed] [Google Scholar]
- Smith BK, Jain SS, Rimbaud S, Dam A, Quadrilatero J, Ventura-Clapier R, Bonen A, Holloway GP. FAT/CD36 is located on the outer mitochondrial membrane, upstream of long-chain acyl-CoA synthetase, and regulates palmitate oxidation. Biochem J. 2011;437:125–134. doi: 10.1042/BJ20101861. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Fernstrom M, Walsh B, Ji LL, Rooyackers O, Hammarqvist F, Wernerman J, Sahlin K. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflugers Arch. 2003;446:261–269. doi: 10.1007/s00424-003-1044-9. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Harris B, Sahlin K. Mitochondrial oxidative function in human saponin-skinned muscle fibres: effects of prolonged exercise. J Physiol. 1998;510:279–286. doi: 10.1111/j.1469-7793.1998.279bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan H, Newsholme EA. Effects of calcium ion and electron-acceptor concentrations on the activity of mitochondrial glycerol 1-phosphate dehydrogenase from insect flight muscle. Biochem J. 1970a;116:31P–32P. doi: 10.1042/bj1160031pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan H, Newsholme EA. The effects of calcium ions and adenosine diphosphate on the activity of nicotinamide-adenine dinucleotide-linked isocitrate dehydrogenase of muscle. Biochem J. 1970b;116:23P. doi: 10.1042/bj1160023pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veksler VI, Kuznetsov AV, Anflous K, Mateo P, van Deursen J, Wieringa B, Ventura-Clapier R. Muscle creatine kinase-deficient mice. II. Cardiac and skeletal muscles exhibit tissue-specific adaptation of the mitochondrial function. J Biol Chem. 1995;270:19921–19929. doi: 10.1074/jbc.270.34.19921. [DOI] [PubMed] [Google Scholar]
- Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta. 1987;892:191–196. doi: 10.1016/0005-2728(87)90174-5. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Vassort G. Role of myofibrillar creatine kinase in the relaxation of rigor tension in skinned cardiac muscle. Pflugers Arch. 1985;404:157–161. doi: 10.1007/BF00585412. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40:1271–1296. doi: 10.1007/s00726-011-0877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B, Tonkonogi M, Malm C, Ekblom B, Sahlin K. Effect of eccentric exercise on muscle oxidative metabolism in humans. Med Sci Sports Exerc. 2001a;33:436–441. doi: 10.1097/00005768-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Walsh B, Tonkonogi M, Sahlin K. Effect of endurance training on oxidative and antioxidative function in human permeabilized muscle fibres. Pflugers Arch. 2001b;442:420–425. doi: 10.1007/s004240100538. [DOI] [PubMed] [Google Scholar]
- Walsh B, Tonkonogi M, Soderlund K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2001c;537:971–978. doi: 10.1111/j.1469-7793.2001.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoll J, Koulmann N, Bahi L, Ventura-Clapier R, Bigard AX. Quantitative and qualitative adaptation of skeletal muscle mitochondria to increased physical activity. J Cell Physiol. 2003a;194:186–193. doi: 10.1002/jcp.10224. [DOI] [PubMed] [Google Scholar]
- Zoll J, N’Guessan B, Ribera F, Lampert E, Fortin D, Veksler V, Bigard X, Geny B, Lonsdorfer J, Ventura-Clapier R, Mettauer B. Preserved response of mitochondrial function to short-term endurance training in skeletal muscle of heart transplant recipients. J Am Coll Cardiol. 2003b;42:126–132. doi: 10.1016/s0735-1097(03)00499-6. [DOI] [PubMed] [Google Scholar]
- Zoll J, Sanchez H, N’Guessan B, Ribera F, Lampert E, Bigard X, Serrurier B, Fortin D, Geny B, Veksler V, Ventura-Clapier R, Mettauer B. Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2002;543:191–200. doi: 10.1113/jphysiol.2002.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


