Abstract
The United Nations is committed to achieving universal access to HIV care, treatment, and prevention. Although the gateway to HIV care and secondary prevention is knowledge of serostatus, use of voluntary counseling and testing in resource-limited settings with the highest burden of HIV/AIDS has been limited. Based on evidence of increased patient uptake and the opportunity to avoid missed HIV testing opportunities in health care facilities, in 2007 the World Health Organization recommended provider-initiated HIV testing as a standard part of medical care in settings of generalized HIV epidemics. While provider-initiated testing has shown promise, optimal implementation strategies which ensure broad coverage, while preserving human rights, remain an active area of research. We review the benefits of knowledge of HIV serostatus and evidence from multiple countries surrounding the successes and pitfalls of provider-initiated testing in health care and home-based settings.
Keywords: HIV, routine HIV testing, resource-limited settings, review
Introduction
In 2006, the United Nations General Assembly issued a political declaration to “scale up significantly” efforts to achieve universal prevention, treatment and care for HIV/AIDS worldwide [1]. In low- and middle-income countries most affected by the epidemic, access to antiretroviral therapy (ART) is increasing, with 3 million people on treatment by the end of 2007 [2]. However, prevention and treatment efforts are severely hampered by poor HIV testing coverage. In a recent 17-country survey, a median of only 11% of women and 10% of men had ever received an HIV test and result [2]. An estimated 20% of people living with HIV in 12 low- and middle-income countries are aware of their seropositive status (Table 1) [2, 3].
Table 1.
Percentages of adults (age 15–49 years) living with HIV who tested and received an HIV test result prior to sero-survey, selected countries, 2005–2007
| Country | Year | % of people living with HIV who tested and received results | ||
|---|---|---|---|---|
| Women | Men | Overall | ||
| Benin | 2006 | 24.9 | -- | 23.5 |
|
| ||||
| Côte d’Ivoire | 2005 | 13.6 | 23.6 | 16.5 |
|
| ||||
| Democratic Republic of Congo | 2007 | 8.7 | -- | 10.7 |
|
| ||||
| Ethiopia | 2005 | 8.4 | 5.6 | 7.6 |
|
| ||||
| Guinea | 2005 | 5.4 | -- | 5.4 |
|
| ||||
| Mali | 2006 | 13.0 | -- | 12.9 |
|
| ||||
| Rwanda | 2005 | 31.3 | 31.6 | 31.4 |
|
| ||||
| Swaziland | 2007 | 44.0 | 28.8 | 38.7 |
|
| ||||
| Zimbabwe | 2005–2006 | 26.3 | 19.3 | 23.7 |
|
| ||||
| Haiti | 2005 | 30.7 | 15.6 | 24.5 |
|
| ||||
| Dominican Republic | 2007 | 72.6 | 49.1 | 60.7 |
| India | 2005–2006 | 6.8 | 12.8 | 10.3 |
Adapted from [2].
Knowledge of HIV status is the gateway to HIV care and stands alone as a prevention measure [4–7]. With increasing ART availability, researchers and policy makers in resource-limited settings advocate aggressive case finding and partner testing in both healthcare and community-based sites [8–10]. In 2007, the World Health Organization recommended provider-initiated HIV testing in health facilities as a standard part of medical care in generalized HIV epidemics, intending to expand upon current practices of client-initiated dedicated voluntary counseling and testing (VCT) [11]. Provider-initiated testing capitalizes on all patient contacts with the medical system, using each as a potential opportunity for HIV testing, diagnosis and linkage to care [8, 11, 12]. HIV testing uptake is affected by multiple factors, including poor access to health facilities or VCT centers, failure of risk-based assessments as the basis for testing, stigma, fear, and practical obstacles such as transportation and cost [10, 13, 14]. We review the literature on the public health benefits of HIV testing and the implications for expanded HIV testing strategies in resource-limited settings.
Public and Individual Health Impact of Voluntary Counseling and Testing
Timely HIV diagnosis is an entry point for receiving ART and opportunistic disease prophylaxis, but it is also increasingly recognized as an opportunity to provide social and behavioral benefits for secondary prevention [4–7]. Voluntary counseling and testing sites, long the cornerstone of HIV testing [11], provide the most evidence for behavioral risk reduction. A randomized controlled trial in Kenya, Tanzania and Trinidad examined VCT versus basic health information. Unprotected sex with non-primary partners declined significantly more at 6 months among those receiving VCT (35–39% reduction with VCT versus 13–17% reduction with health information); the effect was sustained at one year [4]. In Pune, India, counseling and testing at 3-month intervals for men was associated with an increase in consistent condom use with sex workers [15]. In a meta-analysis of 7 studies in developing countries, the odds of VCT recipients engaging in unprotected sex were significantly less compared to their pre-test behavior or to participants who had not received VCT (pooled effect size OR 1.69; 95% CI 1.25–2.31) [7]. These studies illustrate the positive impact of VCT on sexual risk-taking behavior.
The impact of VCT on behavior has been particularly striking among serodiscordant couples, who, without appropriate counseling, may harbor misconceptions about the risk of transmitting HIV to the uninfected partner [16]. Among Zambian cohabiting heterosexual discordant couples, < 3% reported condom use prior to VCT, yet >80% reported condom use in the year following the diagnosis of one partner [5]. Following VCT and a new HIV diagnosis, rural Zimbabwean women reported significantly higher levels of condom use in their regular partnership up to 3 years later [17]. Despite the benefits of knowledge of HIV serostatus on risk behaviors, only a small proportion of HIV-infected individuals know their partner’s status. For example, in Uganda, 21% of HIV-infected adults knew their own HIV status, but only 9% knew their partners’ status [6]. These results are typical, although data from numerous surveys performed in sub-Saharan Africa suggest that testing with a partner is preferred [18, 19].
Participation in client-initiated VCT has improved somewhat in recent years because of the increasing use of rapid HIV testing with same-day results [11]. However, people living with HIV are often testing and presenting for care with advanced clinical disease; among 18 ART programs in low-income countries, the median baseline CD4 count was 108 cells/μl [20]. Even among those who do actively seek VCT, many are self-selected based on socio-demographic features (e.g. can afford transport) and low-risk sexual activity [10, 17, 21]; a broader approach is needed.
Routine and Opt-Out HIV Testing
Provider-initiated testing -- also called routine HIV testing-- refers to HIV testing and counseling recommended at healthcare facilities as a standard component of medical care [11, 22]. In generalized epidemics (HIV prevalence >1% in pregnant women) and more selectively in concentrated and low-level epidemics, the WHO recommends an “opt-out” approach, where a patient must specifically decline an HIV test in the healthcare setting [11]. Opt-out testing promotes simplified pre-test information and advocates verbal, rather than written consent [11]. Data in both well-resourced and resource-limited settings suggest that patients often have multiple medical contacts before an HIV test is offered [23–28]. For example, 88% of newly diagnosed HIV-infected individuals in a Ugandan emergency unit sought medical care in the previous six months and had not been tested [27]. Patients attributed lack of prior testing to no perceived risk (77%) and the cost of testing (25%) [27]. Routine testing dispenses with ineffective risk-based testing referral [12], reduces stigma and discrimination [18], and ultimately increases engagement in HIV care [11]. We review the experience of several countries as examples of routine testing implementation.
The Botswana Experience
Botswana’s efforts to improve rates of HIV testing are exemplary. Its comparatively high per capita income, well-developed health care infrastructure, and strong government commitment to HIV/AIDS programs distinguishes it from other high prevalence African countries [18, 29, 30]. With >30% HIV seroprevalence [29], Botswana established universal access to ART in 2002 for all eligible patients [31], but by the end of 2003, attendance at VCT sites was still lower than expected [32]. In his Christmas radio address of 2003, Botswana President Festus Mogae endorsed “routine by not compulsory” testing at health facilities to make “HIV testing…as simple and as accessible as checking blood pressure” [30].
Eleven months into a routine testing media and public health campaign, a cross-sectional survey of adults in Botswana revealed that 81% were in favor of routine testing because it decreases barriers to testing (89%), HIV-related stigma (60%) and violence toward women (55%), while increasing access to ART (93%) [18]. Though overall impressions were positive, 68% felt unable to refuse the test, and 43% believed that routine testing could lead to avoidance of medical care for fear of being tested [18]. A 2006 household survey found that 81% of respondents had visited a government facility in the 2 years since initiation of the routine testing policy and that 92% were satisfied with their visit; nearly half of those who had visited a government facility reported being HIV tested [33]. The Botswana Ministry of Health reported a tripling of testing rates in the public sector in the first part of 2006 [34]. As a surrogate marker of testing success, one of the National Antiretroviral Program sites reported a concomitant decline in the proportion of patients being assessed for treatment with a CD4 count ≤ 100 cells/μl (49% to 34%) [34].
The Uganda Experience
Uganda predated the WHO with 2005 national guidelines recommending provider-initiated, opt-out HIV testing in health care facilities that have a link with HIV/AIDS services [35]. Mulago and Mbarara Hospitals, the largest public, tertiary hospitals in Uganda, offered HIV testing only on request and for a fee. In November 2004, the hospitals established a routine, rapid testing program, expanded to 25 wards/clinics, that was free of charge to patients using dedicated HIV counselors; they offered testing to >50,000 patients, with an uptake of 98% [36]. The newly diagnosed HIV prevalence was 25%. More than 10,000 patient relatives and household members present in the hospital were also offered HIV testing; 93% accepted, and HIV prevalence among family members was 20%, nearly as high as the index patients.
The South Africa Experience
South Africa has over 5 million people infected [37], the most citizens of any country, with plans to expand provider-initiated testing [38]. Though there are more than 4,000 VCT sites in the country [39], only 30% of South Africans report every having been tested for HIV [40]. One study in the outpatient department of an urban medical center in Durban compared physician referral to a self-pay VCT site in the hospital complex to a free, routine testing intervention. During the 14-week VCT period, physicians referred 435 patients for testing, but only 32% of patients completing the test within 4 weeks of referral; newly diagnosed HIV prevalence was 75%. During the 12-week routine testing period, over 2,000 people were offered testing, 49% of them agreed, and 33% of them were HIV-infected [41]. In this study, routine testing was acceptable to patients and led to a substantially higher rate of newly-identified HIV cases compared to testing based on physician referral.
The Haiti Experience
The Grupe Haitien d’Etude du Sarcome de Kaposi et des Infections Opportunistes (GHESKIO) has provided VCT in Haiti since 1985, the poorest and most heavily HIV-burdened country in the western hemisphere [42]. In a 15-year experience, tuberculosis care, sexually transmitted disease management, family planning, nutritional support, prenatal services, and HIV post-exposure prophylaxis were integrated into VCT services [43]. GHESKIO reports a rapid increase in clients from 142 in 1985 to over 8,000 in 1999, with 36% of patients presenting for VCT benefiting from at least one other offered service [43]. While not strictly provider-initiated testing, this experience demonstrates the successes of comprehensive medical encounters that include HIV testing. Successful implementation of doctor- or nurse-initiated HIV testing lead to few “missed opportunities” in a rural public medical clinic; 85% of HIV-infected patients were diagnosed at the first medical encounter [44].
These examples from several generalized HIV epidemics illustrate that provider-initiated testing integrated into routine health care is acceptable to patients, increases testing participation, and may identify HIV-infected individuals at an earlier disease stage [12, 18, 27, 34, 36, 41]. Despite these encouraging data, in 2007, only 12 of 27 (44%) countries with generalized epidemics had guidelines stating that health care providers should recommend HIV testing and counseling in all encounters [2].
Antenatal and Intrapartum HIV Testing
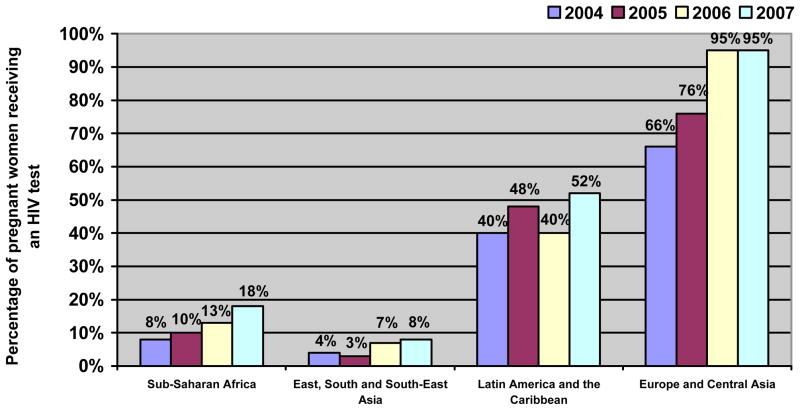
The United Nations Programme on HIV/AIDS has called for the elimination of mother to child HIV transmission in all countries by 2015 [45]. However, in 2007 only 18% of the total estimated number of pregnant women in low- and middle-income countries received an HIV test, the essential gateway to ART and other prevention of mother to child transmission (PMTCT) services (Figure 1).
Figure 1. Percentage of pregnant women in low- and middle-income countries receiving an HIV test, 2004–2007.
No data are available from the Middle East or North Africa.
Adapted from [2].
Despite its failure to reach all women in need, some of the greatest HIV screening successes in less-resourced countries have been in antenatal testing [46]. In 1998, Thailand became the first resource-limited country to implement and financially support a national program for routine HIV testing coupled with PMTCT [47]. Success was evident by 2001, when 96% of women giving birth in Thailand had antenatal care, with 94% of these having a known HIV test result, and 76% of those infected with HIV receiving zidovudine for PMTCT [47, 48]. Routine testing in Botswana’s antenatal clinics resulted in 95% testing acceptance; women were not deterred from seeking care because of the new routine HIV testing policy [49]. Very high rates of HIV test uptake have also been documented in routine antenatal and labor testing programs in Uganda [28], Malawi [50, 51], Zimbabwe [52, 53], South Africa [54], Brazil [55], and India [56]. In rural India, where half of women present in labor without prior antenatal care, 98% of eligible women accepted testing on the labor ward in a round-the-clock program offering rapid oral fluid HIV testing [56].
Women are generally more often aware of their HIV status than men, at least in part due to antenatal services [33, 34, 57]; innovative efforts to reach men are urgently needed. Despite the high rates of discordant serostatus among couples [36, 58, 59], partner testing in the antenatal and labor settings has had variable success. In a Malawi antenatal program where 95% of the mothers were tested for HIV with 22% seropositivity, only 8% of partners of HIV-infected mothers underwent HIV testing [50]. Similarly, a successful Zimbabwean PMTCT program encouraged all women who tested, regardless of the result, to bring their partners for free testing; only 7% of partners tested [52].
Partner testing programs were more successful in Uganda where, among women who tested, 97% and 98% of accompanying men tested in the antenatal clinic and maternity ward, respectively [28]. When partner counseling and testing does occur in the antenatal setting, women are more likely to return for PMTCT interventions [60, 61]; efforts are needed to improve outreach for partner testing.
Home-based Routine HIV Testing
Routine and antenatal HIV testing in health care facilities by definition reach only those who seek medical care. Such services often exclude partners and family members of HIV-infected individuals, rural populations living remote from testing facilities, and those with lower socioeconomic status who cannot afford transport or time off from work [2, 10]. Home-based provision of HIV counseling and testing services has been recommended as a means of achieving “universal” HIV testing in Africa and increasing partner testing [59].
In Malawi, household members in the lowest income quartile were significantly less likely to have ever used facility-based HIV testing than the rest of the population, but were 70% more likely use the home-based rapid HIV testing program [10]. A Ugandan study compared four HIV testing strategies: stand-alone VCT, hospital-based provider-initiated testing, home-based provider-initiated testing, and household member home-based testing [62]. Although hospital-based testing diagnosed the highest proportion of HIV-infected individuals (prevalence 27%), home-based and household member home-based testing reached the largest proportion of previously untested adults (>90% of all clients) [62].
A Zambian trial found that subjects randomized to an optional testing location (most often home testing) were more than four times more likely to accept VCT than those assigned to health facility testing [63]. Similarly, testing participation increased from 10% to 37% in Uganda when people learned their HIV test results at home compared to at a counseling site [64]. District-wide door-to-door HIV testing reached 63% of all households in Bushenyi District, Uganda, increasing the proportions of people ever tested for HIV (20 vs 63%, p<0.001) and reporting disclosure of their serostatus (72% vs 81%, p= 0.04) [65]. Home-based HIV testing was also found to be acceptable in Thailand, where the majority of household contacts (74%) of tuberculosis patients consented to HIV screening in Chiang Rai [66]. These studies illustrate that home-based testing is a feasible and acceptable method for expanding access to HIV testing.
Pre-ART Loss to Follow-Up
An important metric of HIV testing programs’ success is the rate at which patient are linked to and retained in care following a new HIV diagnosis. Many studies do not report data on post-partum linkage to HIV care for mothers’ health; the problem of post-partum loss to follow-up is an emerging concern [50, 67]. For example, although testing uptake was high in a Malawi antenatal clinic, cumulative loss to follow-up rates among HIV-infected women were also high, starting in the antenatal period: 55% by the 36-week antenatal visit, 68% by delivery, 70% by first postnatal visit and 81% by the 6-month postnatal visit [50]. The vast majority (87%) of deliveries occurred at peripheral clinical sites where ART for PMTCT -- or later for maternal health--was not available [50]. In four community care clinics in South Africa, pregnant women with CD4 ≤ 200/μl were three times more likely to be lost compared to men with CD4 > 200/μl [67]. A study in Burkina Faso found that nearly 20% of HIV-infected women diagnosed in antenatal clinic were lost by the time their baby was 18 months old [68].
Pre-ART loss is also increasingly recognized as a problem outside of the PMTCT setting. A Durban study of patients enrolled prior to HIV testing in the medical outpatient department revealed that 45% of patients fail to undergo a CD4 count within 8 weeks of a new HIV diagnosis [69]. One year later, only 39% of those who completed a CD4 count within 90 days of HIV testing and were deemed ART-eligible were known to have initiated ART. Of those who were ART eligible, 20% died by 1 year, with 82% of deaths occurring prior to ART initiation or with unknown ART initiation status [70]. Many HIV testing programs do not report linkage to care outcomes following testing, particularly in home-based settings; this critical area mandates further study.
Costs
Although resources are limited, few studies have reported the costs and cost-effectiveness of HIV screening in these settings. In Uganda, costs per client tested ranged from $8.29 for door-to-door testing to $11.68 for hospital-based testing, to $13.85 for household member testing and $19.26 for stand-alone testing. Because of differential yield in case detection, costs per HIV-infected case identified were most expensive for household testing ($231.65) and least for hospital based testing ($43.10) [62]. In the Durban experience, routine HIV screening cost less than $25 per HIV-infected case identified [41]. A South African-based cost-effectiveness analysis using conservative assumptions regarding HIV-related stigma, linkage to care, and ART access reports that routine HIV screening every 5 years, and even annually, is a very cost-effective intervention ($1,650/YLS and $1,940/YLS, respectively) [71].
Continued Areas of Concern
While routine health care facilities-based and home-based testing increase uptake of HIV testing, areas of uncertainty related to appropriate implementation and scale-up remain. Should testing be “opt-in” or “opt-out” to simultaneously maximize patient understanding, consent, and participation [18]? Which providers should be offering and discussing testing with patients—doctors and nurses [44] or dedicated HIV counselors [41, 52]? Rapid test kits, which have greatly expanded the scope and availability of HIV testing in resource-limited settings, have had disappointing performance in some settings [72–76], necessitating careful in-country validation and labor-intensive evaluation of rapid test kits [77].
Aggressive screening strategies must effectively balance individual rights with public health; true informed consent with the preserved ability to refuse testing is critical [18, 78]. A comparative study of HIV-infected people in India, Indonesia, Thailand and the Phillipines found that discrimination was prominent in the health sector, with high rates of breaches of confidentiality and delays in health care provision [79]. A concern with increased routine antenatal and health-care based testing initiatives is that newly-identified HIV-infected women and girls would be subject to abuse [78]. This has not been reported systematically in the literature thus far. No reports of discrimination, stigmatization, or increased violence as a result of routine testing have been described in Botswana [33, 34]. However, increased rates of partner violence have been reported for HIV-infected women compared to HIV-uninfected women at a VCT site in Tanzania [80], and fear of partner’s reaction has been cited as a barrier to HIV test acceptance in antenatal clinics in Uganda [81]. Ensuring adequate follow-up and social services, at least through local referral, is an essential element of provider-initiated testing and counseling [11].
One need only examine the failed Lesotho experience to understand that scale-up of testing services must be done in concert with crucial attention to detail to prevent harm. Lesotho has the third highest HIV prevalence in the world [82]. On World AIDS Day 2005, the Lesotho government announced plans to offer country-wide community-based testing to 1.3 million citizens twelve years and older, a campaign which was lauded by the WHO HIV/AIDS program [83]. In 2008, Human Rights Watch reported that the program fell short of its goals both in program implementation and in safeguarding the rights of those tested [84]. The report notes poor training and supervision of lay counselors in performing rapid tests, inadequate funding and infrastructure for post-test referral, and insufficient human rights protections [84]. The Lesotho experience poignantly illustrates the need for careful planning, sufficient funding, adequate training and oversight for a successful community-wide testing campaign.
Conclusions
As ART becomes more widely available in resource-limited settings, increasingly the challenge is case identification so that patients may benefit from ART and secondary prevention [12]. Provider-initiated testing in a variety of settings has demonstrated improved HIV testing participation [41, 49, 64], and knowledge of serostatus has been shown to decrease risk behaviors [4, 7]. Survey data indicate that patients seeking health care are in favor of making HIV testing a routine part of medical care [18, 27], and that the offer of HIV testing does not deter individuals from seeking care [49]. By routinely offering HIV testing, the test will become normalized, decreasing stigma, and removing highly personal prerequisite discussions about HIV risk [41]. Testing in the community and at home can broaden coverage to include couples, lower income individuals, rural populations, and men less likely to visit health care facilities [10, 22, 34, 62].
Questions about how best to implement routine testing, including ensuring confidentiality, dignity, and availability of care and treatment services following a new HIV diagnosis remain. Yet, evidence of widespread support for making HIV testing a routine part of care promises to improve access to life-sustaining treatment for millions living with HIV infection around the world.
Acknowledgments
We would like to thank Bethany Morris and Jennifer Chu for their technical assistance.
This work was supported in part by the National Institute of Allergy and Infectious Disease: K23AI068458 (IVB), The National Institute of Mental Health: R01 MH073445 (RPW) and R01 MH65869 (RPW), and The Doris Duke Charitable Foundation, Clinical Scientist Development Award (RPW).
Footnotes
The authors have no conflicts of interest to report.
Literature Cited
- 1.United Nations General Assembly. Political declaration on HIV/AIDS. [Accessed 19 May 2009];United Nations General Assembly document 60/262. Available at: http://data.unaids.org/pub/Report/2006/20060615_HLM_PoliticalDeclaration_ARES60262_en.pdf.
- 2.World Health Organization. [Accessed 19 May 2009];Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report. 2008 Available at: http://www.who.int/hiv/pub/towards_universal_access_report_2008.pdf.
- 3. [Accessed 19 May 2009];Demographic and Health Surveys. Available at: http://www.measuredhs.com/
- 4.Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. The Voluntary HIV-1 Counseling and Testing Efficacy Study Group. Lancet. 2000;356:103–12. [PubMed] [Google Scholar]
- 5.Allen S, Meinzen-Derr J, Kautzman M, et al. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17:733–40. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 6.Bunnell R, Opio A, Musinguzi J, et al. HIV transmission risk behavior among HIV-infected adults in Uganda: results of a nationally representative survey. AIDS. 2008;22:617–24. doi: 10.1097/QAD.0b013e3282f56b53. [DOI] [PubMed] [Google Scholar]
- 7.Denison JA, O’Reilly KR, Schmid GP, Kennedy CE, Sweat MD. HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990–2005. AIDS Behav. 2008;12:363–73. doi: 10.1007/s10461-007-9349-x. [DOI] [PubMed] [Google Scholar]
- 8.De Cock KM, Bunnell R, Mermin J. Unfinished business--expanding HIV testing in developing countries. N Engl J Med. 2006;354:440–2. doi: 10.1056/NEJMp058327. [DOI] [PubMed] [Google Scholar]
- 9.De Cock KM, Marum E, Mbori-Ngacha D. A serostatus-based approach to HIV/AIDS prevention and care in Africa. Lancet. 2003;362:1847–9. doi: 10.1016/S0140-6736(03)14906-9. [DOI] [PubMed] [Google Scholar]
- 10.Helleringer S, Kohler HP, Frimpong JA, Mkandawire J. Increasing uptake of HIV testing and counseling among the poorest in sub-Saharan countries through home-based service provision. J Acquir Immune Defic Syndr. 2009;51:185–93. doi: 10.1097/QAI.0b013e31819c1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization/United Nations Program on HIV/AIDS. [Accessed 19 May 2009];Guidance on provider-initiated HIV testing and counseling in health facilities. Available at: http://whqlibdoc.who.int/publications/2007/9789241595568_eng.pdf.
- 12.Beckwith CG, Flanigan TP, del Rio C, et al. It is time to implement routine, not risk-based, HIV testing. Clin Infect Dis. 2005;40:1037–40. doi: 10.1086/428620. [DOI] [PubMed] [Google Scholar]
- 13.Obermeyer CM, Osborn M. The utilization of testing and counseling for HIV: a review of the social and behavioral evidence. Am J Public Health. 2007;97:1762–74. doi: 10.2105/AJPH.2006.096263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalichman SC, Simbayi LC. HIV testing attitudes, AIDS stigma, and voluntary HIV counselling and testing in a black township in Cape Town, South Africa. Sex Transm Infect. 2003;79:442–7. doi: 10.1136/sti.79.6.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bentley ME, Spratt K, Shepherd ME, et al. HIV testing and counseling among men attending sexually transmitted disease clinics in Pune, India: changes in condom use and sexual behavior over time. AIDS. 1998;12:1869–77. doi: 10.1097/00002030-199814000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Bunnell RE, Nassozi J, Marum E, et al. Living with discordance: knowledge, challenges, and prevention strategies of HIV-discordant couples in Uganda. AIDS Care. 2005;17:999–1012. doi: 10.1080/09540120500100718. [DOI] [PubMed] [Google Scholar]
- 17.Sherr L, Lopman B, Kakowa M, et al. Voluntary counselling and testing: uptake, impact on sexual behaviour, and HIV incidence in a rural Zimbabwean cohort. AIDS. 2007;21:851–60. doi: 10.1097/QAD.0b013e32805e8711. [DOI] [PubMed] [Google Scholar]
- 18.Weiser SD, Heisler M, Leiter K, et al. Routine HIV testing in Botswana: a population-based study on attitudes, practices, and human rights concerns. PLoS Med. 2006;3:e261. doi: 10.1371/journal.pmed.0030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irungu TK, Varkey P, Cha S, Patterson JM. HIV voluntary counselling and testing in Nakuru, Kenya: findings from a community survey. HIV Med. 2008;9:111–7. doi: 10.1111/j.1468-1293.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- 20.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 21.Morin SF, Khumalo-Sakutukwa G, Charlebois ED, et al. Removing barriers to knowing HIV status: same-day mobile HIV testing in Zimbabwe. J Acquir Immune Defic Syndr. 2006;41:218–24. doi: 10.1097/01.qai.0000179455.01068.ab. [DOI] [PubMed] [Google Scholar]
- 22.Matovu JK, Makumbi FE. Expanding access to voluntary HIV counselling and testing in sub-Saharan Africa: alternative approaches for improving uptake, 2001–2007. Trop Med Int Health. 2007;12:1315–22. doi: 10.1111/j.1365-3156.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- 23.Liddicoat RV, Horton NJ, Urban R, Maier E, Christiansen D, Samet JH. Assessing missed opportunities for HIV testing in medical settings. J Gen Intern Med. 2004;19:349–56. doi: 10.1111/j.1525-1497.2004.21251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald EA, Currie MJ, Bowden FJ. Delayed diagnosis of HIV: missed opportunities and triggers for testing in the Australian Capital Territory. Sex Health. 2006;3:291–5. doi: 10.1071/sh06022. [DOI] [PubMed] [Google Scholar]
- 25.Moyer LB, Brouwer KC, Brodine SK, et al. Barriers and missed opportunities to HIV testing among injection drug users in two Mexico--US border cities. Drug Alcohol Rev. 2008;27:39–45. doi: 10.1080/09595230701710845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stekler JD, Golden MR. Learning from the missed opportunities for HIV testing. Sex Transm Infect. 2009;85:2–3. doi: 10.1136/sti.2008.035329. [DOI] [PubMed] [Google Scholar]
- 27.Nakanjako D, Kamya M, Daniel K, et al. Acceptance of routine testing for HIV among adult patients at the medical emergency unit at a national referral hospital in Kampala, Uganda. AIDS Behav. 2007;11:753–8. doi: 10.1007/s10461-006-9180-9. [DOI] [PubMed] [Google Scholar]
- 28.Homsy J, Kalamya JN, Obonyo J, et al. Routine intrapartum HIV counseling and testing for prevention of mother-to-child transmission of HIV in a rural Ugandan hospital. J Acquir Immune Defic Syndr. 2006;42:149–54. doi: 10.1097/01.qai.0000225032.52766.c2. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. [Accessed 19 May 2009];Botswana: Country health system fact sheet. 2006 Available at: http://www.afro.who.int/home/countries/fact_sheets/botswana.pdf.
- 30.President Festus Mogae. Christmas and New Year message to the nation. Gaborone, Botswana: Dec 23, 2003. [Google Scholar]
- 31.de Korte D, Mazonde P, Darkoh E. [Accessed 19 May 2009];Introducing ARV therapy in the public sector in Botswana: Case study. 2004 Available at: http://www.who.int/hiv/pub/prev_care/botswana.pdf.
- 32. [Accessed 19 May 2009];Epidemiological Fact Sheet on HIV and AIDS: Uganda. 2008 Available at: http://apps.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_UG.pdf.
- 33.Cockcroft A, Andersson N, Milne D, Mokoena T, Masisi M. Community views about routine HIV testing and antiretroviral treatment in Botswana: signs of progress from a cross sectional study. BMC Int Health Hum Rights. 2007;7:5. doi: 10.1186/1472-698X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steen TW, Seipone K, de Gomez FL, et al. Two and a half years of routine HIV testing in Botswana. J Acquir Immune Defic Syndr. 2007;44:484–8. doi: 10.1097/QAI.0b013e318030ffa9. [DOI] [PubMed] [Google Scholar]
- 35.Uganda Ministry of Health. [Accessed 19 May 2009];Uganda national policy guidelines for HIV counseling and testing. 2005 Available at: http://www.who.int/hiv/topics/vct/UG_HCT%20Policy%20DRAFTFeb05.pdf.
- 36.Wanyenze RK, Nawavvu C, Namale AS, et al. Acceptability of routine HIV counselling and testing, and HIV seroprevalence in Ugandan hospitals. Bull World Health Organ. 2008;86:302–9. doi: 10.2471/BLT.07.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. [Accessed 19 May 2009];Epidemiological Country Profile on HIV and AIDS: South Africa. 2008 Available at: http://www.who.int/globalatlas/predefinedReports/EFS2008/short/EFSCountryProfiles2008_ZA.pdf.
- 38. [Accessed 19 May 2009];HIV and AIDS and STI Strategic Plan for South Africa. 2007–2011 Available at: http://www.info.gov.za/otherdocs/2007/aidsplan2007/index.html.
- 39.AVERT. [Accessed 19 May 2009];HIV and AIDS in South Africa. Available at: http://www.avert.org/aidssouthafrica.htm.
- 40.Shisana O, Rehle T, Simbayi LC, et al. South African National HIV prevalence, HIV incidence, behaviour and communication survey, 2005. Cape Town: HSRC Press; 2005. [Google Scholar]
- 41.Bassett IV, Giddy J, Nkera J, et al. Routine voluntary HIV testing in Durban, South Africa: the experience from an outpatient department. J Acquir Immune Defic Syndr. 2007;46:181–6. doi: 10.1097/QAI.0b013e31814277c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. [Accessed 19 May 2009];Epidemiological Country Profile on HIV and AIDS: Haiti. 2008 Available at: http://apps.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_HT.pdf.
- 43.Peck R, Fitzgerald DW, Liautaud B, et al. The feasibility, demand, and effect of integrating primary care services with HIV voluntary counseling and testing: evaluation of a 15-year experience in Haiti, 1985–2000. J Acquir Immune Defic Syndr. 2003;33:470–5. doi: 10.1097/00126334-200308010-00007. [DOI] [PubMed] [Google Scholar]
- 44.Ivers LC, Freedberg KA, Mukherjee JS. Provider-initiated HIV testing in rural Haiti: low rate of missed opportunities for diagnosis of HIV in a primary care clinic. AIDS Res Ther. 2007;4:28. doi: 10.1186/1742-6405-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.UNAIDS. [Accessed 21 May 2009];UNAIDS calls for virtual elimination of mother to child transmission of HIV by 2015. Press release: 21 May 2009. Available at: http://data.unaids.org/pub/PressRelease/2009/20090521_pr_priorityareas_en.pdf.
- 46.McIntyre J, Lallemant M. The prevention of mother-to-child transmission of HIV: are we translating scientific success into programmatic failure? Curr Opin HIV AIDS. 2008;3:139–45. doi: 10.1097/COH.0b013e3282f5242a. [DOI] [PubMed] [Google Scholar]
- 47.Kanshana S, Simonds RJ. National program for preventing mother-child HIV transmission in Thailand: successful implementation and lessons learned. AIDS. 2002;16:953–9. doi: 10.1097/00002030-200205030-00001. [DOI] [PubMed] [Google Scholar]
- 48.Chasombat S, Lertpiriyasuwat C, Thanprasertsuk S, Suebsaeng L, Lo YR. The National Access to Antiretroviral Program for PHA (NAPHA) in Thailand. Southeast Asian J Trop Med Public Health. 2006;37:704–15. [PubMed] [Google Scholar]
- 49.Creek TL, Ntumy R, Seipone K, et al. Successful introduction of routine opt-out HIV testing in antenatal care in Botswana. J Acquir Immune Defic Syndr. 2007;45:102–7. doi: 10.1097/QAI.0b013e318047df88. [DOI] [PubMed] [Google Scholar]
- 50.Manzi M, Zachariah R, Teck R, et al. High acceptability of voluntary counselling and HIV-testing but unacceptable loss to follow up in a prevention of mother-to-child HIV transmission programme in rural Malawi: scaling-up requires a different way of acting. Trop Med Int Health. 2005;10:1242–50. doi: 10.1111/j.1365-3156.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- 51.Moses A, Zimba C, Kamanga E, et al. Prevention of mother-to-child transmission: program changes and the effect on uptake of the HIVNET 012 regimen in Malawi. AIDS. 2008;22:83–7. doi: 10.1097/QAD.0b013e3282f163b5. [DOI] [PubMed] [Google Scholar]
- 52.Chandisarewa W, Stranix-Chibanda L, Chirapa E, et al. Routine offer of antenatal HIV testing (“opt-out” approach) to prevent mother-to-child transmission of HIV in urban Zimbabwe. Bull World Health Organ. 2007;85:843–50. doi: 10.2471/BLT.06.035188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez F, Zvandaziva C, Engelsmann B, Dabis F. Acceptability of routine HIV testing (“opt-out”) in antenatal services in two rural districts of Zimbabwe. J Acquir Immune Defic Syndr. 2006;41:514–20. doi: 10.1097/01.qai.0000191285.70331.a0. [DOI] [PubMed] [Google Scholar]
- 54.Van Wyk E, Giddy J, Roberts C, et al. The Efficiency of Opt-out HIV Testing Compared with Provider-initiated Voluntary Counselling and Testing in an Antenatal Clinic in Durban, South Africa [poster 1049]. Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 55.Veloso VG, Portela MC, Vasconcellos MT, et al. HIV testing among pregnant women in Brazil: rates and predictors. Rev Saude Publica. 2008;42:859–67. doi: 10.1590/s0034-89102008000500011. [DOI] [PubMed] [Google Scholar]
- 56.Pai NP, Barick R, Tulsky JP, et al. Impact of round-the-clock, rapid oral fluid HIV testing of women in labor in rural India. PLoS Med. 2008;5:e92. doi: 10.1371/journal.pmed.0050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Coeur S, Collins IJ, Pannetier J, Lelievre E. Gender and access to HIV testing and antiretroviral treatments in Thailand: why do women have more and earlier access? Soc Sci Med. 2009;69:846–53. doi: 10.1016/j.socscimed.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 58.Were WA, Mermin JH, Wamai N, et al. Undiagnosed HIV infection and couple HIV discordance among household members of HIV-infected people receiving antiretroviral therapy in Uganda. J Acquir Immune Defic Syndr. 2006;43:91–5. doi: 10.1097/01.qai.0000225021.81384.28. [DOI] [PubMed] [Google Scholar]
- 59.Bunnell R, Cherutich P. Universal HIV testing and counselling in Africa. Lancet. 2008;371:2148–50. doi: 10.1016/S0140-6736(08)60929-0. [DOI] [PubMed] [Google Scholar]
- 60.Farquhar C, Kiarie JN, Richardson BA, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37:1620–6. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Msuya SE, Mbizvo EM, Hussain A, Uriyo J, Sam NE, Stray-Pedersen B. Low male partner participation in antenatal HIV counselling and testing in northern Tanzania: implications for preventive programs. AIDS Care. 2008;20:700–9. doi: 10.1080/09540120701687059. [DOI] [PubMed] [Google Scholar]
- 62.Menzies N, Abang B, Wanyenze R, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS. 2009;23:395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]
- 63.Fylkesnes K, Siziya S. A randomized trial on acceptability of voluntary HIV counselling and testing. Trop Med Int Health. 2004;9:566–72. doi: 10.1111/j.1365-3156.2004.01231.x. [DOI] [PubMed] [Google Scholar]
- 64.Wolff B, Nyanzi B, Katongole G, Ssesanga D, Ruberantwari A, Whitworth J. Evaluation of a home-based voluntary counselling and testing intervention in rural Uganda. Health Policy Plan. 2005;20:109–16. doi: 10.1093/heapol/czi013. [DOI] [PubMed] [Google Scholar]
- 65.Nuwaha F, Tumwesigye E, Kasasa S, et al. Population-level changes in knowledge of HIV status, stigma and HIV risk behavior after district-wide door-to-door voluntary counseling and testing: Bushenyi District, Uganda [abstract 139]. Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 66.Suggaravetsiri P, Yanai H, Chongsuvivatwong V, Naimpasan O, Akarasewi P. Integrated counseling and screening for tuberculosis and HIV among household contacts of tuberculosis patients in an endemic area of HIV infection: Chiang Rai, Thailand. Int J Tuberc Lung Dis. 2003;7:S424–31. [PubMed] [Google Scholar]
- 67.Wang B, Losina E, Stark R, et al. Loss to Follow-up in Community Clinics in South Africa: Role of CD4 Count, Gender, and Pregnancy [abstract 841]. Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. [Google Scholar]
- 68.Pignatelli S, Simpore J, Pietra V, et al. Factors predicting uptake of voluntary counselling and testing in a real-life setting in a mother-and-child center in Ouagadougou, Burkina Faso. Trop Med Int Health. 2006;11:350–7. doi: 10.1111/j.1365-3156.2006.01564.x. [DOI] [PubMed] [Google Scholar]
- 69.Losina E, Bassett IV, Giddy J, et al. The “ART” of Linkage: Early loss to follow up (LTFU) after HIV diagnosis at two PEPFAR sites in Durban, South Africa [poster TUPE0345]. XVIIth International AIDS Society Meeting; Mexico City. August 3–8, 2008. [Google Scholar]
- 70.Bassett IV, Regan S, Chetty S, et al. Who starts ART in Durban, South Africa?…Not everyone who should. AIDS. doi: 10.1097/01.aids.0000366081.91192.1c. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walensky RP, Fofana MO, Wood R, et al. The Clinical Impact and Cost-effectiveness of Routine, Voluntary HIV Testing in South Africa [abstract 1050]. Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 72.Bassett IV, Chetty S, Giddy J, et al. False Negative Rapid HIV Tests in an Outpatient Department in Durban, South Africa [poster 908]. Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. [Google Scholar]
- 73.Claassen M, van Zyl GU, Korsman SN, Smit L, Cotton MF, Preiser W. Pitfalls with rapid HIV antibody testing in HIV-infected children in the Western Cape, South Africa. J Clin Virol. 2006;37:68–71. doi: 10.1016/j.jcv.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 74.Gray RH, Makumbi F, Serwadda D, et al. Limitations of rapid HIV-1 tests during screening for trials in Uganda: diagnostic test accuracy study. BMJ (Clinical research ed. 2007;335:188. doi: 10.1136/bmj.39210.582801.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walensky RP, Arbelaez C, Reichmann WM, et al. Revising expectations from rapid HIV tests in the emergency department. Ann Intern Med. 2008;149:153–60. doi: 10.7326/0003-4819-149-3-200808050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aghokeng A, Dimodi H, Atem-Tambe A, et al. Inaccurate HIV diagnosis in developing countries: an unresolved issue [abstract 1051]. Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 77.World Health Organization. [Accessed 19 May 2009];Guidelines for Appropriate Evaluations of HIV Testing Technologies in Africa. 2002 Available at: http://wwwn.cdc.gov/dls/pdf/HIV%20Test%20Guidelines%20Africa.pdf.
- 78.Csete J, Schleifer R, Cohen J. Opt-out” testing for HIV in Africa: a caution. Lancet. 2004;363:493–4. doi: 10.1016/S0140-6736(04)15509-8. [DOI] [PubMed] [Google Scholar]
- 79.Paxton S, Gonzales G, Uppakaew K, et al. AIDS-related discrimination in Asia. AIDS Care. 2005;17:413–24. doi: 10.1080/09540120412331299807. [DOI] [PubMed] [Google Scholar]
- 80.Maman S, Mbwambo JK, Hogan NM, et al. HIV-positive women report more lifetime partner violence: findings from a voluntary counseling and testing clinic in Dar es Salaam, Tanzania. Am J Public Health. 2002;92:1331–7. doi: 10.2105/ajph.92.8.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dahl V, Mellhammar L, Bajunirwe F, Bjorkman P. Acceptance of HIV testing among women attending antenatal care in south-western Uganda: risk factors and reasons for test refusal. AIDS Care. 2008;20:746–52. doi: 10.1080/09540120701693990. [DOI] [PubMed] [Google Scholar]
- 82.UNAIDS. [Accessed 19 May 2009];A global view of HIV infection. 2007 Available at: http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/
- 83.Mills EJ, Chong S. Lesotho embarks on universal HIV testing. HIV AIDS Policy Law Rev. 2006;11:27–8. [PubMed] [Google Scholar]
- 84.Human Rights Watch. [Accessed 18 May 2009];A testing challenge: the experience of Lesotho’s universal HIV counseling and testing campaign. 2008 Available at: www.hrw.org/en/reports/2008/11/17/testing-challenge.