Abstract
Prior neuroimaging research has implicated regions within and near the posterior superior temporal sulcus (pSTS) in the visual processing of biological motion and of the intentions implied by specific movements. However, it is unknown whether this region is engaged during the processing of human motion at a conceptual level, such as during story comprehension. Here, we obtained functional magnetic resonance images from subjects reading brief stories that described a human character’s background and then concluded with an action or decision made by the character. Half of the stories contained incidental descriptions of biological motion (such as the character’s walking or grasping) while the remaining half did not. As a second factor, the final action of the story was either congruent or incongruent with the character’s background and implied goals and intentions. Stories that contained biological motion strongly activated the pSTS bilaterally, along with ventral temporal areas, premotor cortex, left motor cortex, and the precuneus. Active regions of pSTS in individual subjects closely overlapped with regions identified with a separate biological motion localizer (point-light display) task. Reading incongruent versus congruent stories activated dorsal anterior cingulate cortex and bilateral anterior insula. These results support the hypothesis that reading can engage higher visual cortex in a content-specific manner, and suggest that the presence of biological motion should be controlled as a potential confound in fMRI studies using story comprehension tasks.
Keywords: biological motion, intention, reading, story comprehension, superior temporal sulcus
Introduction
Humans are able to rapidly infer social meaning from other’s movements, such as eye gaze shifts, hand grasps and gestures, and expression formation. Recent neuroimaging and electrophysiological research has linked this ability, termed social perception, to the posterior superior temporal sulcus (pSTS). A multimodal perceptual region, the pSTS responds strongly while viewing biological motion, or motion generated by animate agents, and is sensitive to the perceived intentionality of specific motions (see review by Allison et. al. 2000).
Brain regions within and near the pSTS respond more strongly to point-light displays (PLDs) depicting human actions than to object motion PLDs, random dot motion, and scrambled human PLDs (Bonda et. al. 1996, Grossman et. al. 2000, Grossman and Blake 2002, Beauchamp et. al. 2003, Saygin et. al. 2004). The pSTS also responds to specific movements performed by real or virtual humans, such as eye gaze shifts (Puce et. al. 1998, Pelphrey, Morris, Michelich et. al. 2005), hand motion (Pelphrey, Morris, et. al. 2004, Pelphrey, Morris, Michelich, et. al. 2005; see also Bonda et. al. 1996), and body motion (Pelphrey et. al. 2003, Pelphrey, Viola, et. al. 2004). Furthermore, this region is engaged by animations of interacting geometric shapes such as Heider-Simmel animations, in which certain motion patterns elicit impressions of animacy and specific social interactions (Castelli et. al. 2000, 2002, Schultz et. al. 2002, Martin and Weisberg 2003, Gobbini et. al. 2007). These activations are typically bilateral but stronger and more consistently observed in the right hemisphere. The pSTS may subserve mechanisms dedicated to the visual processing of biological motion; the existence of such mechanisms could help explain the speed and ease with which humans identify and interpret this rather complex class of motion.
A related literature has demonstrated that pSTS activity is sensitive to the perceived intentionality of human movements. In particular, a series of studies has consistently found a greater right pSTS response to actions that are incongruent versus congruent with the implied intention of the actor: for instance, grasping empty space rather than an adjacent object, or shifting gaze to empty space rather than a target (Pelphrey, Morris, et. al. 2004, Saxe, Xiao, et. al. 2004, Mosconi et. al. 2005, Brass et. al. 2007, Vander Wyk et. al. 2009). It has thus been proposed that this region is involved in inferring information about goals and intentions from visual properties of biological motion and the context in which it occurs (cf. Saxe, Carey, et. al. 2004, Morris et. al. 2005; see also Jellema et. al. 2000, Akitsuki and Decety 2009). Humans rapidly infer intentions from properties of others’ movements—for instance, a grasp toward an object implies interest in or desire for that object—and looking time studies suggest that even young (~15-month-old) infants make such inferences (cf. Saxe, Carey, et. al. 2004). As an area engaged during the perception of biological motion, and whose sensitivity to eye gaze and biological motion is present even in 4–5 month old infants (Grossmann et. al. 2008, Lloyd-Fox et. al. 2009), the pSTS is a candidate neural substrate for inferences about intentions. This hypothesis is consistent with the finding that certain individuals with autism, which involves impairment in the ability to infer others’ intentions (cf. Baron-Cohen 1999), lack a differentiation of pSTS activity evoked by incongruent and congruent actions (Pelphrey, Morris, and McCarthy 2005, Vander Wyk et. al. 2009).
Nearby regions of posterior lateral temporal/parietal cortex are also consistently found active during the processing of mental states at a conceptual level, such as during story or cartoon comprehension (e.g. Fletcher et. al. 1995, Gallagher et. al. 2000, Saxe and Kanwisher 2003, Saxe and Wexler 2005, Saxe and Powell 2006, den Ouden et. al. 2005, Perner et. al. 2006, Ciaramidaro et. al. 2007, Young and Saxe 2008a, 2008b). Some have argued that this region, termed the temporo-parietal junction (TPJ), is centrally involved in representing mental states (e.g. Saxe and Powell 2006, Ciaramidaro et. al. 2007), while others believe that TPJ activation during theory of mind tasks reflects the recruitment of a more general process (Decety and Lamm 2007, Mitchell 2008, Corbetta et. al. 2008).
Studies that have used both biological motion and theory of mind tasks in the same set of subjects have found a dissociation, with the latter engaging a more posterior and superior region (Gobbini et. al. 2007, Saxe et. al. 2009; see also Saxe, Xiao et. al. 2004). This suggests the presence of separate lateral temporal/parietal systems involved in inferring mental states from visual properties, and processing mental states at a conceptual level. However, stimuli used in these studies have also differed in their mode of presentation (visual versus story-based), leaving open the possibility that this distinction accounts for the observed dissociation. Furthermore, many studies using false belief tasks have not adequately controlled for the presence of human motion in vignette stimuli; for instance, the false belief versus false photograph contrast used by Saxe and colleagues (Saxe and Kanwisher 2003, Saxe and Wexler 2005, Saxe and Powell 2006, and others) doesn’t account for such a confound.
The present study aimed to investigate the relative roles of pSTS and TPJ in processing human actions and their implied intentions, as presented in a single task using brief stories, analogous to vignette stimuli used in studies of theory of mind. Subjects underwent functional magnetic resonance imaging (fMRI) while reading brief (paragraph-length) stories describing a human character’s background or current situation, and then an action performed or decision made by that individual (see Appendix for example stimuli). We varied the amount of human motion described in the story (e.g. walking, grasping, swimming, dancing), and the congruency of the action with the prior background description.
We predicted that the posterior STS would be preferentially engaged as subjects read stories containing biological motion. Several studies have suggested that reading passages or individual words can activate perceptual and motor cortex in a modality- and content-specific manner (e.g. Hauk et. al. 2004, Goldberg et. al. 2006, Aziz-Zadeh et. al. 2006, Speer et. al. 2009); we expected this finding to extend to the pSTS, on the hypothesis that this region is involved in the perceptual processing of biological motion.
However, we predicted that the incongruent-congruent contrast would not yield pSTS activation, based on the hypothesis that the pSTS is involved specifically in inferring intentions from visual properties of human motion. Instead, we predicted that incongruent stories would engage the TPJ and possibly other regions active during mental state processing, such as medial prefrontal cortex and precuneus. Humans readily explain others’ actions in terms of intentions and other mental states, so we hypothesized that reading about incongruent (difficult to explain) actions would engage regions putatively involved in theory of mind. Saxe and Wexler (2005) found that reading descriptions of character’s intentions that were incongruent with their personal descriptions preferentially engaged the right TPJ, as defined by a false belief localizer; we believed that the TPJ would also respond differentially to actions or decisions that were incongruent with a background description and implied desires and intentions. Thus, we hoped that our approach would allow the identification and comparison of these two nearby regions with a single task that varied only the nature of the story.
Methods
Subjects and Stimuli
15 subjects (8 male, 7 female, mean age 22, all right handed), with normal vision and no history of neurological or psychiatric illness, participated in the study. All subjects gave written, informed consent and the protocol was approved by the Yale Human Investigations Committee.
Subjects were presented text-based vignettes describing human characters and their actions (mean story length = 70 words, see Appendix for example stimuli). Each story was broken into two sections of similar length, and each section was visually presented for 8s with no delay between the sections, in 24pt black font on a white background. There were 24 different stories presented over 4 runs, each lasting 172s, with a 10s rest period between each story. Stories were presented in pseudorandom order, with conditions counterbalanced across runs and subjects. Subjects were instructed to read and comprehend each story. No overt response was required from the subject. After the scan, subjects were asked whether they had been able to read the stories in the time provided; all subjects confirmed that they had been. Story content was manipulated as follows: (1) stories either did or did not contain at least two explicit descriptions of human motion (e.g. “John walked…” or “Jane grasped…”); and (2) the concluding action or decision described was either congruent or incongruent with the background description of the character’s situation. Stories with biological motion contained motion verbs in both sections, while only the second section of the story contained the congruent/incongruent action or decision.
Each subject was also run in a biological motion localizer task, to determine areas that responded to visually presented biological motion, in individual subjects. In this task, subjects viewed PLDs of moving humans, and scrambled control PLDs. Brief animations of each type were blocked together, with four 32s blocks for each type, all presented in a single run. Scrambled PLDs were created by taking the human PLDs, and randomly permuting the initial positions of dots with given trajectories, to eliminate the percept of human motion (similar to the control stimuli used by Grossman and Blake 2002, Gobbini et. al. 2007).
Image Acquisition and Preprocessing
Brain images were collected using a 3.0 Tesla Siemens TRIO scanner. Functional images were acquired using a standard echo planar pulse sequence (parameters: repetition time TR = 2s, echo time TE = 25ms, flip angle α = 90°, field of view = 240mm, matrix = 642, slice thickness = 3.5mm, 34 slices). Two sets of structural images were collected for registration: coplanar images, collected using a T1 Flash sequence (TR = 300ms, TE = 2.47ms, α = 60°, FOV = 240mm, matrix = 2562, slice thickness = 3.5mm, 34 slices); and high-resolution images, collected using a 3D MP-Rage sequence (TR = 2530ms, TE = 3.34ms, α = 7°, FOV = 256mm, matrix = 2562, slice thickness = 1mm, 160 slices).
Preprocessing and regression analyses were performed using the FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl/). All images were skull-stripped using FSL’s brain extraction tool supplemented, in two cases, by manual masking. The first 3 volumes (6s) of each functional dataset were removed to eliminate MR equilibration effects. Data were temporally realigned to correct for interleaved slice acquisition, and spatially realigned to correct for head motion using FSL’s MCFLIRT linear realignment tool. Images were spatially smoothed with a 5mm FWHM isotropic Gaussian kernel. Each time series was high-pass filtered (.01 Hz cutoff) to remove low-frequency drift. Functional images were registered to coplanar images, which were in turn registered to high-resolution anatomical images, and then normalized to the Montreal Neurological Institute’s MNI152 template.
fMRI Data Analysis
Whole brain voxel-wise regression analyses were performed using FSL’s FEAT. Two first level analyses were computed for each subject, with different models to separately investigate the effects of biological motion and congruency, due to the offset in timing of these two story components. Both models used four regressors, one for each stimulus class (BIO-CONG, BIO-INCONG, NONBIO-CONG, NONBIO-INCONG). For the biological motion analysis, regressors were defined as boxcar functions with value 1 during the full 16s of story presentation for stimuli of a given class, convolved with a single-gamma hemodynamic response function (HRF). For the congruency analysis, regressors were defined similarly, but with boxcars that only peaked during the second section of a given story, where congruency information was presented. For the biological motion analysis, we focused on the contrast (BIO-CONG + BIO-INCONG) − (NONBIO-CONG + NONBIO-INCONG); for the congruency analysis, we focused on (BIO-INCONG + NONBIO-INCONG) − (BIO-CONG + NONBIO-CONG).
For group-level analyses, parameter estimates were assessed with a mixed effects model, with the random effects component of variance estimated using FSL’s FLAME stage 1+2 procedure (cf. Beckmann et. al. 2003). For both individual and group-level analyses, clusters were first defined as contiguous sets of voxels with z (Gaussianized t) > 2.3; clusters were then thresholded using Gaussian random field theory (cluster probability P<.05) to correct for multiple comparisons (cf. Worsley et. al. 1996). The biological motion localizer data were analyzed in an analogous way, using the same HRF and threshold. All group-level analyses are presented on a flattened, inflated representation of cortical surface so that activations within deep sulci can be easily visualized. The flattened brain was derived from the MNI-152 brain using FreeSurfer (http://surfer.nmr.mgh.harvard.edu).
To assess whether the visual and story-level biological versus non-biological motion contrasts activated similar regions of pSTS in individual subjects, we created maps of the set of voxels found active in both contrasts for each subject. These are presented on inflated cortical surfaces generated from individual anatomical images, using FreeSurfer.
We also conducted an anatomical region-of-interest (ROI) analysis to closely investigate the hypothesis that the posterior STS is preferentially engaged by stories with biological motion. Prior studies have found that the posterior crux of the right STS, where it divides into the posterior continuation sulcus and the ascending sulcus that define the angular gyrus, is particularly responsive to biological motion (Pelphrey et. al. 2003, 2004b). We thus defined ROIs in individual subjects as 5-mm-radius spheres centered on a hand-picked coordinate, chosen by identifying this crux on a surface-rendered high-resolution image. For each voxel in each subject’s functional data, fMRI signal was averaged across epochs corresponding to biological and nonbiological story comprehension and converted to percent signal change relative to an inter-trial baseline; time courses of signal change were averaged over the voxels contained in our ROIs, and then averaged across subjects. Two-tailed paired sample t-tests were used to compare percent signal change between the two conditions at each point in time from 2s before story onset to 26s after story onset. ROI analyses were performed using custom-made MATLAB scripts (http://www.mathworks.com). We did not perform ROI analysis for the TPJ, as this region is typically functionally defined, and lacks a precise anatomical marker, as the crux of the pSTS offers.
Results
Stories with and without biological motion
Peak coordinates and z-scores of significant clusters from the BIO versus NONBIO story contrast are given in Table 1. Large regions of bilateral posterior temporal cortex, including posterior STS and adjacent middle temporal gyrus, were found to respond more strongly to stories with biological motion (see Figure 1). Active clusters were also found in bilateral premotor cortex, bilateral fusiform gyrus, precuneus, left postcentral/precentral gyri, bilateral parahippocampal gyrus, bilateral retrosplenial cortex, and right thalamus.
Table 1.
Peak coordinates (in MNI space) from BIO versus NONBIO story contrast.
| Region | Coordinates (mm) | z-score | ||
|---|---|---|---|---|
| x | y | z | ||
| Right precuneus | 6 | −44 | 44 | 4.95 |
| Right pSTS | 58 | −54 | 12 | 4.88 |
| Left pSTS | −60 | −60 | 2 | 4.71 |
| Left parahippocampal gyrus | −32 | −40 | −14 | 4.27 |
| Right parahippocampal gyrus | 24 | −32 | 16 | 4.15 |
| Right premotor cortex | 46 | 4 | 46 | 4.07 |
| Left premotor cortex | −46 | 2 | 46 | 3.44 |
| Right thalamus | 16 | −30 | 6 | 3.83 |
| Left retrosplenial cortex (RSC) | −18 | −60 | 14 | 3.64 |
| Right retrosplenial cortex (RSC) | 18 | −52 | 12 | 3.53 |
| Left fusiform gyrus | −44 | −44 | −20 | 3.40 |
| Right fusiform gyrus | 44 | −42 | −20 | 3.26 |
| Left postcentral gyrus | −42 | 26 | 50 | 3.34 |
Figure 1.
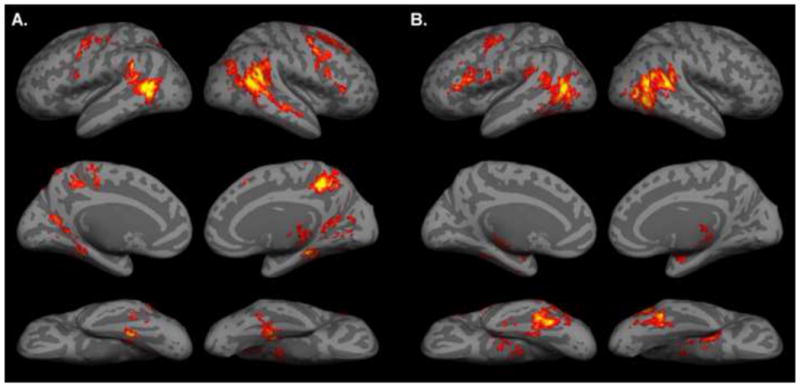
A. Activation map for BIO versus NONBIO contrast in story comprehension task, displayed on a cortical surface representation. B. Activation map for biological motion localizer. In all images, the color bar ranges from z=2.3 (dark red) to 4.0 (bright yellow).
Peak coordinates and z-scores of significant clusters from the biological motion localizer analysis (biological versus nonbiological motion contrast) are given in Table 2. This analysis revealed large patches of active cortex in bilateral posterior STS and adjacent posterior lateral temporal cortex, substantially overlapping with posterior temporal areas active in the story-based biological motion analysis (see Figure 1). Active clusters were also found in left premotor cortex, fusiform gyrus, left inferior frontal gyrus, right thalamus, and right amygdala; these results are in accord with prior studies using human PLD stimuli (cf. Grossman and Blake 2002, Saygin et. al. 2004, Gobbini et. al. 2007). (Note that some of these clusters were broken up in transforming the activation maps to the flattened brain, and thus appear as separate regions on surface renderings; this may be in part artifactual, resulting from the use of volume-based cluster correction followed by surface rendering).
Table 2.
Peak coordinates from the biological motion localizer task.
| Region | Coordinates (mm) | z-score | ||
|---|---|---|---|---|
| x | y | z | ||
| Right pSTS | 56 | −62 | 12 | 4.68 |
| Left pSTS | −50 | −68 | 10 | 4.26 |
| Left fusiform gyrus | −40 | −52 | −22 | 4.24 |
| Right fusiform gyrus | 44 | −46 | −16 | 4.15 |
| Left inferior frontal gyrus | −56 | 26 | 10 | 3.88 |
| Right amygdala | 30 | −4 | −16 | 3.45 |
| Left premotor cortex | −36 | −2 | 48 | 3.26 |
| Right thalamus | 8 | −14 | 6 | 3.02 |
Among the individual subjects’ analyses, significant regions of right posterior STS activated by stories with biological motion were found in 11/15 subjects, and regions of right pSTS active in the localizer were found in 13/15 subjects. Ten subjects had right pSTS in both the visual and story-based biological versus nonbiological contrast; in all ten of these subjects, areas of right pSTS found in with the two contrasts overlapped. Six representative overlap maps (maps of voxels active in both visual and story-based biological motion analyses) are shown in Figure 2.
Figure 2.
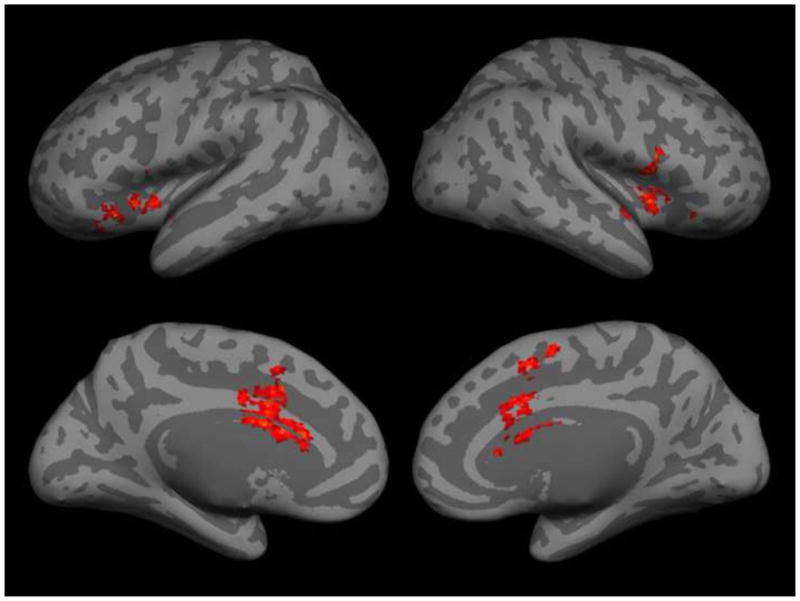
Overlap maps (the set of voxels that were found significant after cluster-correction in both visual and story-based biological motion contrasts) for six representative subjects.
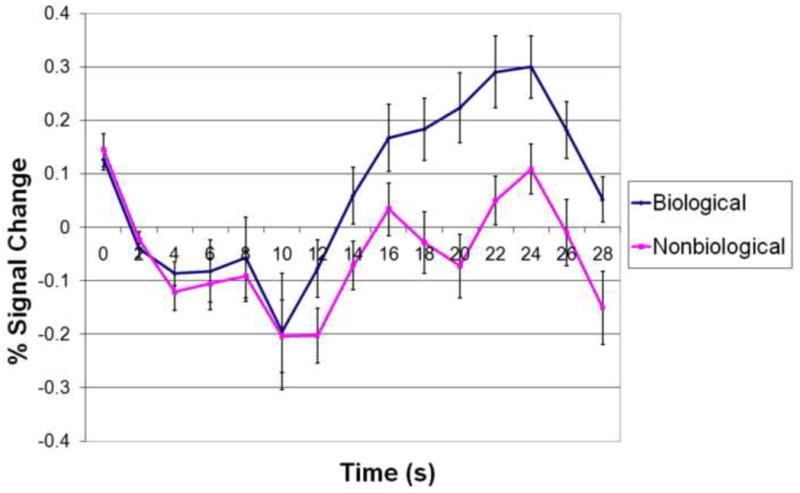
Results from the anatomical ROI analysis are presented in Figure 3. We found that the BOLD response the right posterior STS, defined by anatomical landmarks in individual subjects, was significantly greater to stories with versus without biological motion, at every time point from 12s to 22s (t(14) > 2.145, p < .05, two-tailed) following the onset of story presentation (where stories lasted for 16s). The maximum difference occurred at 18s after story onset (t(14) = 4.263, p < .001, two-tailed).
Figure 3.
Anatomical ROI analysis results: trial- and ROI-averaged time courses of BOLD signal from epochs corresponding to stories with and without biological motion. Error bars indicate standard error of BOLD signal at a given time point. Story presentation begins at 2s and ends at 18s.
Stories with congruent or incongruent endings
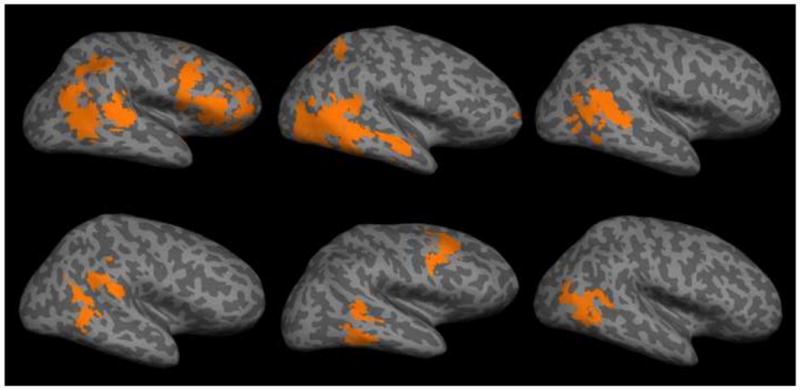
Peak coordinates and z-scores of significant clusters from the INCONG versus CONG story contrast are given in Table 3. Dorsal anterior cingulate cortex (ACC), extending into pre-SMA, and bilateral anterior insula (AI) were found active in this analysis (see Figure 4). No significant voxel clusters were found within the pSTS or the TPJ.
Table 3.
Peak coordinates from INCONG versus CONG story contrast.
| Region | Coordinates (mm) | z-score | ||
|---|---|---|---|---|
| x | y | z | ||
| Dorsal anterior cingulate cortex | −2 | 24 | 20 | 3.62 |
| Right anterior insular cortex | 40 | 10 | −2 | 3.60 |
| Left anterior insular cortex | −30 | 22 | −4 | 3.52 |
Figure 4.
Activation map from INCONG versus CONG contrast.
Discussion
pSTS Response to Stories with Human Motion
These findings demonstrate that the posterior STS is preferentially engaged during comprehension of stories that contain incidental descriptions of human motion. Areas responding to biological motion in stories and in visually perceived PLDs overlapped closely in individual subjects, suggesting that the group level overlap did not result from imperfect registration (cf. Saxe, Carey et. al. 2004), and that the region of pSTS found with the story comprehension task can be identified by the area of pSTS previously implicated in the visual perception of biological motion. The results of our ROI analysis further support the specific anatomical hypothesis that the posterior-most STS responds more strongly to stories with biological motion.
Generally, these results support the hypothesis that reading stories can activate visual cortex in a content-specific manner (cf. Speer et. al. 2009). There are at least two possible cognitive explanations for this phenomenon. One is that such activations result from spontaneous visual imagery of specific objects or places evoked by reading about them. Visual imagery is known to activate higher visual cortex in a content-specific manner (O’Craven and Kanwisher 2000, Ishai et. al. 2000, Grossman and Blake 2001); in particular, imagery of biological motion activates pSTS, relative to imagery of nonbiological motion and fixation control conditions (Grossman and Blake 2001). Another possibility is that activation of visual cortex during reading is directly linked to conceptual processing, and reflects the grounding of certain concepts in perceptual/motor representations (cf. Aziz-Zadeh et. al. 2006); on this hypothesis, our results would imply that the pSTS was involved in the conceptual representation of human motion.
The present results alone cannot distinguish these two explanations, which are generally quite difficult to tease apart. Bedny et. al. (2008) found that reading verbs describing motion relative to verbs that didn’t describe motion (specifically, mental verbs) did not activate any part of lateral temporal cortex, arguing against the hypothesis that the semantic representation of motion verbs draws upon perceptual representations of motion or human motion. This provides some evidence in favor of the imagery interpretation of the present findings, and suggests that full stories, rather than individual words about human motion are required to activate the pSTS. Further research might address this question by seeing whether the strength of pSTS activation during reading about human motion correlates with psychological measures of the strength of visual imagery, such as the Vividness of Visual Imagery Questionnaire (Marks 1973), which has been shown to correlate with V1 activity during visual imagery (Cui et. al. 2007).
Activations around the temporo-parietal junction and posterior STS region have also been consistently found during stimulus-driven shifts of visual attention (cf. Corbetta and Shulman 2002). Such findings suggest a possible confound in neuroimaging studies of biological motion: biological motion is a visually complex and attention-attracting stimulus class, so perhaps the pSTS response to biological motion reflects attentional capture rather than category-specific perceptual processing. However, we argue that the present results are difficult to explain as an effect of visual attention, on the assumption that reading any story of a given length should involve a similar degree of visual attentional engagement. Thus our results support the claim that the pSTS response to biological motion doesn’t result from attentional capture.
To compare our results to past fMRI and PET studies of biological motion and theory of mind, we conducted a meta-analysis. We gathered peak coordinates of TPJ activations from 14 studies of the conceptual-level processing of beliefs or intentions (Fletcher et. al. 1995, Gallagher et. al. 2000, Saxe and Kanwisher 2003, Saxe and Wexler 2005, den Ouden et. al. 2005, Saxe and Powell 2006, Saxe et. al. 2006, Young et. al. 2007, Gobbini et. al. 2007, Ciaramidaro et. al. 2007, Kliemann et. al. 2008, Young and Saxe 2008a, Young and Saxe 2009, Scholz et. al. 2009) and pSTS activations from 18 studies of the perception of biological motion and intentionality (Bonda et. al. 1996, Puce et. al. 1998, Castelli et. al. 2000, Grossman et. al. 2002, Schultz et. al. 2003, Schulz et. al. 2003, Martin and Weisberg 2003, Beauchamp et. al. 2003, Saxe, Xiao et. al. 2004, Pelphrey, Morris, et. al. 2004, Pelphrey, Morris, Michelich, et. al. 2005, Morris et. al. 2005, Thompson et. al. 2005, Mosconi et. al. 2005, Peelen et. al. 2006, Gobbini et. al. 2007, Brass et. al. 2007, Vander Wyk et. al. 2009). Wherever necessary, the inverse Brett transform (the function tal2mni) was used to convert Talairach coordinates into MNI space. Maps of 3×3×3 voxel (6×6×6 mm) cubes centered on each coordinate in MNI152 space were generated and transformed to a cortical surface representation, shown in Figure 5, with our story-level biological motion activation map outlined.
Figure 5.
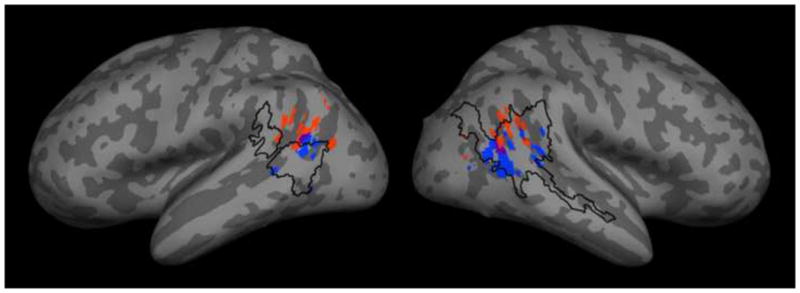
Results from an informal meta-analysis of past activations in studies of theory of mind (red) and the perception of biological motion and intentionality (blue), with overlapping voxels in purple. Our activation (BIO versus NONBIO contrast from the story comprehension task) is outlined in black.
The meta-analysis confirmed prior observations that peak coordinates from theory of mind studies are generally posterior and superior to those from biological motion studies (cf. Gobbini et. al. 2007). The area of pSTS activated by stories with versus without biological motion from the present study overlapped with 15 out of 19 right STS peak coordinates, 5 out of 8 left STS coordinates, 11 out of 15 right TPJ coordinates, and 1 out of 13 left TPJ coordinates. The overlap of our activation with many right TPJ peak coordinates from prior theory of mind studies is particularly intriguing, given that many of these studies used a false belief versus false photograph contrast that may have been confounded by the presence of biological motion. However, we did not run a similar theory of mind task in this set of subjects, barring strong claims regarding overlap between the regions identified in the present study and past theory of mind studies. Future research should explicitly investigate the possible influence of biological motion on theory of mind activations by employing a false belief versus false photograph task with biological motion explicitly controlled as a second factor.
Other regions engaged by stories with biological motion
Beyond the pSTS, reading stories about biological motion activated several other brain regions. Areas of activation were found in premotor cortex and on the fusiform gyrus (consistent with the previously reported location of the fusiform face area, e.g. Puce et al. 1995, McCarthy et. al. 1997), both areas that have been found in prior studies of biological motion using PLD stimuli (e.g. Grossman and Blake 2002, Saygin et. al. 2004, Gobbini et. al. 2007). These activations were less consistent in individual subjects, making it difficult to judge their degree of overlap with similar regions identified by our biological motion localizer; however, we note that at the group level, the larger region on the fusiform gyrus found with the localizer nearly encompassed the area of fusiform gyrus active in the story-level biological motion analysis.
The BIO versus NONBIO contrast also elicited activation in a region of left precentral and postcentral gyri. This activation fell within the hand region of primary motor cortex, roughly anatomically identified as a characteristic crescent in MNI space, and adjacent primary somatosensory cortex. Among our stimuli, 11 out of 12 stories with biological motion contained a description of hand motion of some sort (e.g. grasping, strumming a guitar, throwing a ball, banging a fist, etc). Therefore, this cluster may reflect the activation of a basic motor representation of hand movement while reading stories involving hand actions; this is consistent with the fact that this area was only found on the left (note that all of our subjects were right-handed), and agrees with prior findings that reading verbs describing hand actions preferentially activates the hand region of primary motor cortex (Hauk et. al. 2004, Aziz-Zadeh et. al. 2006).
We also found a strong right-lateralized activation in the precuneus. This was unexpected, but may reflect the putative role of the precuneus in visual imagery. The precuneus has been identified in various studies of visual imagery (reviewed by Cavanna and Trimble 2006): for instance, it is active during visual imagery of objects relative to fixation (Ishai et. al. 2000), during recall of imageable versus nonimageable verbs (Fletcher et. al. 1995), during mental rotation versus simple comparison (Suchan et. al. 2002), and during motor imagery versus execution (Stephan et. al. 1995, Gerardin et. al. 2000, Hanakawa et. al. 2003). These studies typically found an anterior and superior region of precuneus, similar to the present results. Among our stimuli, stories with biological motion described concrete actions that would have been easy to visualize, whereas stories without biological motion were typically more abstract, involving descriptions of an individual’s personality or preferences; thus it is plausible that our contrast would have elicited activation in areas associated with visual imagery. Future research could test this interpretation by controlling the imageability of vignette stimuli or individual sentences, without varying the amount of biological motion.
Unpredicted activations were also found in the parahippocampal gyrus (consistent with the previously reported location of the parahippocampal place area, e.g. Epstein and Kanwisher 1998, Aguirre et. al. 1998) and retrosplenial cortex, both areas implicated in the visual processing of spatial layouts or scenes (e.g. Epstein et. al. 2007, Park and Chun 2009). The biological-motion stories used in the present study described humans interacting in concrete environments; all 12 stories contained at least one description of walking or running, and this was typically described within a spatial context—e.g. walking down the stairs, running across a field, walking into a kitchen, etc. The stories without motion, on the other hand, were abstract and typically didn’t make reference to definite physical locations. Thus, activation of scene-selective regions with our contrast likely resulted from imagery or conceptual processing of spatial layouts described in the stories with motion. This claim could be tested by explicitly controlling the amount of scene description in vignettes or sentences.
Brain response to incongruent stories
We did not find the expected activation in TPJ when contrasting incongruent to congruent stories, but instead found a differentiation of activity in the anterior cingulate cortex and bilateral insula. The lack of TPJ activation was surprising given the similarity of our comparison to that used by Saxe and Wexler (2005). However, Saxe and Wexler (2005) required subjects to answer a question about how the character would evaluate the outcome in each story, and thus forced subjects to think about the character’s intentions. The present study, on the other hand, required no overt response. Thus, it is possible that the design of Saxe and Wexler (2005) was more effective in eliciting the processing of intentions. Theory of mind studies finding TPJ activation have typically explicitly required subjects to think about a mental state, either by asking a question or presenting a story that can only be comprehended by representing a false belief; thus it may be the case that TPJ is only engaged during explicit cognition about others’ mental states. Alternatively, the relevant difference may lie in the type of analysis applied in the two studies: Saxe and Wexler (2005) exclusively used ROI-based analyses, while we used whole-brain voxel-wise regression analyses. Thus on the one hand, our analysis may have lacked the sensitivity necessary to detect a differentiation of TPJ activation; on the other hand, differentiation of ACC and/or insula activity may have been present but gone unnoticed in the experiment reported by Saxe and Wexler (2005).
Our observed activation in ACC and AI demonstrates, however, that the incongruent stories did elicit a consistent, regionally specific brain response, and suggests that our subjects understood the incongruence between the story character’s background and decisions. The ACC and AI form a functional network of regions that frequently co-activate across a range of cognitive and emotional tasks (cf. Craig 2009), and are functionally connected in the resting state (Seeley et. al. 2007). The reason for activation of this network in the present study isn’t completely clear; one possibility is that this activation results from monitoring or processing of conflict while reading incongruent stories. The ACC has been consistently found active during the presence of response conflict across a range of paradigms such as the Stroop task, flanker task, and Go-NoGo task (cf. Botvinick et. al. 2004). Some of these studies have also found activation in the anterior insula (e.g. Menon et. al. 2001, Peterson et. al. 2002). It has been proposed that the ACC is generally involved in conflict monitoring (cf. Botvinick et. al. 2004). The comprehension of incongruent stories used here requires holding in mind two conflicting pieces of information about a human character; thus the ACC response to incongruent stories might reflect the engagement of conflict-monitoring processes. Most studies of the role of the ACC in conflict processing have focused on response conflict, rather than the representation of conflicting conceptual or perceptual information; thus if this interpretation were correct, it would provide support for the notion that the ACC is generally involved in conflict processing. Future studies might test this interpretation by testing whether reading stories with domain-general conceptual violations preferentially engages the ACC and/or AI.
Conclusion
The present study provides clear evidence that the posterior superior temporal sulcus, functionally or structurally defined, is preferentially engaged while reading stories containing human motion. Given these findings, future studies using story comprehension tasks should explicitly control for the presence of biological motion. Our results add to a nascent line of research on content-specific activations during text comprehension (cf. Speer et. al. 2009). Future neuroimaging research should further explore the range of content-specific activations of perceptual, motor, and association cortex that occur while reading.
Acknowledgments
We thank Will Walker, for help in data collection, and Tao Gao, for designing the biological motion localizer stimuli. The present research was supported by NIH grant NS41328.
Appendix: sample stimuli
Congruent/incongruent endings are indicated by the bracketed phrase.
No biological motion
The Hastings school board is voting on Bill 43A, to establish a special education program targeted to those with autism and Asperger’s syndrome. Susan is sympathetic to children with disabilities, having met many while volunteering in a pediatric clinic in college. She understands that autism is a severe and increasingly problematic condition. After weighing the options extensively, Susan votes [yes/no] on Bill 43A.
Rich is a programmer in his 40’s; he has recently lost his job in the finance industry, and is looking for new employment. Rich’s greatest assets are his analytical and problem-solving skills: given a computational task, he can come up with a highly efficient program to perform this task, in no time. After mining the job market, Rich decides to [work for Google/become a construction worker].
Biological motion
Christmas was approaching, and all that Johnny could think about was the one gift he had asked for that year: a Wii video game system. Earlier that week he had written a letter to Santa asking for it. On Christmas morning, Johnny ran down the stairs to the tree. He opened his one gift, and lo and behold, it was a [Nintendo Wii video game system/box full of clothes from Old Navy]. Johnny’s eyes lit up, and he jumped up to give his mom a hug.
Ronald is a professor at the University of Chicago, and has always been passionate about music. As he walks through a CD store, grabbing a CD here and there to read its song listing, he is reminded of his years playing clarinet in high school and college, and feels eager to play a Bach chorale again. After circling the store several times, he makes a choice—[a “Gershwin’s greatest” compilation/Metallica’s Master of Puppets]—and brings it to the register.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, Zarahn E, D’Esposito M. An area within human ventral cortex sensitive to “building” stimuli: evidence and implications. Neuron. 1998;21:373–383. doi: 10.1016/s0896-6273(00)80546-2. [DOI] [PubMed] [Google Scholar]
- Akitsuki Y, Decety J. Social context and perceived agency modulate brain activity in the neural circuits underpinning empathy for pain: an event-related fMRI study. NeuroImage. 2009;47:722–734. doi: 10.1016/j.neuroimage.2009.04.091. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Sciences. 2000;4(7):267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Wilson SM, Rizzolatti G, Iacoboni M. Congruent embodied representations for visually presented actions and linguistic phrases describing actions. Current Biology. 2006;16(18):1818–1823. doi: 10.1016/j.cub.2006.07.060. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Campbell R, Karmiloff-Smith A, Grant J, Walker J. Are children with autism blind to the mentalistic significance of the eyes? Br J Dev Psychol. 1999;13:379–398. [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. fMRI responses to video and point-light displays of moving humans and manipulable objects. Journal of Cognitive Neuroscience. 2003;15(7):991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in fMRI. NeuroImage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bedny M, Caramazza A, Grossman E, Pascual-Leone A, Saxe R. Concepts are more than percepts: the case of action verbs. The Journal of Neuroscience. 2008;28 (44):11347–11353. doi: 10.1523/JNEUROSCI.3039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Ostry D, Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. The Journal of Neuroscience. 1996;16(11):3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brass M, Schmitt RM, Spengler S, Gergely G. Investigating action understanding: Inferential processes versus action simulation. Current Biology. 2007;17:2117–2121. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happé F, Frith U, Frith C. Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. NeuroImage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Ciaramidaro A, et al. The intentional network: How the brain reads varieties of intentions. Neuropsychologia. 2007;45:3105–3113. doi: 10.1016/j.neuropsychologia.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:206–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel – now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Cui X, Jeter CB, Yang D, Montague PR, Eagleman DM. Vividness of mental imagery: Individual variability can be measured objectively. Vision Research. 2007;47:474–478. doi: 10.1016/j.visres.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporo-parietal junction in social interaction: How low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13(6):580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- den Ouden HEM, Frith U, Frith C, Blakemore SJ. Thinking about intentions. NeuroImage. 2005;28:787–796. doi: 10.1016/j.neuroimage.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Parker WE, Feiler AM. Where am I now? Distinct roles for parahippocampal and retrosplenail cortices in place recognition. The Journal of Neuroscience. 2007;27(23):6141–6149. doi: 10.1523/JNEUROSCI.0799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, et al. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RSJ, Dolan RJ. The mind’s eye—Precuneus activation in memory-related imagery. NeuroImage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gerardin E, et al. Partially overlapping neural networks for real and imagined hand movements. Cerebral Cortex. 2000;10:1093–1104. doi: 10.1093/cercor/10.11.1093. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: a comparison of theory of mind tasks. Journal of Cognitive Neuroscience. 2007;19(11):1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Perfetti CA, Schneider W. Perceptual knowledge retrieval activates sensory brain regions. The Journal of Neuroscience. 2006;26(18):4917–4921. doi: 10.1523/JNEUROSCI.5389-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain activity evoked by inverted and imagined biological motion. Vision Research. 2001;41:1475–1482. doi: 10.1016/s0042-6989(00)00317-5. [DOI] [PubMed] [Google Scholar]
- Grossman E, Blake R. Brain areas active during visual perception of biological motion. Neuron. 2002;35:1167–1175. doi: 10.1016/s0896-6273(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, et al. Brain areas involved in perception of biological motion. Journal of Cognitive Neuroscience. 2000;12(5):711–720. doi: 10.1162/089892900562417. [DOI] [PubMed] [Google Scholar]
- Grossmann T, et al. Early cortical specialization for face-to-face communication in human infants. Proc R Soc B. 2008;275:2803–2811. doi: 10.1098/rspb.2008.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T, Immish I, Toma K, Dimyan MA, Van Gelderen P, Hallett M. Functional properties of brain areas associated with motor execution and imagery. Journal of Neurophysiology. 2003;89:989–1002. doi: 10.1152/jn.00132.2002. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermüller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Haxby JV. Distributed neural systems for the generation of visual images. Neuron. 2000;28:979–990. doi: 10.1016/s0896-6273(00)00168-9. [DOI] [PubMed] [Google Scholar]
- Jellema T, Baker CI, Wicker B, Perrett DI. Neural representation for the perception of the intentionality of actions. Brain and Cognition. 2000;44:280–302. doi: 10.1006/brcg.2000.1231. [DOI] [PubMed] [Google Scholar]
- Kliemann D, Young L, Scholz J, Saxe R. The influence of prior record on moral judgment. Neuropsychologia. 2008;46:2949–2957. doi: 10.1016/j.neuropsychologia.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Volein A, Everdell N, Elwell CE, Johnson MH. Social perception in infancy: a near-infrared spectroscopy study. Child Development. 2009;80:986–999. doi: 10.1111/j.1467-8624.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- Marks DF. Visual imagery differences in the recall of pictures. British Journal of Psychology. 1973;1:17–24. doi: 10.1111/j.2044-8295.1973.tb01322.x. [DOI] [PubMed] [Google Scholar]
- Martin A, Weisberg J. Neural foundations for understanding social and mechanical concepts. Cogn Neuropsychol. 2003;20(3–6):575–587. doi: 10.1080/02643290342000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Gore JC, Allison T. Face-specific processing in the human fusiform gyrus. Journal of Cognitive Neuroscience. 1997;9(5):605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cerebral Cortex. 2008;18(2):262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Regional brain activation evoked when approaching a virtual human on a virtual walk. Journal of Cognitive Neuroscience. 2005;17(11):1744–1752. doi: 10.1162/089892905774589253. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Mack PB, McCarthy G, Pelphrey KA. Taking an “intentional stance” on eye-gaze shifts: A functional neuroimaging study of social perception in children. NeuroImage. 2005;27:247–252. doi: 10.1016/j.neuroimage.2005.03.027. [DOI] [PubMed] [Google Scholar]
- O’Craven KM, Kanwisher N. Mental imagery of faces and places activates corresponding stimulus-specific brain regions. Journal of Cognitive Neuroscience. 2000;12(6):1013–1023. doi: 10.1162/08989290051137549. [DOI] [PubMed] [Google Scholar]
- Park S, Chun MM. Different roles of the parahippocampal place area (PPA) and retrosplenial cortex (RSC) in scene perception. NeuroImage. 2009;47:1747–1756. doi: 10.1016/j.neuroimage.2009.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Wiggett AJ, Downing PE. Patterns of fMRI activity dissociate overlapping functional brain areas that respond to biological motion. Neuron. 2006;49:815–822. doi: 10.1016/j.neuron.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Mitchell TV, McKeown MJ, Goldstein J, Allison T, McCarthy G. Brain activity evoked by the perception of human walking: Controlling for meaningful coherent motion. The Journal of Neuroscience. 2003;23(17):6819–6825. doi: 10.1523/JNEUROSCI.23-17-06819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Grasping the intentions of others: The perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. Journal of Cognitive Neuroscience. 2004a;16(10):1706–1716. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005b;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G. Functional anatomy of biological motion perception in posterior temporal cortex: an fMRI study of eye, mouth, and hand movements. Cerebral Cortex. 2005;15:1866–1876. doi: 10.1093/cercor/bhi064. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Viola RJ, McCarthy G. When strangers pass: Processing of mutual and averted social gaze in the superior temporal sulcus. Psychological Science. 2004;15 (9):598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Perner J, Aichorn M, Kronbichler M, Staffen W, Ladumer G. Thinking of mental and other representations: the roles of left and right temporo-parietal junction. Social Neuroscience. 2006;1:245–258. doi: 10.1080/17470910600989896. [DOI] [PubMed] [Google Scholar]
- Peterson BS, et al. An event-related functional MRI study comparing interference effects in the Simon and Sroop tasks. Cognitive Brain Research. 2002;13:427–440. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. The Journal of Neuroscience. 1998;18(6):2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Gore JC, McCarthy G. Face-sensitive regions in human extrastriate cortex studied by functional MRI. Journal of Neurophysiology. 1995;74(3):1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- Saxe R, Carey S, Kanwisher N. Understanding other minds: Linking developmental psychology and functional neuroimaging. Annu Rev Psychol. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: The role of the temporo-parietal junction in “theory of mind. NeuroImage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It’s the thought that counts: Specific brain regions for one component of theory of mind. Psychological Science. 2006;17(8):692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Schulz LE, Jiang YV. Reading minds versus following rules: Dissociating theory of mind and executive control in the brain. Social Neuroscience. 2006;1 (3–4):284–298. doi: 10.1080/17470910601000446. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: The role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Saxe RR, Whitfield-Gabrieli S, Scholz J, Pelphrey KA. Brain regions for perceiving and reasoning about other people in school-aged children. Child Development. 2009;80(4):1197–1209. doi: 10.1111/j.1467-8624.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Xiao DK, Kovacs G, Perrett DI, Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42:1435–1446. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Saygin AP, Wilson SM, Hagler DJ, Bates E, Sereno MI. Point-light biological motion perception activates human premotor cortex. The Journal of Neuroscience. 2004;24(27):6181–6188. doi: 10.1523/JNEUROSCI.0504-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, Saxe R. Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS One. 2009;4(3):e4869. doi: 10.1371/journal.pone.0004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, et al. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Phil Trans R Soc Lond B. 2003;358:415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J, Friston KJ, O’Doherty J, Wolpert DM, Frith CD. Activation in posterior superior temporal sulcus parallels parameter inducing the percept of animacy. Neuron. 2005;45:625–636. doi: 10.1016/j.neuron.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and Executive Control. The Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer NK, Reynolds JR, Swallow KM, Zacks JM. Reading stories activates neural representations of visual and motor experiences. Psychological Science. 2009;20(8):989–999. doi: 10.1111/j.1467-9280.2009.02397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KM, et al. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. Journal of Neurophysiology. 1995;73:373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Suchan B, et al. Hemispheric dissociation of visuo-spatial processing and visual rotation. Cognitive Brain Research. 2002;136:533–544. doi: 10.1016/s0166-4328(02)00204-8. [DOI] [PubMed] [Google Scholar]
- Thompson JC, Clarke M, Stewart T, Puce A. Configural processing of biological motion in human superior temporal sulcus. The Journal of Neuroscience. 25(39):9059–9066. doi: 10.1523/JNEUROSCI.2129-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Wyk BC, Hudac CM, Carter EJ, Sobel DM, Pelphrey KA. Action understanding in the superior temporal sulcus region. Psychological Science. 2009;20(6):771–777. doi: 10.1111/j.1467-9280.2009.02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for fMRI group analysis using Bayesian inference. NeuroImage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Young L, Cushman F, Hauser M, Saxe R. The neural basis of the interaction between theory of mind and moral judgment. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Saxe R. An fMRI investigation of spontaneous mental state inference for moral judgment. Journal of Cognitive Neuroscience. 2008a;21(7):1396–1405. doi: 10.1162/jocn.2009.21137. [DOI] [PubMed] [Google Scholar]
- Young L, Saxe R. The neural basis of belief encoding and integration in moral judgment. NeuroImage. 2008b;40(4):1912–1920. doi: 10.1016/j.neuroimage.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Young L, Saxe R. Innocent intentions: A correlation between forgiveness for accidental harm and neural activity. Neuropsychologia. 2009;47:2065–2072. doi: 10.1016/j.neuropsychologia.2009.03.020. [DOI] [PubMed] [Google Scholar]


