Abstract
L-selectin is a key molecule that participates in neutrophil tethering and subsequent rolling. It is cleaved from the surface of neutrophils activated in the presence of lipopolysaccharides, N-formyl-methionine-leucine-phenylalanine (fMLP), or Interleukin-8 (IL-8). We previously showed that L- selectin is also shed from the neutrophil surface during rolling on sialyl Lewis-x coated surfaces in a force-, ADAM-17 sheddase-, and p38 MAP kinase- dependent manner under flow. c-Abl tyrosine kinase is phosphorylated when L-selectin on the surface of neutrophils is cross-linked with anti-L-selectin antibodies. Here, we study the effect of c-Abl inhibition on L-selectin shedding from primary human neutrophils in static conditions following exposure to fMLP, IL-8, and hypotonic buffer and under flow through sialyl Lewis-x coated microtubes. Results indicate that c-Abl inhibition by STI571 significantly affects neutrophil adhesion via L-selectin, by decreasing the average rolling velocity and increasing the flux of rolling cells. The change in surface receptor expression was verified by flow cytometry. Interestingly, other forms of L-selectin shedding induced by fMLP, IL-8 or osmotic swelling were unaffected by STI571 treatment. These findings implicate the c-Abl signaling molecule in regulating L-selectin mechanical shedding in response to shear stress, setting this type of signaling apart from those triggered by the presence of a hypotonic environment, fMLP, or IL-8. This study sheds light on the role of c-Abl in neutrophil adhesion not previously reported in the literature.
Keywords: adhesion, inflammation, shear stress, neutrophil activation
Introduction
The capture and rolling of leukocytes along the endothelium are essential steps leading to the firm adhesion, transmigration, and trafficking of leukocytes to wound or infection sites as part of the inflammation cascade [1; 2; 3]. L-selectin is a key molecule that participates in neutrophil tethering and subsequent rolling [4]. For example, L-selectin deficient mice showed impaired leukocyte recruitment into the inflamed endothelium, virtually no lymphocyte migration into the lymph nodes, and impaired neutrophil sequestration in the microvasculature of the lungs in the presence of bacterially induced pneumonia [5; 6]. While E-selectin and P-selectin are expressed on activated endothelium, L-selectin is constitutively expressed at the tips of microvilli on the neutrophil surface [7; 8]. L-selectin binds to Gly-CAM1, CD34, and podocalyxn found on the endothelial surface of high venules in lymph nodes [9; 10; 11]. Heparin sulfate also acts as a primary ligand for L-selectin on the inflamed vascular endothelium [12]. In addition, L-selectin interacts with the single O-linked sialyl Lewis × and three adjacent moieties on P-selectin glycoprotein ligand 1 (PSGL-1), a sialomucin expressed on the surface of neutrophils. PSGL-1 acts as the dominant ligand for E- and P-selectin and interacts with L-selectin leading to secondary leukocyte recruitment via leukocyte-leukocyte interactions [13; 14].
In addition to playing a mechanical role in mediating neutrophil adhesion, L-selectin also acts as a signal-transducing receptor [15; 16; 17]. Unlike E- and P-selectin, L-selectin is cleaved from the leukocyte surface as a result of cellular activation and inflammatory stimuli [18]. L-selectin ligation in neutrophils has been shown to activate cells as measured by increases in Ca2+ flux, superoxide generation, increased adhesiveness and activation of the mitogen activated protein kinase (MAPK) and tyrosine phosphorylation pathways [15; 19; 20; 21; 22]. The downregulation of L-selectin and conformational change of β2 integrins leading to increased binding affinity are characteristic results of neutrophil activation [23; 24]. Stimulus by IL-8, PMA, LPS, or fMLP leads to L-selectin shedding and conformational change in β2 integrins [7; 25; 26]. TNF-α-converting enzyme (TACE or ADAM-17) was identified as the protease responsible for L-selectin cleavage [27; 28]. Crosslinking of L-selectin with anti-L-selectin mAb or stimulation with sialyl Lewis-x under static conditions leads to the activation of a signaling cascade from tyrosine kinase p56lck to Sos, Ras, MAPK, and Rac2 [29; 30].
We previously found that L-selectin is shed from the neutrophil surface as a result of the mechanical stimulus stemming from neutrophil rolling on a sialyl Lewis-x coated surface under shear flow [31]. This form of L-selectin shedding is dependent on the rate of flow, indicating mechanical force plays a role in L-selectin cleavage from the neutrophil surface. The activity of ADAM-17 during L-selectin shedding under flow has been studied both in vivo [32] and in vitro [31]. It was also shown that the pharmacological inhibition of either ADAM-17 or p38 MAP kinase was sufficient to prevent mechanically-induced L-selectin shedding [31]. Mice with ADAM-17 conditionally knocked out exhibit a reduction of L-selectin shedding and an increase in neutrophil adhesion to the blood vessel wall [32]. The increase in L-selectin mediated neutrophil adhesion modified the inflammatory response of mice enough to significantly increase the survival rate of those with E. coli-mediated peritoneal sepsis [32].
The propagation of signaling instigated by L-selectin cross-linking is dependent on c-Abl, a non-receptor tyrosine kinase [17]. c-Abl has a catalytic domain, polyproline-rich regions and SH2 and SH3 domains that are involved in protein-protein interactions and regulate the kinase activity itself. The C-terminal end has nuclear localization and nuclear export signals, allowing the protein to have activity inside and outside of the nucleus. Additionally, c-Abl has F-actin and G-actin-binding domains that participate in the regulation of actin polymerization [33; 34]. Inhibition of c-Abl through the use of either the pharmaceutical inhibitor STI571 (also known as Imatinib methanesulfonate salt, Imatinib mesylate, or trade name Gleevec®) or conditional knockout mice with the gene truncation solely found in the T cell lineage causes a reduction in F-actin polymerization in T cells [35]. Exposure to thermal cycles or L-selectin ligation by monoclonal antibodies on the neutrophil surface leads to a significant increase in c-Abl kinase activity spatially localized near F-actin [16]. The role of c-Abl in propagating cell signaling in neutrophils under shear flow has not been examined. Thus, the purpose of this study was to examine the effect of c-Abl inhibition on L-selectin shedding from neutrophils during activation and under shear flow.
Materials and Methods
Reagents
APC-CD62L mAb, clone Dreg-56, specific for human L-selectin and APC conjugated mouse IgG1 isotype control antibody were obtained from Becton Dickinson, San Jose, CA, USA. FITC conjugated mAb for anti-human CD11b clone CBRM1/5 and FITC conjugated mouse IgG1 isotype control were purchased from eBioscience, San Diego, CA, USA. The c-Abl inhibitor STI571, >99% (LC Laboratories, Woburn, MA, USA) was purchased. Ca2+ and Mg2+ free HBSS (Invitrogen, Camarillo, CA, USA), Ca2+ and Mg2+ free DPBS (Invitrogen, Camarillo, CA, USA), calcium carbonate (Sigma Chemical Co., St. Louis, MO, USA), endotoxin free water (MO BIO Laboratories, Carlsbad, CA, USA), endotoxin free human serum albumin (Sigma Chemical Co., St. Louis, MO, USA), and low endotoxin (1 ng/mg), essentially globulin-free BSA (Sigma Chemical Co., St. Louis, MO, USA) were used to make buffer solutions for neutrophil isolation and flow experiments.
Neutrophil Isolation
Human peripheral blood was collected from healthy adult donors after informed consent. Neutrophils were isolated by centrifugation at 480g for 50 min at 23°C in a Marathon 8K centrifuge (Fisher Scientific, Fair Lawn, NJ, USA) using 1-Step Polymorphs (Accurate Chemical and Scientific Corporation, Westbury, NY, USA.) This zonal centrifugation method uses a density gradient to create separate visible layers of plasma, mononuclear cells, neutrophils, and erythrocytes and platelets. The neutrophil layer was extracted and washed twice in Ca2+ and Mg2+ free HBSS to remove the polymorph, and any remaining red blood cells were lysed by osmotic swelling. Neutrophils were resuspended at a concentration of 1 × 106 cells/mL. Half the neutrophils were incubated at RT for 30 minutes in 10 μM STI571. These incubation conditions have been previously used to inhibit c-Abl signaling in neutrophils in vitro [16; 17; 36; 37], and it falls within the range of the peak plasma concentrations found in patients taking 400 to 800 mg STI571 as part of a chemotherapy regime [38]. Following the incubation, the cells were resuspended in HBSS containing 0.5% HSA, 2mM Ca2+, 10 mM HEPES, buffered to 7.4 with or without 10 μM STI571.
Neutrophil Activation Under Static Conditions
Isolated neutrophils were treated with STI571 and incubated with IL-8 or fMLP (R&D Systems Inc., Minneapolis, MN, USA) to determine the effect of STI571 treatment on L-selectin shedding during neutrophil activation under static conditions. IL-8 was dissolved at a concentration of 100 μg/mL in endotoxin free water. fMLP was dissolved at a concentration of 100 μM in DMSO. Both STI571-treated and untreated neutrophils were suspended at a concentration of 1 × 106 cells/mL in HBSS containing 0.5% HSA, 2mM Ca2+, 10 mM HEPES, buffered to 7.4. Cells were then incubated in either 1 nM IL-8 or 5 nM fMLP for 2 minutes at RT or in 0.5x Ca2+and Mg2+ free HBSS at RT for 30 minutes. Control samples were treated with equivalent volumes of endotoxin free water or DMSO. Neutrophils were then labeled with anti-L-selectin and CBRM1/5 antibodies at 4°C, washed with cold Ca2+and Mg2+ free DPBS, and fixed in cold 4% paraformaldehyde for 30 minutes before analysis by flow cytometry as described below. Experiments were conducted using neutrophils from at least three different donors.
Microtube Preparation
Polyurethane microtubes with an inner diameter of 300 μm and external diameter of 600 μm (Braintree Scientific Inc., Braintree, MA, USA) were cut to a length of 50 cm. Two tubes were prepared by drawing up 200 μg/mL NeutrAvidin biotin-binding protein (Thermo Fisher Scientific Inc., Rockford, IL, USA) with insulin needle syringes (Becton Dickinson, San Jose, CA, USA Biosciences) followed by an overnight incubation at 4°C. Next the tubes were incubated with 20 μg/mL sialyl Lewis-x-PAA-biotin (GlycoTech Corporation, Gaithersburg, MD, USA) for 2 hours at room temperature (RT). Finally, the tubes were incubated with 1% BSA at RT for 1 hour to block non-specific adhesion. Two BSA control tubes were incubated with 1% BSA for 1 hour at RT.
Microtube Flow Experiment
Coated microtubes were mounted on an inverted microscope, Olympus IX81 (Olympus America Inc., Melville, NY, USA). Neutrophils were perfused through the microtubes using a syringe pump at a wall shear stress of 1.5 dyne/cm2. Untreated and STI571-treated neutrophils were perfused through either sialyl Lewis-x coated- or (control) BSA coated microtubes. Videos were recorded for 30 seconds at 10 random locations along the length of each microtube after 5 minutes of neutrophil perfusion. After 30 minutes of flow, HBSS containing 0.5% HSA and 10 mM HEPES buffered to 7.4 was used to collect the remaining neutrophils from the microtubes.
Data Acquisition
Videos of the rolling neutrophils were recorded using a microscope-linked Hitachi CCD camera KP-M1AN (Hitachi, Japan) and a Sony DVD Recorder DVO-1000MD (Sony Electronics Inc., San Diego, California, USA). DVD chapters were converted to 640 × 480 pixels at 30 fps using FFMPEGX (Fabrice Bellard, France). Rolling flux, rolling velocity and detachment rate were determined using ImageJ (U. S. National Institutes of Health, Bethesda,3MD, USA). Rolling cells were defined as any cell translating along the tube surface for longer than 2 seconds at a velocity less than 50% the free stream velocity of a non-interacting cell near the tube wall. Rolling flux was determined by drawing a line across the view field, perpendicular to the direction of flow, and counting the number of cells that crossed the line over a period of 20 seconds. Rolling velocity was quantified by recording the amount of time required for a rolling cell to move halfway across the field of view. The neutrophil detachment rate was determined by counting the number of rolling cells that detached from the surface of the microtube in a 165 × 228 μm area.
Flow Cytometry
Neutrophils perfused through the microtubes were immediately collected in syringes mounted on the syringe pump. Perfused and nonperfused neutrophils from both STI571 treated and untreated samples were labeled with APC-conjugated anti-human CD62L mAb and FITC-conjugated anti-human CBRM1/5 mAb to quantify L-selectin and β2 integrin molecules that have exposed the activation-epitope. Control samples of both treated and untreated neutrophils were incubated with APC- and FITC- conjugated mouse IgG1 control antibodies. Labeled neutrophils were incubated for 30 minutes at 4°C, washed with cold Ca2+ and Mg2+ free DPBS, and fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) in Ca2+ and Mg2+ free DPBS at 4°C for 30 minutes. Samples were analyzed using an Accuri C6 flow cytometer (Accuri Cytometers Inc., Ann Arbor, Michigan, USA) and plots were created using the FlowJo v. 7.6.3 (Tree Star Inc., Ashland, Oregon) software package.
Statistical Analysis
Rolling velocity, rolling flux, neutrophil detachment rate, and L-selectin expression data were plotted and analyzed using Prism 5.0b for Microsoft (GraphPad Software, San Diego, CA, www.graphpad.com). Two-tailed paired t-tests were employed to analyze results with a significance level of α=0.05 where applicable.
Results
c-Abl is not involved in L-selectin shedding from activated neutrophils under static conditions
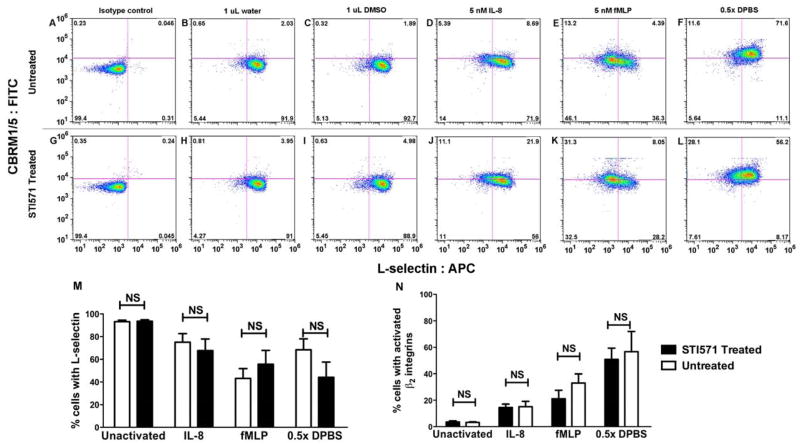
Previous studies have shown that L-selectin crosslinking leads to c-Abl activation under static conditions [17]. However, the effect of c-Abl inhibition on L-selectin shedding (the reverse cause-effect sequence) during chemically- or hypotonically-induced neutrophil activation has not been documented. Figure 1A through 1L contains representative data from one experiment that shows that STI571 treated and untreated neutrophils shed L-selectin as a result of chemical stimulation with IL-8, fMLP, or hypotonic activation. Figure 1M and 1N are the cumulative results from 5 independent experiments using blood from 5 different donors. Figure 1M shows that c-Abl inhibition does not significantly change the amount of L-selectin remaining on neutrophils after 5 minutes of activation. Figure 1N shows the amount of activated β2 integrins expressed on the surface of neutrophils after 5 minutes of activation by various stimuli. In either case, c-Abl does not play a role in the mechanism that leads to proteolytic cleavage of L-selectin from the neutrophil surface during osmotic swelling or chemical activation with IL-8 or fMLP under static conditions.
Fig 1. c-Abl inhibition does not alter L-selectin shedding from neutrophils following activation with IL-8, fMLP, or osmotic swelling under static conditions.
untreated (A-F) and STI571 treated (G-L) neutrophils were stimulated with 1 μL water (B, H), 5 μL DMSO, 1 nM IL-8 (D, J), 5 nM fMLP (E, K), or by osmotic swelling (F, L) and then labeled with anti- L-selectin and CBRM1/5 antibodies. STI571 treated (G) and untreated neutrophils (A) were also labeled with APC- and FITC- conjugated isotype controls. Neutrophils were fixed and analyzed via flow cytometry. STI571 treatment did not have a significant effect on L-selectin shedding (M) and β2 integrin activation (N) due to exposure to IL-8, fMLP, or osmotic swelling under static conditions. In (M) and (N), all activation conditions were significantly different from the unactivated samples (p<0.05). These results are representative of five separate experiments using cells from different consenting donors. Statistical evaluation of the results were conducted using two-tailed paired t-tests with a significance level of α=0.05 for each type of cell stimulation. Error bars displayed are SEM.
L-selectin mechanical shedding is inhibited by STI571
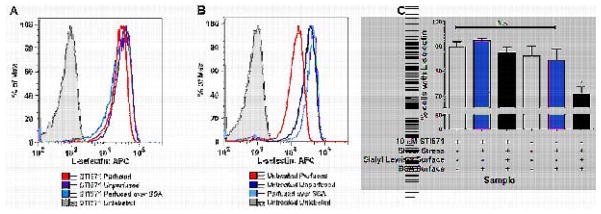
L-selectin mechanical shedding under shear flow leads to a significant reduction in L-selectin expression that can be quantified by flow cytometry [31]. Flow cytometry results indicated that untreated neutrophils perfused through a sialyl Lewis-x coated microtube shed approximately 10 times more L-selectin than STI571 treated neutrophils that were also perfused under identical conditions. In contrast, both STI571-treated and untreated neutrophils that were perfused through the BSA coated control tubes showed a minimal shift in L-selectin surface expression. Figures 2A and 2B are histograms from a representative experiment showing such a shift in L-selectin expression. Neutrophils that were considered to be L-selectin positive were any cells that showed higher APC fluorescence than 99% of the isotype control sample for that same experiment. The flow cytometry results from three independent experiments quantifying the percent of neutrophils expressing L-selectin are shown in Figure 2C. Neutrophil isolation involved density centrifugation through the use of One-Step Polymorph, a proprietary mix of sodium diatrizoate, ficoll, and dextran 500. Dextran centrifugation followed by hypotonic lysis of red blood cells has been shown to cause a small but significant amount of L-selectin downregulation [39]. L-selectin expression levels and β2 integrin activation in the unstimulated control samples shown in Figures 1 and 2 with or without STI571 treatment are consistent with the expression levels found in primary human neutrophils isolated by a dextran based density centrifugation step followed by a hypotonic wash as found by Macey, et al. [39]. This indicates that any downregulation measured following neutrophil stimulation is due to the specific stimuli and not prior cellular activation. These data confirm that c-Abl inhibition prevents L-selectin mechanical shedding from the surface of neutrophils under shear flow.
Fig 2. L-selectin mechanical shedding is hindered by c-Abl inhibition.
A) STI571 treated neutrophils show no appreciable change in L-selectin expression following perfusion through either sialyl Lewis-x or BSA coated microtubes. B) Untreated neutrophils that were perfused through a sialyl Lewis-x coated tube exhibit a decrease in L-selectin expression. C) Flow cytometry results were compiled from experiments conducted with samples from three different consenting donors. Untreated neutrophils consistently exhibit a statistically significant 10 fold greater loss of L-selectin expression in contrast with STI571 treated neutrophils that exhibit no significant change in L-selectin expression. All samples were analyzed using two-tailed paired t-tests with a significance level of α=0.05. Error bars displayed are SEM.
c-Abl inhibition alters the rolling behavior of neutrophils under shear flow
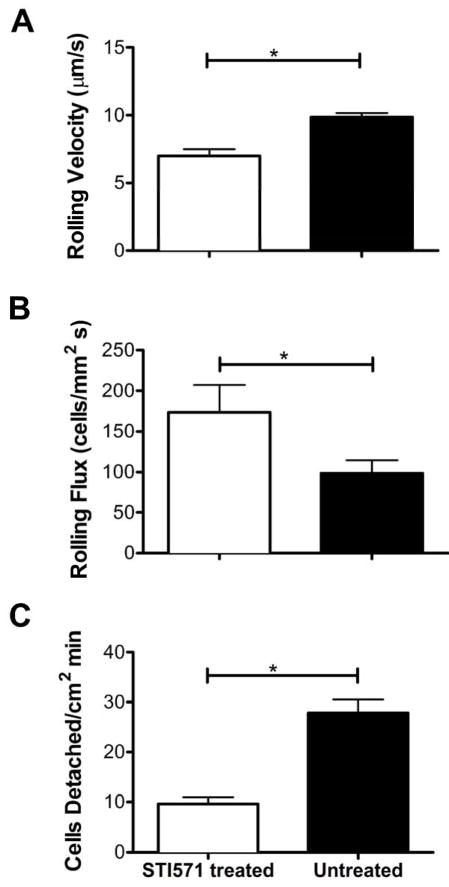
L-selectin shedding from the surface of neutrophils under shear flow is associated with an increase in average cell rolling velocity [31]. Therefore, it is reasonable to expect that the inhibition of L-selectin mechanical shedding would lead to a marked decrease in cell rolling velocity. This was previously shown to be true through the inhibition of both ADAM-17 and p38 MAP kinase [31]. Figure 3A shows the rolling velocities of STI571 treated and untreated neutrophils from six different consenting donors. Video recordings taken during neutrophil perfusion through sialyl Lewis-x coated microtubes show that neutrophils pretreated with STI571 rolled at a significantly lower velocity than untreated cells. The rolling velocities from six separate experiments were analyzed using a paired one-tailed t-test to determine whether the change in rolling velocity was statistically significant. Untreated neutrophils roll 40% faster than STI571 treated neutrophils. This result further implicates c-Abl in a cell-signaling cascade that ultimately leads to L-selectin mechanical shedding from the neutrophil surface.
Fig 3. c-Abl inhibition causes significant changes in neutrophil rolling velocity, rolling flux, and rate of cell detachment from the microtube wall.
Isolated human neutrophils were incubated in 10 μM STI571 for 30 min. Untreated control samples were treated with endotoxin-free water. Samples were perfused at a wall shear stress of 1.5 dyne/cm2 through a 300 μm polyurethane microtube coated with sialyl Lewis-x. (A) Cell rolling velocity was calculated from video recordings of cell interaction with the microtube wall. (B) Video recordings collected during cell perfusion were analyzed to quantify the number of cells rolling across a plane within the viewing field perpendicular to flow over a time interval of 30 seconds. (C) Videos of cell interactions with the microtube wall were analyzed to calculate the number of cells that detach during 30 s from a 0.04752 mm2 area of the view field. All samples were analyzed using a two-tailed paired t-tests with a significance level of α=0.05. All p-values are < 0.05. Error bars displayed are SEM.
L-selectin participates in the recruitment and adhesion of neutrophils in blood vessels [1; 2; 3]. It has been shown previously that the inhibition of L-selectin shedding through the pharmacological inhibition of p38 MAP kinase leads to an increase in cell rolling flux [31]. Conceptually, if L-selectin is not cleaved from the surface of the neutrophil it will remain available for binding to the sialyl Lewis-x surface for a longer duration. This expectation would be confirmed through a significant change in neutrophil rolling flux. The rolling flux of STI571 treated and untreated neutrophils from six different consenting donors is shown in Figure 3B. STI571 treated neutrophils have a significantly greater rolling flux compared to untreated neutrophils, as confirmed by a paired one-tailed t-test. These results agree with the neutrophil rolling velocity results in suggesting that c-Abl plays an important role in L-selectin mechanical shedding.
The third method used to characterize the rolling behavior of neutrophils under shear flow was to quantify the rate at which rolling neutrophils detach from the sialyl Lewis-x coated microtubes. The inhibition of L-selectin mechanical shedding is expected to allow more L-selectin sialyl Lewis-x bonds to form, thereby causing neutrophils to adhere more firmly to the sialyl Lewis-x coated microtube wall. Figure 3C is a compilation of data collected from experiments conducted with STI571 treated and untreated neutrophils from six different donors. STI571 treated neutrophils were found to detach at a lower rate than untreated neutrophils as expected.
β2 integrins on neutrophils remain unactivated throughout rolling experiments
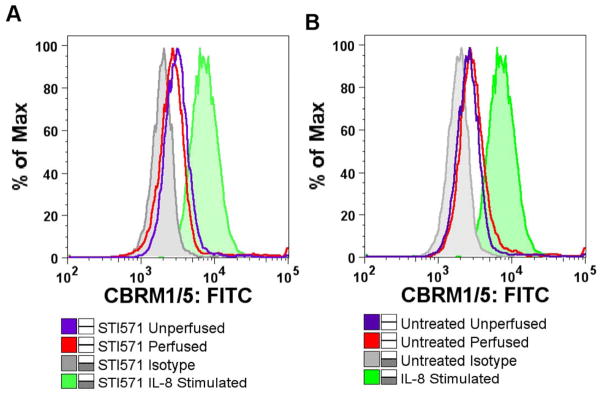
The L-selectin shedding and the conformational change in β2 integrins leading to the exposure of the CBRM1/5-binding domain are characteristic of neutrophil activation [23; 24]. Figure 4 contains representative flow cytometry data showing that β2 integrins on both STI571 treated and untreated neutrophils were not significantly activated throughout the perfusion experiment. In contrast, CBRM1/5 labeling significantly increased and L-selectin expression decreased as a result of IL-8 treatment, showing that neutrophil activation was not generally suppressed during the perfusion experiments.
Fig 4. Neutrophils perfused through sialyl Lewis-x coated microtubes are not activated.
STI571 treated (A) and untreated neutrophils (B) were labeled with FITC-conjugated anti-human CBRM1/5 antibody following cell perfusion through sialyl Lewis-x coated microtubes to evaluate the resulting integrin activation states. These results are representative of three separate experiments that were analyzed using two-tailed paired t-tests with a significance level of α=0.05. None of the samples showed significant signs of β2 integrin conformational change from baseline. IL-8 activation was used as a positive control.
Discussion
The consequences of c-Abl inhibition on the downregulation of L-selectin in neutrophils have not previously been documented to our knowledge. The current work shows that the inhibition of c-Abl through the use of STI571 does not change the extent of L-selectin shedding from neutrophils activated with IL-8, fMLP, or hypotonic buffer under static conditions. This suggests that c-Abl does not participate in the cell-signaling cascade leading to L-selectin downregulation that is triggered by these stimuli. However, c-Abl inhibition created a significant reduction in the mechanical L-selectin down-regulation from the surface of neutrophils perfused through a microtube coated with sialyl Lewis-x. This result implies that c-Abl is part of the same cell signaling cascade triggered by mechanical force exerted on the sialyl Lewis-x bond with L-selectin. Results of the neutrophil adhesion dynamics measurements also support this implication. If c-Abl inhibition leads to the retention of L-selectin on the neutrophil surface, then one would expect neutrophils treated with the c-Abl inhibitor to exhibit the tendency to adhere more firmly to the microtube walls coated with sialyl Lewis-x. This is indeed the observation shown in Figure 3 through the comparison of cell rolling velocity, rolling flux, and detachment rate.
Ideally, one would like to more directly quantify the level of c-Abl activity through the use of traditional methods such as western blot or confocal microscopy with activation-specific antibodies. However, conducting such an experiment requires the isolation and flow of large numbers of neutrophils over a very short period of time in order to capture rapid cell signaling states. However, L-selectin shedding represents a longer-lived record of this signaling activity, since L-selectin cannot be re-expressed over the time frame of the experiment. STI571 is a well-recognized, effective tyrosine kinase inhibitor that prevents c-Abl phosphorylation, thus the observed changes in cell adhesion and L-selectin expression on the neutrophil surface can be attributed to the specific inhibition of c-Abl [35; 40].
c-Abl inhibition with STI571 alters the rolling dynamics of neutrophils. Treatment with STI571 led to a decrease in rolling velocity and cell detachment rate while increasing the rolling flux of neutrophils in the microtube. The consequences of this change in adhesive behavior should be studied in vivo to determine the effect it might have on the innate immune system’s response to infection. This study could contribute to an understanding of the consequences of STI571 chemotherapy at the cellular level.
Conclusions
In conclusion, we have shown evidence that c-Abl may participate in both an “outside-in” and “inside-out” signaling cascade that regulates L-selectin shedding in neutrophils perfused over a sialyl Lewis-x coated surface under shear flow. The L-selectin downregulation signaling cascades initiated by IL-8, fMLP, or osmotic swelling are distinct from the mechanisms triggered by the exertion of mechanical force on the transient bond formed by L-selectin and sialyl Lewis-x under flow and do not demonstrate this c-Abl dependence. We have also shown that c-Abl inhibition changes the capture and cell rolling behavior of neutrophils by decreasing cell-rolling velocity and detachment rate while increasing the rolling flux of cells adhered to the surface of a sialyl Lewis-x coated microtube. Taken together, this work shows that c-Abl plays a key role in L-selectin cleavage from the neutrophil surface under shear flow.
Acknowledgments
This work was funded by the National Institutes of Health grant number HL018128.
Abbreviations
- ABL1
c-Abl
- CML
Chronic myelogenous leukemia
- DPBS
Dubelcco’s phosphate buffered saline
- Gly-CAM1
Glycosylation-dependent cell adhesion molecule 1
- IL-8
Interleukin-8
- MEKK1
MAP kinase kinase kinase 1
- Mac-1
Membrane attack complex type 1
- MAPK
Mitogen-activated protein kinase
- MLH1
mutL homologue 1
- fMLP
N-formyl-Methionine-Leucine-Phenylalanine
- PMA
phorbol 12-myristate 13-acetate
- PSGL-1
P-selectin glycoprotein ligand 1
- RT
Room Temperature
- TACE
TNF-α-converting enzyme
Footnotes
Conflicts of Interest
There were no financial or commercial conflicts of interest in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Springer TA. Traffic signals for lymphocyte recirculation and luekocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 2.McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 3.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 4.von Andrian UH, Chambers JD, Berg EL, Michie SA, Brown DA, Karolak D, Ramezani L, Berger EM, Arfors KE, Butcher EC. L-selectin mediates neutrophil rolling in inflamed venules through sialyl Lewis-x- dependent and -independent recognition pathways. Blood. 1993;82:182–191. [PubMed] [Google Scholar]
- 5.Doyle NA, Bhagwan SD, Meek BB, Kutkoski GJ, Steeber DA, Tedder TF, Doerschuk CM. Neutrophil margination, sequestration, and emigration in the lungs of L-selectin-deficient mice. J Clin Invest. 1997;99:526–533. doi: 10.1172/JCI119189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tedder TF, Steeber DA, Pizcueta P. L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181 doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto TK, Jutila MA, Butcher EC. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci USA. 1990;87:2244–8. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai Y, Singer MS, Fennie C, Lasky LA, Rosen SD. Identification of a carbohydrate-based endothelial ligand for a lymphocyte homing receptor. J Cell Biol. 1991;113:1213–1221. doi: 10.1083/jcb.113.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puri KD, Finger EB, Gaudernack G, Springer TA. Sialomucin CD34 is the major L-selectin ligand in human tonsil high endothelial venules. J Cell Biol. 1995;131:261–270. doi: 10.1083/jcb.131.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sassetti C, Tangemann K, Singer MS, Kershaw DB, Rosen SD. Identification of podocalyxin-like protein as a high endothelial venule ligand for L-selectin: Parallels to CD34. J Exp Med. 1998;187:1965–1975. doi: 10.1084/jem.187.12.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6:902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- 13.McEver RP, Cummings RD. Perspectives Series: Cell Adhesion in Vascular Biology. J Clin Invest. 1997;100:485–492. doi: 10.1172/JCI119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walcheck B, Alexander SR, St Hill CA, Matala E. ADAM-17-independent shedding of L-selectin. J Leukoc Biol. 2003;74:389–394. doi: 10.1189/jlb.0403141. [DOI] [PubMed] [Google Scholar]
- 15.Waddell TK, Fialkow L, Chan CK, Kishimoto TK, Downey GP. Signaling Functions of L-selectin. J Biol Chem. 1995;270:15403–15411. doi: 10.1074/jbc.270.25.15403. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Ba X, Xu T, Cui L, Hao S, Zeng X. c-Abl is involved in the F-actin assembly triggered by L-selectin crosslinking. J Biochem. 2006;140:229–235. doi: 10.1093/jb/mvj149. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Shang X, Xu T, Cui L, Luo J, Ba X, Hao S, Zeng X. c-Abl is required for the signaling transduction induced by L-selectin ligation. Eur J Immunol. 2007;37:3246–3258. doi: 10.1002/eji.200737221. [DOI] [PubMed] [Google Scholar]
- 18.Palecanda A, Walcheck B, Bishop DK, Jutila MA. Rapid activation-independent shedding of leukocyte L-selectin induced by crosslinking of the surface antigen. Eur J Immunol. 1992;22:1279–1286. doi: 10.1002/eji.1830220524. [DOI] [PubMed] [Google Scholar]
- 19.Laudanna C, Constantin G, Baron P, Scarpini E, Scarlato G, Cabrini G, Dechecchi C, Rossi F, Cossatella MA, Berton G. Sulfatides trigger increase of cytosolic free calcium and enhanced expression of tumor necrosis factor-α and interleukin-8 mRNA in human neutrophils. J Biol Chem. 1994;269:4021–4026. [PubMed] [Google Scholar]
- 20.Waddell TK, Fialkow L, Chan CK, Kishimoto TK, Downey GP. Potentiation of the oxidative burst of human neutrophils. J Biol Chem. 1994;269:18485–18491. [PubMed] [Google Scholar]
- 21.Smolen JE, Peterson TK, Koch C, O'Keefe SJ, Hanlon WA, Seo S, Pearson D, Fossett MC, Simon SI. L-selectin signaling of neutrophil adhesion and degranulation involves p38 mitogen-activated protein kinas. J Biol Chem. 2000;275:15876–15884. doi: 10.1074/jbc.M906232199. [DOI] [PubMed] [Google Scholar]
- 22.Dwir O, Kansas GS, Alon R. Cytoplasmic anchorage of L-selectin controls leukocyte capture and rolling by increasing the mechanical stability of the selectin tether. J Cell Biol. 2001;155:145–56. doi: 10.1083/jcb.200103042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elemer GS, Edgington TS. Monoclonal antibody to an activation neoepitope of αMβ2 inhibits multiple αMβ2 functions. J Immunol. 1994;152:5836–5844. [PubMed] [Google Scholar]
- 24.Diamond MS, Springer TA. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993;120:545. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan H, Derynck R. Ectodomain shedding of TGF-α and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. EMBO J. 1999;18:6962–6972. doi: 10.1093/emboj/18.24.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jutila MA, Rott L, Berg EL, Butcher EC. Function and regulation of the neutrophil MEL-14 antigen in vivo: comparison with LFA-1 and Mac-1. J Immunol. 1989;143:3318–3324. [PubMed] [Google Scholar]
- 27.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzer JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 28.Moss ML, Jin SLC, Milla ME, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchenll J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Warner J, Willard D, Becherer JD. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 29.Brenner B, Gulbins E, Busch GL, Koppenhoefer U, Lang F, Linderkamp O. L-selectin regulates actin polymerisation via activation of the small G-protein Rac2. Biochem Biophys Res Commun. 1997;231:802–807. doi: 10.1006/bbrc.1997.6191. [DOI] [PubMed] [Google Scholar]
- 30.Brenner B, Gulbins E, Koppenhoefer U, Busch GL, Walzog B, Steinhausen M, Coggeshall KM, Linderkamp O, Lang F. L-selectin activates the Ras pathway via the tyrosine kinase p56lck. Proc Natl Acad Sci USA. 1996;93:15376–15381. doi: 10.1073/pnas.93.26.15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee D, Schultz JB, Knauf PA, King MR. Mechanical shedding of L-selectin from the neutrophil surface during rolling on sialyl Lewis × under flow. J Biol Chem. 2007;282:4812–20. doi: 10.1074/jbc.M609994200. [DOI] [PubMed] [Google Scholar]
- 32.Long C, Wang Y, Herrera AH, Horiuchi K, Walcheck B. In vivo role of leukocyte ADAM17 in the inflammatory and host responses during E. coli-mediated peritonitis. J Leukoc Biol. 2010;87:1097–1101. doi: 10.1189/jlb.1109763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Etten RA, Jackson PK, Baltimore D, Sanders MC, Matsudaira PT, Janmey PA. The COOH terminus of the c-Abl tyrosine kinase contains distinct F- and G-actin binding domains with bundling activity. J Cell Biol. 1994;124:325–340. doi: 10.1083/jcb.124.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodring PJ, Hunter Tony, Wang Jean YJ. Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases. J Cell Sci. 2003;116:2613–2626. doi: 10.1242/jcs.00622. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y, Comiskey EO, Dupree RS, Li S, Koleske AJ, Burkhardt JK. The c-Abl tyrosine kinase regulates actin remodeling at the immune synapse. Blood. 2008;112:111–119. doi: 10.1182/blood-2007-10-118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui L, Chen C, Xu T, Zhang J, Shang X, Luo J, Chen L, Ba X, Zeng X. c-Abl kinase is required for β2 integrin-mediated neutrophil adhesion. J Immunol. 2009;182:3233–3242. doi: 10.4049/jimmunol.0802621. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Ma Q, Wang J, Liu X, Yang Y, Zhao H, Wang Y, Jin Y, Zeng J, Li J, Song L, Li P, Qian X, Cao C. c-Abl and Arg tyrosine kinases regulate lysosomal degradation of the oncoprotein Galectin-3. Cell Death Differ. 2010;17:1277–1287. doi: 10.1038/cdd.2010.8. [DOI] [PubMed] [Google Scholar]
- 38.Coutre P, Kreuzer KA, Pursche S, Bonin M, Leopold T, Baskaynak G, Dörken B, Ehninger G, Ottmann O, Jenke A, Bornhäuser M, Schleyer E. Pharmacokinetics and cellular uptake of imatinib and its main metabolite CGP74588. Cancer Chemotherapy and Pharmacology. 2004;53:313–323. doi: 10.1007/s00280-003-0741-6. [DOI] [PubMed] [Google Scholar]
- 39.Macey MG, McCarthy DA, Vordermeier S, Newland AC, Brown KA. Effects of cell purification methods on CD11b and L-selectin expression as well as the adherence and activation of leucocytes. Journal of Immunological Methods. 1995;181:211–219. doi: 10.1016/0022-1759(95)00003-s. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien SG, Gathmann I, Cornelissen JJ, Hughes T, Rousselot P, Shepherd J, Goldman JM, Verhoef G, Druker BJ. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]