Abstract
In just the last few years, plant geneticists have made tremendous progress in identifying the molecular genetic basis of postzygotic reproductive isolation. With more than a dozen genes now cloned, it is clear that plant hybrid incompatibilities usually evolve via two or more mutational steps, as is predicted by the Dobzhansky-Muller model. There is evidence that natural selection or random genetic drift can be responsible for these incompatibilities.
Introduction
The goal of explaining the origin of species has inspired more than two centuries of scientific inquiry, involving early naturalists through to modern evolutionary biologists. Though only hinted at by Darwin [1], the idea that the evolution of reproductive isolation is central to the process of speciation [2] is now widely recognized [3-5]. Reproductive isolating mechanisms that restrict gene exchange between diverging species include prezygotic barriers that limit the potential for mating or zygote formation (e.g. habitat and flowering time differences or pollen-pistil incompatibilities) and postzygotic barriers that reduce the viability or fertility of hybrid offspring if interbreeding does occur. Whereas the evolution of prezygotic isolation is often easily understood as a byproduct of differential adaptation to variable ecological conditions or of selection to reduce the production of low-fitness hybrids, the evolution of postzygotic isolation has been a long-standing mystery [1] because the production of dead or sterile hybrids cannot be favored by natural selection.
The Dobzhansky-Muller model
Dobzhansky [6] and Muller [7] outlined a solution to this puzzle, explaining that if postzygotic isolation is caused by incompatible gene interactions between diverging species then natural selection need not oppose its evolution. A new mutation might function perfectly well in the context of its native genetic background, increasing in frequency until it becomes fixed within the species. But because the fitness of this new variant has only been “tested” against its own genetic background, it might be functionally incompatible with divergent alleles present in foreign genetic backgrounds, resulting in hybrid sterility or inviability. The key insight of the so-called Dobzhansky-Muller model is that, if hybrid incompatibilities are caused by two or more mutational differences between species, there can be transitional genotypes that are adaptive or neutral in ancestral populations and, therefore, not eliminated by natural selection. In contrast, if reduced hybrid fitness is caused by a single mutational step (resulting in heterozygote disadvantage), natural selection is expected to impede the initial spread of the mutation within species. Nevertheless, in some situations, random genetic drift can overwhelm selection and lead to the fixation of such a mutation, particularly if the reduction in fitness is modest. Indeed, it is not uncommon for partial hybrid sterility in plants to be caused by heterozygous chromosomal rearrangements that evolved in a single mutational step [4,8]. Differentiating between these alternative genetic pathways to postzygotic isolation – one versus multiple mutational steps – can help identify which evolutionary processes contribute to species divergence.
To determine whether hybrid incompatibilities evolve in multiple steps, as predicted by the Dobzhansky-Muller model, it is necessary to pinpoint the causal genetic changes at the molecular level. But even if the Dobzhansky-Muller model is confirmed, it provides no expectation as to which kinds of molecular genetic changes cause postzygotic isolation. Are there predictable genetic pathways to incompatibilities? What types of molecular interactions cause hybrid dysfunction? The Dobzhansky-Muller model is also agnostic about the evolutionary processes that cause divergence within species. Do the initial mutations increase in frequency by random genetic drift or by natural selection because they benefit the native species for some reason that is incidental to their eventual contribution to reproductive isolation? In several plant systems, the molecular variants that cause hybrid inviability and sterility have recently been identified, providing some of the first hints at answers to these fundamental evolutionary questions. Here, we focus on exclusively nuclear-encoded hybrid incompatibility genes but point the reader to several excellent reviews of cytoplasmic male sterility [9,10] and the population genetic theory for how cytonuclear incompatibilities might evolve [11,12].
Recent advances
Two-locus hybrid incompatibilities
In the classic model of speciation, hybrid incompatibilities are thought to evolve as a by-product of adaptation to different environments [13]. Some of the best evidence for this idea comes from recent work on hybrid necrosis, a form of plant hybrid inviability characterized by necrotic lesions, wilting, and inhibited growth [14]. A remarkable commonality has emerged among cloned hybrid necrosis genes: all encode proteins involved in plant defense against bacterial or fungal pathogens, and incompatible allelic combinations appear to induce autoimmune-like responses [15]. The plant immune system is a tightly coordinated network of proteins that recognize pathogen invasions and elicit a suite of cellular defense responses [16,17]. One might imagine that plant-pathogen molecular coevolution, occurring in independent populations with unique pathogen communities, could lead to hybrid mismatches between interacting components of the immune system that result in hyperactivation of defense responses in hybrid offspring. Indeed, two-locus incompatibilities between pathogen resistance genes and their interacting partners have been implicated in hybrid necrosis between divergent strains of Arabidopsis thaliana [18-20], between the indica and japonica subspecies of Asian cultivated rice [21], and between wild and domesticated species of tomato [22] and lettuce [23]. For a few of these loci, alleles that confer necrosis differ by several amino acid changes from ancestral alleles, perhaps consistent with a sustained fine-tuning of these proteins to improve pathogen recognition.
Other recent studies suggest that two-locus hybrid incompatibilities can also readily evolve in the absence of natural selection. Based on his early genetic analyses of hybrid sterility between the indica and japonica varieties of Oryza sativa, Oka suggested that defects in pollen development might be caused by loss-of-function alleles at different duplicate genes in each parent [24]. Postzygotic isolation might evolve passively then, due to degenerative mutations and genetic drift acting on different paralogs (genes related by duplication within a genome) in diverging populations [25,26]. Only recently has the cloning of several plant hybrid incompatibility genes provided empirical evidence for this idea. Indeed, hybrids between different strains of A. thaliana arrest as early embryos when they carry loss-of-function alleles at duplicate copies of the essential histidinol-phosphate amino-transferase gene (hpa1 and hpa2) [27]. Likewise, in two distinct Oryza crosses, null alleles at duplicate genes confer gametic male sterility [28,29]. Mizuta et al. [28] showed that pollen from indica-japonica hybrids fails to germinate when it carries loss-of-function alleles – one from each parent – at two duplicate genes of unknown function, DOPPELGANGER1 and 2 (DPL1 and DPL2). Similarly, pollen sterility in hybrids between O. sativa japonica and an Amazonian wild rice, Oryza glumaepatula, is caused by nonfunctional alleles at duplicate loci S27 and S28 that both encode the mitochondrial ribosomal L27 protein (mtRPL27) [29]. In this case, one lineage – O. glumaepatula – lacks the duplication; pollen is defective when it carries a loss-of-function allele at S28 from O. sativa and is missing the S27 paralog (by virtue of inheriting the corresponding genomic region from O. glumaepatula). An extraordinary feature of each of these systems is that loss-of-function alleles have evolved repeatedly. In A. thaliana, roughly three-quarters of plant collections carry one of six possible loss-of-function alleles at either hpa1 or hpa2 [27]. In Oryza, several different disruptive mutations have arisen in closely related species for both the DPL1/DPL2 and S27/S28 incompatibilities [28,30]. These findings, along with the rich history of whole genome duplication in plants, suggest that the divergent resolution of gene duplicates via mutation and genetic drift might be a common source of postzygotic isolation in plants.
Single-locus hybrid incompatibilities
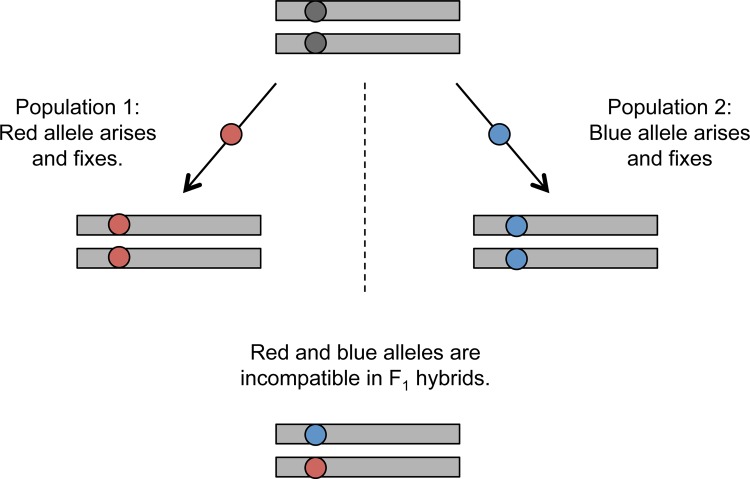
In addition to the cases described above, studies have occasionally discovered hybrid incompatibilities that map to single genetic loci [31,32], which might be taken as prima facie evidence that postzygotic isolation can evolve via a single mutational step. Like the fixation of an underdominant chromosomal rearrangement, the establishment of a new sterility- or inviability-causing allele requires a hefty contribution from genetic drift to outweigh selection against unfit heterozygotes. But, if instead, a single-locus hybrid incompatibility is conferred by two or more alleles that evolve independently – implying multiple mutational steps – it is possible to circumvent any initial fitness valley within species [33] (Figure 1). The recent cloning of the single-locus hybrid incompatibility gene OAK [34], which encodes a receptor-like kinase (RLK) and causes reduced growth and developmental abnormalities in interpopulational hybrids of A. thaliana, supports just such a multiple-step scenario. Indeed, the two incompatible OAK alleles are 9% divergent at the amino acid level, with 55 of 152 residues differing in a key malectin-like domain [34]. With such extreme levels of sequence diversity, it is reasonable to infer that intermediate, viable genotypes predated the current incompatible pair of alleles. Although its adaptive significance is unclear, the occurrence of OAK within a highly variable tandem array of RLK genes suggests that high rates of gene conversion and illegitimate recombination may have contributed to its evolution [34].
Figure 1. The Dobzhansky-Muller model for a single-locus hybrid incompatibility.
An ancestral population splits into two geographically isolated populations that diverge genetically and eventually fix different alleles (red or blue) at the same locus. In the F1 hybrid, these two derived alleles are incompatible. Note that hybrid incompatibilities can just as easily arise between ancestral and derived alleles if two or more rounds of mutation and fixation occur within a lineage.
The molecular characterization of three additional, single-locus incompatibilities between indica and japonica rice varieties also suggests the involvement of multiple mutational steps. The S5 incompatibility locus causes embryo sac abortion in indica-japonica heterozygotes, but certain “wide-compatibility” varieties carry neutral alleles and can, therefore, produce fertile progeny when crossed to either subspecies. Originally described by Ikehashi and Araki as a simple, three-allele system [31], a reasonable assumption has been that the neutral allele represents an intermediate step that allowed the indica-japonica incompatibility to evolve without a fitness cost [35,36]. Upon closer inspection, however, genetic mapping of the S5 locus has revealed that hybrid female sterility is regulated by three, tightly linked genes – ORF3, ORF4, and ORF5 – and that haplotypes vary substantially among strains of indica, japonica, and wild rice [37,38,39]. During female sporogenesis, “killer” alleles at ORF4 and ORF5 cause endoplasmic reticulum stress that results in premature programmed cell death and embryo sac abortion, but these effects can be rescued by a “protector” allele at the adjacent ORF3 gene. This killer-protector combination appears to be the ancestral genotype and is at high frequency in the wild species Oryza rufipogon and Oryza nivara, as well as in the outgroup species O. glumaepatula [39]. In contrast, the typical indica S5 haplotype has a deletion in ORF4 that incapacitates its killing function, and the typical japonica haplotype carries mutations that disable both the ORF5 killer and ORF3 protector. The S5 incompatibility causes an aberrant, gain-of-function phenotype in indica-japonica hybrids that carry killer alleles at ORF4 and ORF5 but lack the protector allele at ORF3.
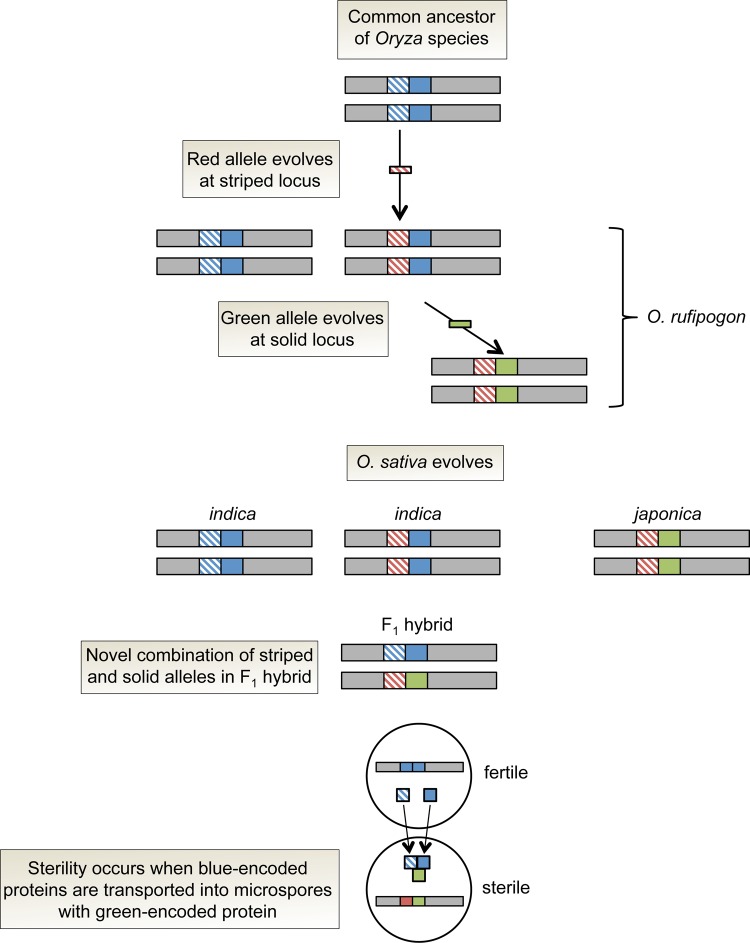
In the second case, Long et al. [40] fine-mapped the Sa locus to two distinct, tightly linked loci: SaM encodes a small ubiquitin-like modifier (SUMO) E3 ligase-like protein, and SaF encodes an F-box protein. Hybrid plants that are heterozygous at SaM and also carry indica alleles at SaF are semi-sterile because haploid pollen that inherits the japonica SaM allele fails to develop properly. To explain the surprising result that epistasis among three alleles is required for abortion of japonica SaM-carrying microspores, the authors propose a complicated model in which indica-encoded SaM and SaF proteins are transported between haploid microspores during early pollen development to kill gametes that inherit the japonica SaM allele [40]. The idea is that the japonica SaM protein is unable to move between microspores, so pollen grains that carry indica alleles at SaM and SaF – but do not receive the interacting japonica SaM protein – remain viable. For this incompatibility system to have evolved unopposed by natural selection, the japonica SaM allele must have spread after a “permissive” allele at SaF had fixed initially (Figure 2). Indeed, the current geographic distribution of SaM/F haplotypes in O. sativa and its wild ancestor, O. rufipogon, is consistent with this scenario [40]. Moreover, patterns of SaM/F haplotype variation – particularly, the finding that japonica-like haplotypes segregate in O. rufipogon but not indica populations – support the hypothesis of independent origins of domestication for indica and japonica [41,42]. An important, unanswered question is what caused the japonica haplotype to become fixed.
Figure 2. Model for the evolution of Sa hybrid sterility in rice.
A classic, single-locus incompatibility between Oryza sativa indica and Oryza sativa japonica is conferred by two adjacent genes (depicted here as striped and solid). Semi-sterility occurs in F1 hybrids that carry blue alleles at the striped and solid genes (indica haplotype) in combination with a green allele at the solid gene (japonica haplotype). The wild progenitor Oryza rufipogon carries all three haplotypes: ancestral (blue, blue), “permissive” (red, blue), and japonica-like (red, green). Figure adapted from [40].
In the third case, Zhao et al. [43] mapped the S24 pollen sterility locus to an ankyrin-3 (ANK-3) gene, which Kubo et al. [44] later showed is dependent on an additional, unlinked locus, Epistatic Factor for S24 (EFS). Like S5 and Sa, the S24-EFS hybrid incompatibility is a gain-of-function: pollen grains with japonica S24 alleles are aborted in S24-heterozygotes, but only when the plant is also homozygous for japonica alleles at EFS. As with Sa, it is possible that the otherwise deleterious japonica S24 allele was only able to spread in a permissive, indica-like EFS background, but the necessary phylogeographic analyses have not yet been done. It is also worth noting that for both Sa and S24, certain wide compatibility varieties carry neutral alleles that rescue pollen sterility [43,45], indicating that the evolutionary histories of these incompatibilities might include alternate mutational routes.
Conclusions and future challenges
In just the last few years, plant geneticists have made tremendous progress in identifying the molecular genetic basis of postzygotic reproductive isolation. With more than a dozen genes now cloned, it is clear that plant hybrid incompatibilities usually evolve via two or more mutational steps, as is predicted by the Dobzhansky-Muller model. This conclusion flows rather easily from those cases in which hybrid dysfunction is caused by epistasis between two or more unlinked genes, but it has been less obvious for a number of classic, single-locus incompatibilities [31]. Because of several recent detailed genetic studies, however, we now know that even these single-locus incompatibilities arose by mutations in two tightly linked genes, or, at the very least, two or more amino acid changes encoded within the same gene. Thus, it is possible that most of these incompatibility alleles appeared without any reductions in fitness within species.
Of course, the key question for speciation is which evolutionary forces allow incompatibility alleles to increase in frequency and eventually become fixed within species. For a few of the cases discussed above there is evidence that natural selection (e.g. hybrid necrosis) or random genetic drift (e.g. divergent resolution of duplicate genes) may have played a role. It also appears that there are some common genetic routes to plant hybrid dysfunction. Indeed, immune system genes seem to contribute disproportionately to hybrid necrosis. As we have seen, it is even possible for the very same gene to be involved in multiple cases of hybrid dysfunction: divergent duplicate loci carry a striking number of independently derived, loss-of-function mutations. Yet, there are many hybrid incompatibilities that do not involve disease resistance or duplicate genes, and, for these systems, we are only beginning to understand the evolutionary causes of divergence. Remarkably, at least 50 loci contribute to hybrid sterility between the closely related subspecies Oryza sativa indica and Oryza sativa japonica [46]. So does this large number reflect a special propensity in rice species for evolving hybrid sterility? Given that Oryza flowers have six anthers with many pollen grains per ovule, a gametic pollen killer that lowers fertility by 50% might entail little or no reduction in heterozygote seed fertility. Although our current sample of plant hybrid incompatibility genes comes from only two genera (Oryza and Arabidopsis), there are a number of classic [46,47] and more recently identified [48-51] incompatibility systems that are not yet molecularly defined. As advances in genomics and DNA sequencing technologies enable rigorous genetic analysis of reproductive isolation in a number of emerging model systems, we should gain new insight into which factors affect the number and nature of hybrid incompatibilities that accumulate between plant species.
Abbreviations
- ANK-3
ankyrin-3
- DPL
DOPPELGANGER
- EFS
Epistatic Factor for S24
- mtRPL27
mitochondrial ribosomal L27 protein
- RLK
receptor-like kinase
- SUMO
small ubiquitin-like modifier
Competing interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/4/23
Contributor Information
Andrea L. Sweigart, Email: sweigart@uga.edu.
John H. Willis, Email: jwillis@duke.edu.
References
- 1.Darwin C. On the Origin of Species. Murray; London: 1859. [Google Scholar]
- 2.Mayr E. Systematics and the Origin of Species. Columbia University Press; New York: 1942. [Google Scholar]
- 3.Schluter D, Conte GL. Genetics and ecological speciation. Proc Natl Acad Sci USA. 2009;106(Suppl 1):9955–62. doi: 10.1073/pnas.0901264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyne JA, Orr HA. Speciation. Sinauer; Sunderland, Massachusetts: 2004. [Google Scholar]
- 5.Sobel JM, Chen GF, Watt LR, Schemske DW. The biology of speciation. Evolution. 2010;64:295–315. doi: 10.1111/j.1558-5646.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- 6.Dobzhansky T H. Genetics and the Origin of Species. Columbia University Press; New York: 1937. [Google Scholar]
- 7.Muller HJ. Isolating mechanisms, evolution, and temperature. Biol Symp. 1942;6:71–125. [Google Scholar]
- 8.Rieseberg LH, Willis JH. Plant speciation. Science. 2007;317:910–4. doi: 10.1126/science.1137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chase CD. Cytoplasmic male sterility: a window to the world of plant mitochondrial-nuclear interactions. Trends Genet. 2007;23:81–90. doi: 10.1126/science.1137729. [DOI] [PubMed] [Google Scholar]
- 10.Hanson MR, Bentolila S. Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell. 2004;16:S154–69. doi: 10.1105/tpc.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurst LD, Atlan A, Bengtsson BO. Genetic conflicts. Q Rev Biol. 1996;71:317–64. doi: 10.1086/419442. [DOI] [PubMed] [Google Scholar]
- 12.Burt A, Trivers R. Genes in Conflict: The Biology of Selfish Genetic Elements. Harvard University Press; Cambridge, MA: 2006. [Google Scholar]
- 13.Schluter D, Conte GL. Colloquium Papers: Genetics and ecological speciation. Proceedings of the National Academy of Sciences. 2009;106:9955–62. doi: 10.1073/pnas.0901264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bomblies K, Weigel D. Hybrid necrosis: autoimmunity as a potential gene-flow barrier in plant species. Nat Rev Genet. 2007;8:382–93. doi: 10.1038/nrg2082. [DOI] [PubMed] [Google Scholar]; www.f1000.com/prime/717963314
- 15.Bomblies K. Doomed lovers: mechanisms of isolation and incompatibility in plants. Annu Rev Plant Biol. 2010;61:109–24. doi: 10.1146/annurev-arplant-042809-112146. [DOI] [PubMed] [Google Scholar]; www.f1000.com/prime/717963315
- 16.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–9. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 17.Dodds PN, Rathjen JP. Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet. 2010;11:539–48. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 18.Bomblies K, Lempe J, Epple P, Warthmann N, Lanz C, Dangl JL, Weigel D. Autoimmune response as a mechanism for a Dobzhansky-Muller-type incompatibility syndrome in plants. PLoS Biol. 2007;5:e236. doi: 10.1371/journal.pbio.0050236. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.f1000.com/prime/1091250
- 19.Alcázar R, García AV, Parker JE, Reymond M. Incremental steps toward incompatibility revealed by Arabidopsis epistatic interactions modulating salicylic acid pathway activation. Proc Natl Acad Sci USA. 2009;106:334–9. doi: 10.1073/pnas.0811734106. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.f1000.com/prime/717963240
- 20.Alcázar R, García AV, Kronholm I, de Meaux J, Koornneef M, Parker JE, Reymond M. Natural variation at Strubbelig Receptor Kinase 3 drives immune-triggered incompatibilities between Arabidopsis thaliana accessions. Nat Genet. 2010;42:1135–9. doi: 10.1038/ng.704. [DOI] [PubMed] [Google Scholar]; www.f1000.com/prime/6542957
- 21.Yamamoto E, Takashi T, Morinaka Y, Lin S, Wu J, Matsumoto T, Kitano H, Matsuoka M, Ashikari M. Gain of deleterious function causes an autoimmune response and Bateson-Dobzhansky-Muller incompatibility in rice. Mol Genet Genomics. 2010;283:305–15. doi: 10.1007/s00438-010-0514-y. [DOI] [PubMed] [Google Scholar]; www.f1000.com/prime/717963241
- 22.Krüger J, Thomas CM, Golstein C, Dixon MS, Smoker M, Tang S, Mulder L, Jones JDG. A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science. 2002;296:744–7. doi: 10.1126/science.1069288. [DOI] [PubMed] [Google Scholar]; www.f1000.com/prime/1006815
- 23.Jeuken MJW, Zhang NW, McHale LK, Pelgrom K, den Boer E, Lindhout P, Michelmore RW, Visser RGF, Niks RE. Rin4 causes hybrid necrosis and race-specific resistance in an interspecific lettuce hybrid. Plant Cell. 2009;21:3368–78. doi: 10.1105/tpc.109.070334. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.f1000.com/prime/717963242
- 24.Oka H. Analysis of genes controlling f(1) sterility in rice by the use of isogenic lines. Genetics. 1974;77:521–34. doi: 10.1093/genetics/77.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.f1000.com/prime/717963243
- 25.Lynch M, Force A. The probability of duplicate gene preservation by subfunctionalization. Genetics. 2000;154:459–73. doi: 10.1093/genetics/154.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.f1000.com/prime/717963244
- 26.Werth CR, Windham MD. A Model for Divergent, Allopatric Speciation of Polyploid Pteridophytes Resulting from Silencing of Duplicate-Gene Expression. The American Naturalist. 1991;137:515–26. doi: 10.1086/285180. [DOI] [Google Scholar]
- 27.Bikard D, Patel D, Le Metté C, Giorgi V, Camilleri C, Bennett MJ, Loudet O. Divergent evolution of duplicate genes leads to genetic incompatibilities within A. thaliana. Science. 2009;323:623–6. doi: 10.1126/science.1165917. [DOI] [PubMed] [Google Scholar]; www.f1000.com/prime/1147338
- 28.Mizuta Y, Harushima Y, Kurata N. Rice pollen hybrid incompatibility caused by reciprocal gene loss of duplicated genes. Proc Natl Acad Sci USA. 2010;107:20417–22. doi: 10.1073/pnas.1003124107. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.f1000.com/prime/8935957
- 29.Yamagata Y, Yamamoto E, Aya K, Win KT, Doi K, Sobrizal, Ito T, Kanamori H, Wu J, Matsumoto T, Matsuoka M, Ashikari M, Yoshimura A. Mitochondrial gene in the nuclear genome induces reproductive barrier in rice. Proc Natl Acad Sci USA. 2010;107:1494–9. doi: 10.1073/pnas.0908283107. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.f1000.com/prime/717963245
- 30.Win KT, Yamagata Y, Miyazaki Y, Doi K, Yasui H, Yoshimura A. Independent evolution of a new allele of F1 pollen sterility gene S27 encoding mitochondrial ribosomal protein L27 in Oryza nivara. Theor Appl Genet. 2011;122:385–94. doi: 10.1007/s00122-010-1454-y. [DOI] [PubMed] [Google Scholar]
- 31.Sano Y. The genic nature of gamete eliminator in rice. Genetics. 1990;125:183–91. doi: 10.1093/genetics/125.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.f1000.com/prime/717963246
- 32.Ikehashi H, Araki H. Genetics of F1 sterility in remote crosses of rice. In: IRRI, editor. Rice Genetics. Philippines International Rice Research Institute; 1986. pp. 119–30. [Google Scholar]
- 33.Nei M, Maruyama T, Wu CI. Models of evolution of reproductive isolation. Genetics. 1983;103:557–79. doi: 10.1093/genetics/103.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith LM, Bomblies K, Weigel D. Complex evolutionary events at a tandem cluster of Arabidopsis thaliana genes resulting in a single-locus genetic incompatibility. PLoS Genet. 2011;7:e1002164. doi: 10.1371/journal.pgen.1002164. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.f1000.com/prime/12206956
- 35.Nei M, Nozawa M. Roles of mutation and selection in speciation: from Hugo de Vries to the modern genomic era. Genome Biology and Evolution. 2011;3:812–29. doi: 10.1093/gbe/evr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieseberg LH, Blackman BK. Speciation genes in plants. Annals of Botany. 2010;106:439–55. doi: 10.1093/aob/mcq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Ding J, Ouyang Y, Du H, Yang J, Cheng K, Zhao J, Qiu S, Zhang X, Yao J, Liu K, Wang L, Xu C, Li X, Xue Y, Xia M, Ji Q, Lu J, Xu M, Zhang Q. A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica-japonica hybrids in rice. Proc Natl Acad Sci USA. 2008;105:11436–41. doi: 10.1073/pnas.0804761105. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.f1000.com/prime/1119873
- 38.Du H, Ouyang Y, Zhang C, Zhang Q. Complex evolution of S5, a major reproductive barrier regulator, in the cultivated rice Oryza sativa and its wild relatives. New Phytol. 2011;191:275–87. doi: 10.1111/j.1469-8137.2011.03691.x. [DOI] [PubMed] [Google Scholar]; www.f1000.com/prime/717963247
- 39.Yang J, Zhao X, Cheng K, Du H, Ouyang Y, Chen J, Qiu S, Huang J, Jiang Y, Jiang L, Ding J, Wang J, Xu C, Li X, Zhang Q. A killer-protector system regulates both hybrid sterility and segregation distortion in rice. Science. 2012;337:1336–40. doi: 10.1126/science.1223702. [DOI] [PubMed] [Google Scholar]; www.f1000.com/prime/717963249
- 40.Long Y, Zhao L, Niu B, Su J, Wu H, Chen Y, Zhang Q, Guo J, Zhuang C, Mei M, Xia J, Wang L, Wu H, Liu Y. Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. Proc Natl Acad Sci USA. 2008;105:18871–6. doi: 10.1073/pnas.0810108105. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.f1000.com/prime/717963248
- 41.Londo JP, Chiang Y, Hung K, Chiang T, Schaal BA. Phylogeography of Asian wild rice, Oryza rufipogon, reveals multiple independent domestications of cultivated rice, Oryza sativa. Proc Natl Acad Sci USA. 2006;103:9578–83. doi: 10.1073/pnas.0603152103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sang T, Ge S. The puzzle of rice domestication. Journal of Integrative Plant Biology. 2007;49:760–8. doi: 10.1111/j.1744-7909.2007.00510.x. [DOI] [Google Scholar]
- 43.Zhao ZG, Zhu SS, Zhang YH, Bian XF, Wang Y, Jiang L, Liu X, Chen LM, Liu SJ, Zhang WW, Ikehashi H, Wan JM. Molecular analysis of an additional case of hybrid sterility in rice (Oryza sativa L.) Planta. 2011;233:485–94. doi: 10.1007/s00425-010-1313-8. [DOI] [PubMed] [Google Scholar]
- 44.Kubo T, Yoshimura A, Kurata N. Hybrid male sterility in rice is due to epistatic interactions with a pollen killer locus. Genetics. 2011;189:1083–92. doi: 10.1534/genetics.111.132035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Zhong ZZ, Zhao ZG, Jiang L, Bian XF, Zhang WW, Liu LL, Ikehashi H, Wan JM. Fine mapping of a gene causing hybrid pollen sterility between Yunnan weedy rice and cultivated rice (Oryza sativa L.) and phylogenetic analysis of Yunnan weedy rice. Planta. 2010;231:559–70. doi: 10.1007/s00425-009-1063-7. [DOI] [PubMed] [Google Scholar]
- 46.Ouyang Y, Liu Y, Zhang Q. Hybrid sterility in plant: stories from rice. Curr Opin Plant Biol. 2010;13:186–92. doi: 10.1016/j.pbi.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Rick CM. Abortion of male and female gametes in the tomato determined by allelic interaction. Genetics. 1966;53:85–96. doi: 10.1093/genetics/53.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moyle LC, Graham EB. Genetics of hybrid incompatibility between Lycopersicon esculentum and L. hirsutum. Genetics. 2005;169:355–73. doi: 10.1534/genetics.104.029546. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.f1000.com/prime/1014691
- 49.Macnair MR, Christie P. Reproductive isolation as a pleiotropic effect of copper tolerance in Mimulus guttatus. Heredity. 1983;50:295–302. doi: 10.1038/hdy.1983.31. [DOI] [Google Scholar]
- 50.Sweigart AL, Fishman L, Willis JH. A simple genetic incompatibility causes hybrid male sterility in mimulus. Genetics. 2006;172:2465–79. doi: 10.1534/genetics.105.053686. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.f1000.com/prime/1030623
- 51.Burkart-Waco D, Josefsson C, Dilkes B, Kozloff N, Torjek O, Meyer R, Altmann T, Comai L. Hybrid incompatibility in Arabidopsis is determined by a multiple-locus genetic network. Plant Physiol. 2012;158:801–12. doi: 10.1104/pp.111.188706. [DOI] [PMC free article] [PubMed] [Google Scholar]