Abstract
Objective
To investigate whether the severity of cystoid macular edema (CME) in neonates who were 31 to 36 weeks’ postmenstrual age, as viewed by spectral-domain optical coherence tomography (SD-OCT) imaging, predicts the severity of retinopathy of prematurity (ROP) or is related to systemic health.
Design
Of 62 prematurely born neonates in a prospective institutional review board–approved study, 42 met the following inclusion criteria: at least 1 SD-OCT imaging session prior to 37 weeks’ postmenstrual age and prior to ROP laser treatment, if a laser treatment was performed, and an ophthalmic ROP examination at or after 41 weeks’ postmenstrual age, evidence of complete retinal vascularization in zone III, or documentation through telephone report of such information after transfer of care. Measures of CME severity, including central foveal thickness, retinal layer thicknesses, and foveal-to-parafoveal thickness ratio in 1 eye per subject, were compared with ROP outcomes: laser treatment, maximum plus disease, and maximum ROP stage. Systemic health factors were also correlated.
Results
Cystoid macular edema was present in 50% of neonates. Multiple elongated cystoid structures within the inner nuclear layer were most common. The presence of CME was not associated with ROP outcomes. The central foveal thickness, the thickness of the inner retinal layers, and the foveal-to-parafoveal thickness ratio were higher in eyes that required laser treatment or that developed plus disease or ROP stage 3. Cystoid macular edema was not clearly associated with systemic factors.
Conclusions
Cystoid macular edema is common in premature infants screened for ROP before 37 weeks’ postmenstrual age, with the most common SD-OCT phenotype of a bulging fovea from multiple elongated cystoid spaces. Detection of CME is not associated with ROP severity; however, tomographic thickness measurements could potentially predict a higher risk of requiring laser treatment or developing plus disease or ROP stage 3. Systemic health factors are probably not related to the development of CME.
Retinopathy of prematurity (ROP) is a multifactorial vascular disease and is the second most common cause of childhood blindness in the United States.1 Timely treatment of ROP is important to prevent disease progression and improve visual outcomes.2 Current guidelines for ROP screening recommend a dilated ophthalmologic examination of the retina.3,4 However, ophthalmoscopic examinations have a degree of subjectivity.5 Wallace et al6 found disagreement in the diagnosis of plus and pre-plus disease among expert examiners. Standard ROP care also has medicolegal liability issues and logistic difficulties that have motivated researchers to seek objective methods to evaluate the severity of ROP. Thus, approaches such as telemedicine,7,8 video indirect recording,9 RetCam imaging (Clarity Medical Systems),10 and computerized image analysis have gained support.11,12
Another imaging technique, widely used for the diagnosis of retinal disease in adults, is spectral-domain optical coherence tomography (SD-OCT). This technique provides cross-sectional images of retinal architecture, allowing detection of subclinical anatomic changes in infants, although data on its use in ROP screening are limited.13–17
In a study18 of premature infants at risk for ROP, we detected cystoid macular edema (CME) when using SD-OCT but not when using indirect ophthalmoscopy. In the present study, we investigate the significance of CME assessed by use of SD-OCT in premature infants at risk of developing ROP. In addition to gestational age, birth weight, and race,19 other factors such as high oxygen levels,20 poor weight gain in the first 6 weeks of life,21 elevated blood glucose level,22 vascular endothelial growth factor (VEGF) level,23 and, lately, insulinlike growth factor 1 have been postulated as predictors of ROP progression.24,25 We hypothesize that the macular edema may be an imaging biomarker of active intravitreal VEGF levels and thus might be related to ROP progression or severity; however, we recognize that CME might also reflect systemic disease unrelated to ROP. With the goal of bringing objectivity and providing complementary information to ROP examinations, we studied SD-OCT–detectible CME in neonates with mild and advanced ROP.18,26
METHODS
STUDY PARTICIPANTS
Between January 2009 and May 2010, a total of 62 consecutive preterm neonates who had an ROP screening at the Duke University Medical Center’s neonatal intensive care unit were enrolled in an SD-OCT imaging study with parental consent. From that study, the subjects included in the analysis of CME in ROP were required to have at least 1 SD-OCT imaging session prior to 37 weeks’ postmenstrual age (PMA) and prior to ROP laser treatment, if a laser treatment was performed, and either an ophthalmic ROP examination at or after 41 weeks’ PMA, evidence of complete retinal vascularization in zone III, or documentation through telephone report of such information after transfer of care. We selected imaging prior to 37 weeks’ PMA to identify markers before onset of advanced disease or before laser treatment because the median onset of stage 3 and plus disease is 36 weeks’ PMA.27 We selected follow-up ophthalmic ROP examinations at or after 41 weeks’ PMA because, by this time, most subjects would have developed ROP requiring laser treatment.28 Of the 62 subjects, 20 did not meet eligibility criteria and were excluded from analysis.
We recorded birth weight, gestational age, race, and sex. We also examined the neonatal intensive care unit clinical records for systemic factors commonly occurring in premature neonates that we hypothesized could be related to CME, including an Apgar score at 1 minute and at 5 minutes; surgery for patent ductus arteriosus; culture-proven sepsis; surgery for necrotizing enterocolitis; and the presence of intraventricular hemorrhage, periventricular leukomalacia, bronchopulmonary dysplasia, or hydrocephalus. The ROP data (zone, stage, and plus/pre-plus status) were determined at each ophthalmic examination by 1 of 2 ROP examination experts (S.F.F. or D.K.W.) and recorded using a case report form. The ROP examiners were masked to SD-OCT findings.
After an ophthalmoscopic examination, SD-OCT imaging was performed (R.S.M.) using a portable handheld SD-OCT unit (Bioptigen Inc). Previously described customized SD-OCT imaging parameters were applied to allow for high-resolution imaging of all infants without sedation or use of a eyelid speculum.16
The SD-OCT images were converted to the digital imaging and communications in medicine format and graded using OsiriX medical imaging software (OsiriX Foundation). The best scan containing the fovea was evaluated by masked SD-OCT graders for the quantity, location, morphology, and distribution of macular cystoid structures and retinal layers.
The foveal scan was segmented semiautomatically using a custom program, the Duke OCT Retinal Analysis Program version 2.1-SF, based in MATLAB (MathWorks). The foveal center was selected manually. MATLAB was used to compute thickness values from segmentation at each A-scan. The thickness of the inner retinal layers (IRLs; from the internal limiting membrane to the outer plexiform layer), the inner nuclear layer (INL), and the photoreceptor layer (PRL) and the central foveal thickness (CFT; from the internal limiting membrane to the inner border of the retinal pigment epithelium) were calculated. To obtain a quantitative measure of foveal contour, a fovea-to-parafoveal (FP) thickness ratio was computed by dividing the CFT by the average of 2 parafoveal measurements, 1000 μm on either side of the fovea. An FP thickness ratio of less than 1 suggests the presence of a foveal depression, whereas an FP thickness ratio of greater than 1 suggests a bulging fovea (Figure 1).
Figure 1.
Macular spectral-domain optical coherence tomographic scan segmentation to obtain quantitative measurements. Boundary lines were semiautomatically segmented. A, A foveal-to-parafoveal (FP) thickness ratio provided a quantitative measure of foveal contour and was calculated by dividing the central foveal thickness (CFT) by an average of the retinal thickness values at 1000 μm on either side of the fovea (white parafoveal vertical bars): inner retinal layer (IRL) thickness (green to magenta), inner nuclear layer (INL) thickness (yellow to magenta), and CFT (green to cyan). A premature infant with edema had a CFT of 370 μm and an FP thickness ratio of 1.35 (A), whereas a premature infant without edema had a CFT of 113 μm and an FP thickness ratio of 0.42 (B).
STATISTICAL ANALYSIS
Outcome measures included ROP laser treatment (yes or no), most severe ROP vascular abnormality detected (plus disease, pre-plus disease, or no plus disease), and most severe ROP grade detected. The number of SD-OCT imaging sessions per subject ranged from 1 to 12, and 1 eye visit per subject from the pre–37-week PMA time frame was randomly selected for all study analysis except that, if only 1 eye was imaged, that eye was used; if only 1 eye had laser treatment, that eye was selected; or if the patient had CME at any eligible visit, the CME visit was selected. For subjects with foveal SD-OCT scans in the fellow eye at the time of the study eye visit, we compared the CFT in the fellow eye with that in the study eye. Continuous variables were compared using the Wilcoxon rank sum test for equal medians in 2 groups, and the Kruskal-Wallis test was used for multiple group comparisons. Categorical variables were analyzed using the Fisher exact test. The relationship between CME and systemic health factors was analyzed using a multivariate logistic regression. A Bonferroni correction factor was used to adjust for multiple comparisons across systemic health factors. A P value of less than .05 was otherwise considered statistically significant.
RESULTS
The median birth weight was 760 g, and the median gestational age was 26 weeks (Table 1). Of the 42 neonates, 12 (29%) required laser treatment by 41 weeks’ PMA. Two subjects required laser treatment in only 1 eye. Subjects receiving ROP laser treatment had a lower birth weight and an earlier gestational age compared with subjects who did not receive laser treatment (P=.046 and P=.005, respectively). The Apgar scores and the frequency of systemic health factors were similar in the laser and nonlaser groups (Table 1). Eleven subjects had a final outcome of plus disease, and 6 developed pre-plus disease. The maximum ROP stage for the study eyes was as follows: stage 0 (19%), stage 1 (7%), stage 2 (43%), and stage 3 (31%).
Table 1.
Description of Cohort of Neonates to Investigate Whether Severity of CME Predicts Severity of ROP
| Characteristic | All Subjectsa | Presence of CME in Study Eye
|
Progression to Laser Treatment in Study Eye
|
||||
|---|---|---|---|---|---|---|---|
| No | Yesb | P Value | No | Yesc | P Value | ||
| Demographic characteristics | |||||||
| Neonates, No. (%) | 42 (100) | 21 (50) | 21 (50) | 30 (71) | 12 (29) | ||
| Sex, No. (%) | |||||||
| Males | 23 (54.8) | 10 (47.6) | 13 (61.9) | .16 | 15 (50.0) | 8 (66.7) | .17 |
| Females | 19 (45.2) | 11 (52.4) | 8 (38.1) | 15 (50.0) | 4 (33.3) | ||
| Birth weight, median (SD), g | 760 (272) | 750 (272) | 770 (278) | .65 | 855 (283) | 637 (207) | .047 |
| Gestational age, median (SD), wk | 26.0 (1.8) | 27.0 (1.9) | 25.0 (1.6) | .04 | 26.0 (1.7) | 24.0 (1.5) | .005 |
| Age at imaging, median (SD), wk | 34.0 (1.6) | 34.0 (1.6) | 35.0 (1.6) | .15 | 35.0 (1.8) | 34.0 (1.1) | .69 |
| Race, No. (%) | |||||||
| African American | 22 (52.4) | 11 (52.4) | 11 (52.4) | .90 | 17 (56.7) | 5 (41.7) | .32 |
| White | 17 (40.5) | 8 (38.1) | 9 (42.9) | 10 (33.3) | 7 (58.3) | ||
| Hispanic | 3 (7.1) | 2 (9.5) | 1 (4.8) | 3 (10.0) | 0 (0) | ||
| Systemic characteristics | |||||||
| Apgar score, median (SD) | |||||||
| At 1 min | 4 (2) | 4.0 (2.3) | 3.0 (2.5) | .78 | 4.0 (2.3) | 3.5 (2.5) | .08 |
| At 5 min | 7 (2) | 7 (1.5) | 7 (2) | .10 | 7.0 (1.7) | 7.5 (1.7) | .61 |
| Surgery for PDA, No. (%) | 8 (19.0) | 2 (9.5) | 6 (28.5) | .24 | 4 (13.3) | 4 (33.3) | .99 |
| Culture-proven sepsis, No. (%) | 7 (16.7) | 3 (14.3) | 4 (19.0) | .99 | 4 (13.3) | 3 (25.0) | .99 |
| Surgery for NEC, No. (%) | 5 (11.9) | 3 (14.3) | 2 (9.5) | .99 | 3 (10.0) | 2 (16.7) | .99 |
| Presence of IVH, No. (%) | 17 (40.5) | 7 (33.3) | 10 (47.6) | .53 | 10 (33.3) | 7 (58.3) | .53 |
| Grade 1 | 7 (41.2) | 3 (42.9) | 4 (40.0) | 4 (40.0) | 3 (42.9) | ||
| Grade 2 | 4 (23.5) | 3 (42.9) | 1 (10.0) | 3 (30.0) | 1 (14.3) | ||
| Grade 3 | 1 (5.9) | 0 (0.0) | 1 (10.0) | 1 (10.0) | 0 (0.0) | ||
| Grade 4 | 5 (29.4) | 1 (14.3) | 4 (40.0) | 2 (20.0) | 3 (25.0) | ||
| Presence of PVL, No. (%) | 5 (11.9) | 1 (20) | 4 (19.0) | .34 | 2 (6.7) | 3 (25.0) | .99 |
| Presence of BPD, No. (%) | 13 (31.0) | 5 (38) | 8 (38.1) | .32 | 10 (33.3) | 3 (25.0) | |
| Presence of hydrocephalus, No. (%) | 5 (11.9) | 2 (40) | 3 (14.3) | .60 | 3 (10.0) | 2 (16.7) | |
| Ocular characteristics in study eye | |||||||
| Age at imaging, median (SD), weeks’ PMA | 34.0 (1.61) | 33.5 (1.6) | 34.5 (1.53) | .16 | 35.0 (1.79) | 34.0 (1.1) | .86 |
| ROP stage at imaging, No. (%) | |||||||
| Stage 0 | 13 (30.9) | 10 (47.6) | 3 (14.3) | .02 | 12 (40.0) | 1 (8.3) | .04 |
| Stage 1 | 6 (14.3) | 1 (4.8) | 5 (23.8) | .08 | 4 (13.3) | 2 (16.7) | .35 |
| Stage 2 | 17 (40.5) | 8 (38.1) | 9 (42.9) | .24 | 14 (46.7) | 3 (25.0) | .13 |
| Stage 3 | 6 (14.3) | 2 (9.5) | 4 (19.0) | .24 | 0 (0) | 6 (50.0) | <.001 |
| Vascular status at imaging, No. (%) | |||||||
| No plus disease | 31 (73.8) | 18 (85.7) | 13 (61.9) | .06 | 27 (90.0) | 4 (33.3) | .001 |
| Pre-plus disease | 6 (14.3) | 1 (4.8) | 5 (23.8) | .08 | 3 (10.0) | 3 (25.0) | .17 |
| Plus disease | 5 (11.9) | 2 (9.5) | 3 (14.3) | .33 | 0 (0) | 5 (41.7) | .001 |
Abbreviations: BPD, bronchopulmonary dysplasia; CME, cystoid macular edema; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; PMA, postmenstrual age; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity.
One eye per subject was randomly chosen.
Two subjects had unilateral imaging with CME and were included in the CME group.
Two subjects had unilateral laser and were included in the laser group.
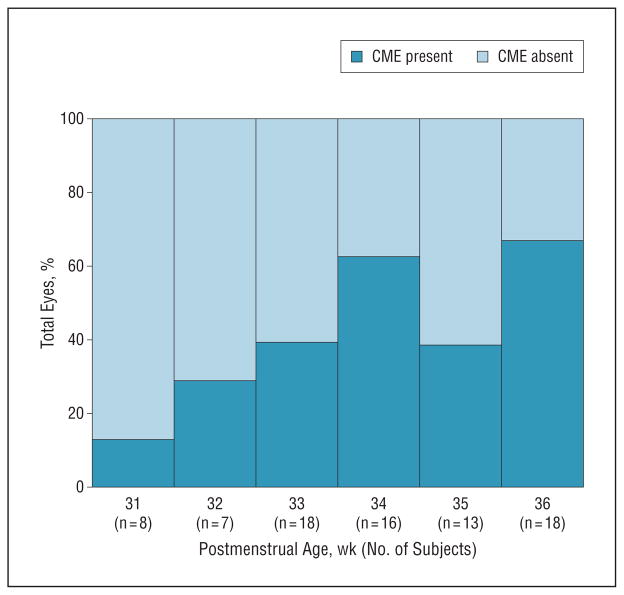
Cystoid macular edema was not detected by clinical examination in any of the eyes of these subjects. Half of all subjects (n = 21) had CME detected on SD-OCT images during at least 1 visit and in at least 1 eye (CME group). Cystoid macular edema was bilateral in all 19 subjects who underwent bilateral SD-OCT imaging. Cystoid macular edema was documented in 1 eye of 2 subjects (both at 35 weeks’ PMA) who did not undergo an adequate SD-OCT imaging session for the fellow eye. The prevalence of CME ranged from 13% of eyes at 31 weeks’ PMA to 60% of eyes at 34 weeks’ PMA to 65% of eyes at 36 weeks’ PMA, although with very small numbers of eyes per weekly interval (Figure 2).
Figure 2.
Prevalence of cystoid macular edema (CME) in the study population, as a percentage of total eyes imaged in which CME was either present (dark blue) or absent (light blue), per postmenstrual age.
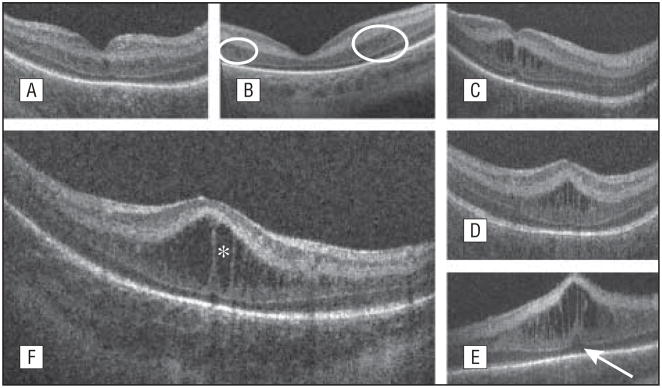
Cystoid structures, on SD-OCT imaging, in the setting of ROP were identified as hyporeflective round or elongated structures separated in most instances by a vertical hyperreflective band (Figure 3). These structures were either single or multiple and were always located exclusively at the level of the INL. In a review of study eyes, a single central cystoid structure was infrequent (5% of eyes), whereas multiple cystoid structures were found in 95% of eyes. The shape of the cystoid structures was elongated in 81% of eyes and extended across the macula (involving the foveal and parafoveal area) in 76% of eyes. Cystoid macular edema also had an effect on the foveal contour, creating a bulging fovea in 62% of eyes and also producing an elevated PRL at the foveal center in 28% of eyes (Table 2 and Figure 3).
Figure 3.
Morphologic characteristics and phenotypes of cystoid macular edema (CME) observed by use of spectral-domain optical coherence tomography (SD-OCT). Three CME phenotypes were observed in our subjects: single central (A), parafoveal (when cystoid structures were grouped around the foveal center, as shown within the white encircled areas) (B), and multiple elongated cystoid structures when the parafoveal and central fovea contained cystoid structures (C-E). For the multiple elongated CME phenotype, severity was scored as mild (C) if the foveal pit was present, moderate (D) if the fovea was bulging but the photoreceptor layer was not affected, and severe (E) if the fovea and the photoreceptor layer had a bulging shape (white arrow). A magnified SD-OCT scan shows the morphologic characteristics found in severe CME (F). The white asterisk is located within 1 cystoid space.
Table 2.
CME Morphologic Characteristics in the Study Eyes
| Characteristic | Eyes, No. (%)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eyes With CME | Progression to Laser Treatment in Study Eyes With CME
|
Vascular Outcome in Study Eyes With CME
|
Maximum ROP Stage in Study Eyes With CME
|
|||||||
| No | Yes | No Plus | Pre-plus | Plus | Stage 0 | Stage 1 | Stage 2 | Stage 3 | ||
| Eyes, Total No. (%) | 21 (100) | 14 (67) | 7 (33) | 11 (52) | 4 (19) | 6 (29) | 3 (14) | 1 (5) | 8 (38) | 9 (43) |
| Quantity | ||||||||||
| Single | 1 (5) | 1 (7) | 0 (0) | 1 (9) | 0 (0) | 0 (0) | 0 | 0 | 1 (12) | 0 |
| Multiple | 20 (95) | 13 (93) | 7 (100) | 10 (91) | 4 (100) | 6 (100) | 3 (100) | 1 (100) | 7 (88) | 9 (100) |
| Shape | ||||||||||
| Predominantly round | 4 (19) | 1 (7) | 1 (14) | 2 (18) | 1 (25) | 1 (17) | 1 (33) | 0 | 1 (12) | 2 (22) |
| Predominantly elongated | 17 (81) | 13 (93) | 6 (86) | 10 (91) | 3 (75) | 5 (83) | 2 (67) | 1 (100) | 7 (88) | 7 (78) |
| Lateral location | ||||||||||
| Foveal only | 1 (5) | 1 (7) | 0 (0) | 1 (9) | 0 (0) | 0 (0) | 0 (0) | 0 | 1 (12) | 0 |
| Parafoveal only | 4 (19) | 3 (21) | 1 (14) | 1 (9) | 2 (50) | 1 (17) | 1 (33) | 0 | 1 (12) | 2 (22) |
| Continuous | 16 (76) | 10 (72) | 6 (86) | 9 (82) | 2 (50) | 5 (83) | 2 (67) | 1 (100) | 6 (75) | 7 (78) |
| Axial location | ||||||||||
| INL only | 21 (100) | 14 (100) | 7 (100) | 11 (100) | 4 (100) | 6 (100) | 3 (100) | 1 (100) | 8 (100) | 9 (100) |
| INL and PRL | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 0 | 0 | 0 |
| Foveal contour | ||||||||||
| Deep | 4 (19) | 3 (21) | 1 (14) | 2 (18) | 1 (25) | 1 (17) | 1 (33) | 0 (0) | 2 (25) | 1 (11) |
| Shallow | 4 (19) | 4 (29) | 0 (0) | 3 (27) | 1 (25) | 0 (0) | 1 (33) | 0 (0) | 2 (25) | 1 (11) |
| Bulging | 13 (62) | 7 (50) | 6 (86) | 6 (55) | 2 (50) | 5 (83) | 1 (33) | 1 (100) | 4 (50) | 7 (78) |
Abbreviations: CME, cystoid macular edema; INL, inner nuclear layer; PRL, photoreceptor layer; ROP, retinopathy of prematurity.
Cystoid macular edema was present in 57% of male neonates and 42% of female neonates (P = .16). Those in the CME group had similar birth weights compared with the non-CME group (P = .65) but earlier gestational ages (P = .04). Race was evenly distributed among these groups (P = .90). The subjects with CME and the subjects without CME had similar Apgar scores and similar clinical records for the following systemic factors: surgery for patent ductus arteriosus, culture-proven sepsis, surgery for necrotizing enterocolitis, and the presence of intraventricular hemorrhage or periventricular leukomalacia. Cystoid macular edema was detected in subjects with maximum ROP stages 0, 1, 2, and 3. The presence of CME, per se, was not associated with an increased likelihood of laser treatment in the study eye (odds ratio, 1.6 [95% CI, 0.4–6.2]), of plus disease (odds ratio, 1.3 [95% CI, 0.3–5.0]), or of maximum ROP stage in the study eye (Table 1). The morphological characteristics of CME, except foveal contour, did not differ between those who received laser treatment and those who did not, between those with plus disease and those without, or between ROP stage subgroups (Table 2). There appeared to be a trend toward more severe CME with a bulging fovea in eyes that underwent laser treatment, that had plus disease, or that had a higher stage of ROP (Table 2).
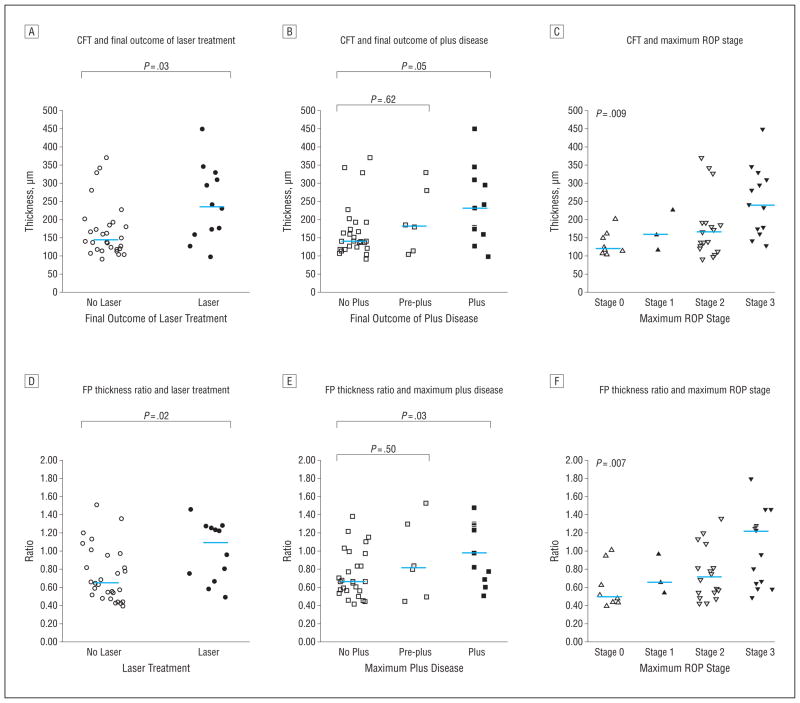
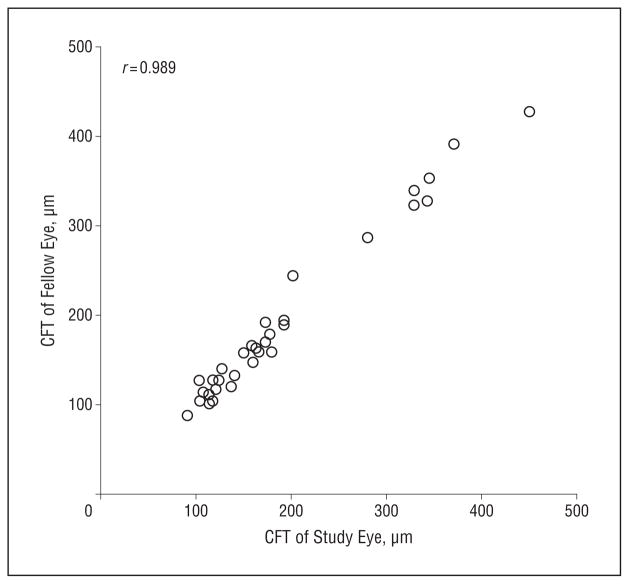
The severity of CME as measured by the thickness of retinal layers at the fovea or by the ratio of foveal-to-parafoveal thickness (ie, the FP thickness ratio), which provided a quantitative measure of foveal contour, appeared to be symmetric and to be associated with measures of severity of ROP (Figure 4). The mean (SD) absolute difference between repeated measures of the CFT and the FP ratio for the same eye and visit were 7 (6) μm and 0.045 (0.045), respectively. The CFT was notably similar between the 2 eyes for each subject, across the range of CME severity, with a mean (SD) absolute difference of 10.0 (8.8) μm between the 2 eyes (Figure 5). The presence of CME was associated with a significantly greater IRL thickness, INL thickness, PRL thickness, CFT, and FP thickness ratios (Table 3). The median CFT, the median IRL thickness, and the median FP thickness ratio were significantly higher in the laser group than in the nonlaser group (Table 4). The median CFT, IRL thickness, and FP thickness ratio were also significantly higher in the plus disease group than in the other groups (Table 4). The median CFT, IRL thickness, and FP thickness ratio were significantly higher in eyes with maximum ROP stage 3 than in the other eyes (Table 4).
Figure 4.
Central foveal thickness (CFT) and foveal-to-parafoveal (FP) thickness ratio data distribution by final retinopathy of prematurity (ROP) outcome. The median CFT and the median FP thickness ratio were greater in the laser group than in the nonlaser group (A and D), in the plus disease group than in the normal vasculature group (B and E), and in maximum stage 3 group than in subjects with stages 0, 1, or 2 (C and F).
Figure 5.
Correlation of control foveal thickness between study and fellow eyes. The central foveal thickness (CFT) was highly correlated in both eyes of the same subject, with a correlation coefficient of 0.989 and a mean (SD) absolute difference of 10.0 (8.8) μm.
Table 3.
Quantitative Assessment of CME Severity
| Retinal Measurementa | Median (Range)
|
|||
|---|---|---|---|---|
| All Eyes (n = 42) | Presence of CME in Study Eye
|
|||
| No (n = 21) | Yes (n = 21) | P Valueb | ||
| CFT, μm | 166 (91–449) | 132 (91–231) | 227 (113–449) | <.001 |
| IRL thickness, μm | 117 (58–384) | 94 (62–186) | 168 (58–384) | <.001 |
| INL thickness, μm | 68 (32–316) | 57 (39–101) | 110 (32–316) | <.001 |
| PRL thickness, μm | 33 (13–65) | 29 (13–55) | 38 (20–65) | .009 |
| FP thickness ratio | 0.75 (0.40–1.51) | 0.55 (0.40–0.96) | 1.08 (0.48–1.51) | <.001 |
Abbreviations: CFT, central foveal thickness; CME, cystoid macular edema; FP, foveal-to-parafoveal; INL, inner nuclear layer; IRL, inner retinal layer; PRL, photoreceptor layer.
Continuous CME outcome variables.
Determined by use of the Wilcoxon rank sum test.
Table 4.
Association of Quantitative Markers of CME Severity to ROP Outcome
| Retinal Measurement | Median (Range)
|
P Value
|
Median (Range)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progression to Laser Treatment in Study Eye
|
Vascular Outcome in Study Eye
|
Maximum ROP Stage in Study Eye
|
|||||||||||
| No (n = 30) | Yes (n = 12) | P Valuea | No Plus (n = 25) | Pre-plus (n = 6) | Plus (n = 11) No Plus | Plus vs No Plus | Pre-plus vs No Plus | Stage 0 (n = 8) | Stage 1 (n = 3) | Stage 2 (n = 18) | Stage 3 (n = 13) | P Valuea | |
| CFT, μm | 145 (91–370) | 236 (98–449) | .03 | 140 (91–370) | 182 (104–329) | 231 (98–449) | .046 | .62 | 121 (103–202) | 160 (117–227) | 139 (91–370) | 240 (127–449) | .009 |
| IRL thickness, μm | 108 (58–328) | 194 (68–384) | .02 | 106 (65–328) | 130 (58–215) | 194 (68–384) | .02 | .74 | 80 (68–160) | 111 (78–175) | 106 (58–328) | 194 (68–384) | .04 |
| INL thickness, μm | 65 (32–263) | 102 (46–316) | .11 | 65 (39–263) | 55 (32–160) | 102 (46–316) | .14 | .40 | 63 (39–117) | 62 (62–127) | 65 (32–263) | 102 (46–316) | .20 |
| PRL thickness, μm | 33 (13–65) | 42 (22–59) | .20 | 29 (13–65) | 46 (36–55) | 42 (22–59) | .10 | .80 | 29 (23–36) | 33 (26–49) | 31 (13–65) | 46 (22–59) | .10 |
| FP ratio | 0.65 (0.40–1.51) | 1.09 (0.48–1.46) | .02 | 0.64 (0.40–1.36) | 0.80 (0.43–1.51) | 0.96 (0.48–1.46) | .03 | .50 | 0.50 (0.40–1.01) | 0.66 (0.54–0.97) | 0.66 (0.42–1.36) | 1.22 (0.58–1.51) | .007 |
Abbreviations: CFT, central foveal thickness; CME, cystoid macular edema; FP, foveal-to-parafoveal; INL, inner nuclear layer; IRL, inner retinal layers; PRL, photoreceptor layer; ROP, retinopathy of prematurity.
Determined by use of the Wilcoxon rank sum test.
We performed a broad search among systemic factors hypothesized to be related to CME. For the large number of factors, this exploratory study was not powered to identify weak associations (Table 5). The presence or absence of CME did not vary with any of the prematurity or systemic health factors assessed in Table 5. After applying a Bonferroni correction, we found that only 1 systemic variable appeared associated with a CME measure; bronchopulmonary dysplasia was associated with PRL thickness in eyes with CME (P ≤ .001) (Table 5).
Table 5.
Relationship Between Neonatal Systemic Health Factors and CMEa
| Factor | Presence of CME in Study Eye
|
P Value for Retinal Measurement in Study Eyeb
|
|||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | CFT | IRL Thickness | INL Thickness | PRL Thickness | FP Thickness Ratio | |
| Prematurity factors | |||||||
| Gestational age | 0.68 (0.47–0.98) | .04 | .014 | .03 | .15 | .06 | .005 |
| Birth weight | 1.00 | .72 | .37 | .51 | .76 | .41 | .30 |
| Demographic factors | |||||||
| Male vs female | 1.79 (0.52–6.11) | .35 | .08 | .16 | .18 | .03 | .08 |
| White vs African American | 1.12 (0.32–3.99) | .83 | .60 | .74 | .65 | .69 | .61 |
| Systemic factors | |||||||
| Apgar score of 1 | 0.90 (0.69–1.17) | .43 | .14 | .14 | .11 | .86 | .03 |
| Apgar score of 5 | 0.84 (0.58–1.23) | .38 | .35 | .30 | .23 | .88 | .03 |
| Surgery for PDA | 3.80 (0.67–21.60) | .13 | .01 | .03 | .05 | .15 | .02 |
| Culture-proven sepsis | 1.33 (0.17–10.25) | .91 | .35 | .46 | .29 | .08 | .50 |
| Surgery for NEC | 0.63 (0.09–4.23) | .64 | .54 | .88 | .47 | .27 | .53 |
| Presence of IVH | 1.82 (0.52–6.33) | .35 | .08 | .25 | .20 | .013 | .09 |
| Presence of PVL | 4.71 (0.48–46.22) | .18 | .09 | .09 | .14 | .03 | .012 |
| Presence of BPD | 1.97 (0.52–7.49) | .32 | .65 | .81 | .10 | .004 | .33 |
| Presence of hydrocephalus | 1.58 (0.24–10.60) | .64 | .44 | .54 | .60 | .011 | .15 |
Abbreviations: BPD, bronchopulmonary dysplasia; CFT, central foveal thickness; CME, cystoid macular edema; FP, foveal-to-parafoveal; INL, inner nuclear layer; IRL, inner retinal layer; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; OR, odds ratio; PDA, patent ductus arteriosus; PRL, photoreceptor layer; PVL, periventricular leukomalacia.
P ≤ .003 is considered statistically significant.
Continuous CME outcome variables.
Of the 21 subjects with CME, 7 had previous SD-OCT examinations free of CME. Cystoid macular edema did not resolve in any subject prior to 36 weeks’ PMA. Although our study was not designed for long-term follow-up, we observed the resolution of CME in 9 of 17 neonates who were examined after 37 weeks’ PMA. The earliest age of resolution of CME was at 36 weeks’ PMA, and the oldest age at which CME persisted was 43 weeks’ PMA (Table 6).
Table 6.
Characteristics of the 21 Subjects With CME at Any Visit Divided by Final Outcome of Laser Treatment
| Subject | Laser Treatment in Study Eye | Retinal Measurementa
|
Age at First Detection of CME, wk | Vascular Outcome in Study Eye | Duration of Plus or Pre-plus, wk | Maximum ROP Stage in Study Eye | ROP Stage When CME First Detected | Systemic Factors | Age of CME Resolution | Age of Persistent CME | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CFT, μm | FP Thickness Ratio | ||||||||||
| 1 | No | 113 | 0.48 | 36 | Pre-plus | 33–36 | 2 | 2 | BPD, sepsis | 47 wk | |
| 2 | No | 138 | 0.57 | 32 | No plus | 2 | 1 | PDA, sepsis | U | ||
| 3 | No | 150 | 1.01 | 34 | No plus | 0 | 0 | N | 39 wk | ||
| 4 | No | 163 | 0.63 | 36 | No plus | 0 | 0 | N | 40 wk | ||
| 5 | No | 166 | 0.82 | 36 | No plus | 2 | 2 | BPD, IVH, PVL | 3 mo | ||
| 6 | No | 173 | 0.75 | 32 | No plus | 2 | 2 | N | 38 wk | ||
| 7 | No | 179 | 0.78 | 36 | Pre-plus | 37–42 | 3 | 2 | BPD, surgery for NEC | 41 wk | |
| 8 | No | 192 | 1.08 | 33 | No plus | 2 | 2 | BPD, PDA | N | 42 wk | |
| 9 | No | 202 | 0.95 | 35 | No plus | 0 | 0 | IVH | 5 mo | ||
| 10 | No | 227 | 0.97 | 31 | No plus | 1 | 1 | U | |||
| 11 | No | 280 | 1.51 | 33 | Pre-plus | 33–38 | 3 | 2 | BPD, IVH, PVL, sepsis | U | |
| 12 | No | 329 | 1.13 | 33 | No plus | 2 | 1 | BPD, IVH, PDA | N | 38 wk | |
| 13 | No | 343 | 1.20 | 34 | No plus | 2 | 2 | N | 38 wk | ||
| 14 | No | 370 | 1.36 | 34 | No plus | 2 | 2 | U | |||
| 15 | Yes | 127 | 0.58 | 35 | Plus | 35–37 | 3 | 3 | IVH | 38 wk | |
| 16 | Yes | 240 | 1.25 | 33 | Plus | 36–39 | 3 | 1 | IVH | 65 wk | |
| 17 | Yes | 294 | 1.22 | 36 | Plus | 35–39 | 3 | 1 | BPD, PVL | 8 mo | |
| 18 | Yes | 309 | 1.23 | 33 | Plus | 33–36 | 3 | 2 | BPD, IVH, PDA, PVL, sepsis | N | 40 wk |
| 19 | Yes | 329 | 1.28 | 34 | Pre-plus | 34–35 | 3 | 3 | IVH, surgery for NEC, PDA | 36 wk | |
| 20 | Yes | 345 | 1.28 | 36 | Plus | 35–38 | 3 | 3 | IVH | 1 y | |
| 21 | Yes | 449 | 1.46 | 33 | Plus | 33–37 | 3 | 2 | IVH, PDA | N | 43 wk |
Abbreviations: BPD, bronchopulmonary dysplasia; CFT, central foveal thickness; CME, cystoid macular edema; FP, foveal-to-parafoveal; INL, inner nuclear layer; IRL, inner retinal layer; IVH, intraventricular hemorrhage; N, CME was not resolved by the week stated in the last column; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; PRL, photoreceptor layer; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity; U, unable to determine age of CME resolution because no follow-up imaging was obtained.
Continuous CME outcome variables.
COMMENT
In premature neonates screened for ROP in a tertiary neonatal intensive care unit, CME was common, did not differ by race, and, when present, was almost always bilateral. Increased severity of CME, identified by greater CFT, IRL thickness, and FP thickness ratio, was associated with subsequent laser treatment, with plus disease, and with higher ROP stage. Although follow-up imaging visits were limited, CME resolved in 9 of 17 subjects imaged after 36 weeks’ PMA, and CME resolved as early as 36 weeks’ PMA in 1 subject and was still present in numerous subjects after 40 weeks’ PMA (Table 6). It is unclear whether CME in premature neonates is pathologic, and the high frequency of this finding might suggest a transient stage of foveal development.
The CME in these neonates, imaged between 31 and 36 weeks’ PMA and before any progression to severe ROP, was not visible during indirect ophthalmoscopy. It is also notable that CME has not been noted by ROP experts in studies that use color fundus photography to assess the premature infant macula.29 Furthermore, Lee et al17 and Vinekar et al26 both found CME on SD-OCT images of neonates undergoing routine ROP screening (Lee et al17 from 31 through 42 weeks and Vinekar et al26 from 35 to 52 weeks) but not with indirect ophthalmoscopy (In Lee et al,17 one case of CME was observed with ophthalmoscopy).
Lee et al17 found CME in 61% of eyes from neonates with a median birth weight of 810 g and median gestational age of 26 weeks’ PMA, whereas Vinekar et al26 reported CME in 15% of eyes from infants with ROP of stage 2 or less, with a weighted birth-weight average of 1255 g, and a weighted gestational-age average of 30.4 weeks’ PMA. Although CME was found across all ROP stages (stages 0, 1, 2, and 3) in Lee et al17 (in neonates with a very early gestational age), in the larger and older infants in Vinekar et al,26 CME was not found in eyes with ROP stage 0 or 1. Although this might be due to a different study design and study population, it also suggests that CME may relate to the severity of ROP or the developmental stage of the retina.
In adults, CME is a pathologic entity associated with a common mechanism of alteration of the blood-retinal barrier in diseases such as diabetic retinopathy and vascular occlusions.30 The pigment epithelium derived–factor level and the VEGF level have also been reported to play a role in the pathogenesis of adult CME.31,32 In our study, the severity of CME was associated with the severity of ROP and was prominent between 31 and 36 weeks’ PMA in extremely low-birth-weight infants. This coincides with the timing of ROP phase II, when VEGF activity increases and insulinlike growth factor 1 levels decrease.24 We hypothesize that CME is a manifestation of the retinal effects of these neurohumoral factors23,24 and that cellular metabolic stress from the rapid development of the foveal cones during this time frame may also further contribute to the CME.18,33 Cystoid macular edema in premature neonates may also reflect the contribution of increased intracapillary hydrostatic pressure as a result vascular congestion from plus disease. Hence, it would be interesting to view SD-OCT images of the infant macula before and after anti-VEGF therapy for ROP. We would predict resolution of VEGF-driven edema after such treatment.
In contrast to the cases of CME found in adults, in which round and elongated cystoid structures coexist and reside in multiple retinal layers,34 the cases of CME found in infants had cystoid structures confined exclusively to the INL with multiple elongated cystoid structures across the central macula (Figure 3). In adults with CME, there is evidence for both extracellular fluid accumulation and intracellular swelling of Muller cells,35 whereas, in infants with CME, the underlying pathophysiology may predominantly be the swelling of Muller cells or perhaps the accumulation of extracellular fluid bridged by the Muller cells.
The effect of CME on foveal development, active at this period of time, is of interest. Older children and adults with a history of ROP show persisting IRLs at the foveal center on OCT scans. This may be related to prematurity, or, noting the relationship between increased IRL thickness and CME (Table 3), we feel that it might be influenced by the CME.36 In addition, between 31 and 36 weeks’ PMA, during normal foveal development, the PRL should be thinnest at the foveal center,18 representing the cone monolayer,33 but in our study, 28% of subjects with CME presented with an elevated, “spiky” PRL at the foveal center, which could represent some type of photoreceptor swelling or traction. Because the integrity of the PRL is associated with good visual outcomes in adults with CME,37 the bulging PRL (Figure 3) is a concern for possible chronic photoreceptor alteration, despite a return to normal morphology on later SD-OCT imaging. Future studies are needed to evaluate visual function in children with and without a history of CME after premature birth.
Greater CME severity was associated with laser treatment, plus disease, and maximum ROP stage (Table 4), and, to our knowledge, this has not been shown in previous studies. The association between CFT and ROP stage has been shown in studies involving adults with a history of ROP36,38 and premature infants at a slightly older age.26 It is striking to note that this association is established prior to laser treatment and before 37 weeks’ PMA and thus would not likely be secondary to treatment. Early SD-OCT imaging may be an important tool that could contribute to an ROP evaluation and may provide an early indicator of the mature foveal morphology. Although we did not find an association between the presence of CME and laser treatment or between the presence of CME and the presence of plus disease (Table 1), because of the low frequency of laser treatments in the available study population, our study was not well suited for assessing a relationship between presence of CME and outcome of laser treatment. Our findings may also be affected by the different CME phenotypes found in our study; for instance, a single central cystoid structure might not represent the same event as prominent CME with a bulging fovea (Figure 3).
The limitations of this research in a neonatal intensive care unit setting included difficulty in contacting parents to obtain consent and the fact that SD-OCT imaging was only permitted in parallel with an ROP examination and the early transfer of subjects to other institutions. Thus, subjects could not be imaged at regular intervals and could have undetected CME or CME that was not monitored to resolution. We did not obtain blood samples and could not obtain ocular fluid samples to test for systemic or ocular factors that might explain the processes behind the edema. Furthermore, we did not pursue fluorescein angiography in this vulnerable population, although it could have added to our understanding of the vascular status and source of fluid in the macula.
To our knowledge, this study is the first attempt to investigate the association between transient CME in infants and ROP severity, with the aim of motivating further research in the area. We have explored CME findings at an age of prematurity when intensive metabolic, humoral, and vascular changes occur, with the intent to test the predictive value of SD-OCT imaging at this stage of ocular development. A multicenter study of SD-OCT imaging outside a single nursery is necessary to test our findings. For these subjects, subsequent visual testing studies should be done later in life to understand the effect of macular edema on vision at this important stage of visual development.
Acknowledgments
Funding/Support: This research was made possible by the grants from Angelica and Euan Baird, from The Hartwell Foundation, from Research to Prevent Blindness, from the National Center for Research Resources (grant 1UL1 RR024128-01), a component of the National Institutes of Health (NIH), and from the NIH Road-map for Medical Research.
Footnotes
Author Contributions: Dr Toth had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the opinions of the National Center for Research Resources or the NIH.
Financial Disclosure: Dr Toth receives research support through Duke University from Alcon Laboratories, Genentech, the National Institutes of Health, and the North Carolina Biotechnology Center; she is a consultant for Alcon Laboratories and Genentech; and she receives royalties for ophthalmic surgical technologies through a Duke University agreement with Alcon Laboratories. Drs Toth and Farsiu have a patent pending on OCT image-processing techniques.
References
- 1.Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J AAPOS. 1999;3(1):26–32. doi: 10.1016/s1091-8531(99)70091-1. [DOI] [PubMed] [Google Scholar]
- 2.Good WV, Hardy RJ, Dobson V, et al. Early Treatment for Retinopathy of Prematurity Cooperative Group. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2010;128(6):663–671. doi: 10.1001/archophthalmol.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123(7):991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 4.Fierson WM, Palmer EA, Biglan AW, Flynn JT, Petersen RA, Phelps DL. Retinopathy of prematurity guidelines. Pediatrics. 1998;101(6):1093. doi: 10.1542/peds.101.6.1093. [DOI] [PubMed] [Google Scholar]
- 5.Phelps DL. It’s plus disease, isn’t it? Arch Ophthalmol. 2007;125(7):963–964. doi: 10.1001/archopht.125.7.963. [DOI] [PubMed] [Google Scholar]
- 6.Wallace DK, Quinn GE, Freedman SF, Chiang MF. Agreement among pediatric ophthalmologists in diagnosing plus and pre-plus disease in retinopathy of prematurity. J AAPOS. 2008;12(4):352–356. doi: 10.1016/j.jaapos.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang MF, Jiang L, Gelman R, Du YE, Flynn JT. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol. 2007;125 (7):875–880. doi: 10.1001/archopht.125.7.875. [DOI] [PubMed] [Google Scholar]
- 8.Lorenz B, Spasovska K, Elflein H, Schneider N. Wide-field digital imaging based telemedicine for screening for acute retinopathy of prematurity (ROP): six-year results of a multicentre field study. Graefes Arch Clin Exp Ophthalmol. 2009;247(9):1251–1262. doi: 10.1007/s00417-009-1077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace DK, Jomier J, Aylward SR, Landers MB., III Computer-automated quantification of plus disease in retinopathy of prematurity. J AAPOS. 2003;7(2):126–130. doi: 10.1016/mpa.2003.S1091853102000150. [DOI] [PubMed] [Google Scholar]
- 10.Cabrera MT, Freedman SF, Kiely AE, Chiang MF, Wallace DK. Combining ROP-tool measurements of vascular tortuosity and width to quantify plus disease in retinopathy of prematurity. J AAPOS. 2011;15(1):40–44. doi: 10.1016/j.jaapos.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace DK, Freedman SF, Zhao Z, Jung SH. Accuracy of ROPtool vs individual examiners in assessing retinal vascular tortuosity. Arch Ophthalmol. 2007;125(11):1523–1530. doi: 10.1001/archopht.125.11.1523. [DOI] [PubMed] [Google Scholar]
- 12.Chiang MF, Gelman R, Martinez-Perez ME, et al. Image analysis for retinopathy of prematurity diagnosis. J AAPOS. 2009;13(5):438–445. doi: 10.1016/j.jaapos.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116(12):2448–2456. doi: 10.1016/j.ophtha.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinekar A, Sivakumar M, Shetty R, et al. A novel technique using spectral-domain optical coherence tomography (Spectralis, SD-OCT+HRA) to image supine non-anaesthetized infants: utility demonstrated in aggressive posterior retinopathy of prematurity. Eye (Lond) 2010;24(2):379–382. doi: 10.1038/eye.2009.313. [DOI] [PubMed] [Google Scholar]
- 15.Muni RH, Kohly RP, Charonis AC, Lee TC. Retinoschisis detected with handheld spectral-domain optical coherence tomography in neonates with advanced retinopathy of prematurity. Arch Ophthalmol. 2010;128(1):57–62. doi: 10.1001/archophthalmol.2009.361. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado RS, Izatt JA, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci. 2010;51(5):2678–2685. doi: 10.1167/iovs.09-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AC, Maldonado RS, Sarin N, et al. Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina. 2011;31(8):1470–1482. doi: 10.1097/IAE.0b013e31821dfa6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maldonado RS, O’Connell RV, Sarin N, et al. Dynamics of human foveal development after premature birth [published online ahead of print September 20, 2011] Ophthalmology. doi: 10.1016/j.ophtha.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akkoyun I, Oto S, Yilmaz G, et al. Risk factors in the development of mild and severe retinopathy of prematurity. J AAPOS. 2006;10(5):449–453. doi: 10.1016/j.jaapos.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Kinsey VE, Arnold HJ, Kalina RE, et al. PaO2 levels and retrolental fibroplasia: a report of the cooperative study. Pediatrics. 1977;60(5):655–668. [PubMed] [Google Scholar]
- 21.Wallace DK, Kylstra JA, Phillips SJ, Hall JG. Poor postnatal weight gain: a risk factor for severe retinopathy of prematurity. J AAPOS. 2000;4(6):343–347. doi: 10.1067/mpa.2000.110342. [DOI] [PubMed] [Google Scholar]
- 22.Blanco CL, Baillargeon JG, Morrison RL, Gong AK. Hyperglycemia in extremely low birth weight infants in a predominantly Hispanic population and related morbidities. J Perinatol. 2006;26(12):737–741. doi: 10.1038/sj.jp.7211594. [DOI] [PubMed] [Google Scholar]
- 23.Hartnett ME. Studies on the pathogenesis of avascular retina and neovascularization into the vitreous in peripheral severe retinopathy of prematurity (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2010;108:96–119. [PMC free article] [PubMed] [Google Scholar]
- 24.Smith LE. Pathogenesis of retinopathy of prematurity. Semin Neonatol. 2003;8 (6):469–473. doi: 10.1016/S1084-2756(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 25.Löfqvist C, Andersson E, Sigurdsson J, et al. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol. 2006;124(12):1711–1718. doi: 10.1001/archopht.124.12.1711. [DOI] [PubMed] [Google Scholar]
- 26.Vinekar A, Avadhani K, Sivakumar M, et al. Understanding clinically undetected macular changes in early retinopathy of prematurity on spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):5183–5188. doi: 10.1167/iovs.10-7155. [DOI] [PubMed] [Google Scholar]
- 27.Austeng D, Källen KB, Hellström A, Tornqvist K, Holmström GE. Natural history of retinopathy of prematurity in infants born before 27 weeks’ gestation in Sweden. Arch Ophthalmol. 2010;128(10):1289–1294. doi: 10.1001/archophthalmol.2010.234. [DOI] [PubMed] [Google Scholar]
- 28.Palmer EA, Flynn JT, Hardy RJ, et al. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Incidence and early course of retinopathy of prematurity. Ophthalmology. 1991;98(11):1628–1640. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 29.Chiang MF, Thyparampil PJ, Rabinowitz D. Interexpert agreement in the identification of macular location in infants at risk for retinopathy of prematurity. Arch Ophthalmol. 2010;128(9):1153–1159. doi: 10.1001/archophthalmol.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bringmann A, Reichenbach A, Wiedemann P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004;36(5):241–249. doi: 10.1159/000081203. [DOI] [PubMed] [Google Scholar]
- 31.Noma H, Funatsu H, Mimura T, Eguchi S, Shimada K, Hori S. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Curr Eye Res. 2011;36(3):256–263. doi: 10.3109/02713683.2010.513090. [DOI] [PubMed] [Google Scholar]
- 32.Ishida S, Usui T, Yamashiro K, et al. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;44(5):2155–2162. doi: 10.1167/iovs.02-0807. [DOI] [PubMed] [Google Scholar]
- 33.Hendrickson AE, Yuodelis C. The morphological development of the human fovea. Ophthalmology. 1984;91(6):603–612. doi: 10.1016/s0161-6420(84)34247-6. [DOI] [PubMed] [Google Scholar]
- 34.Hee MR, Puliafito CA, Wong C, et al. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol. 1995;113(8):1019–1029. doi: 10.1001/archopht.1995.01100080071031. [DOI] [PubMed] [Google Scholar]
- 35.Yanoff M, Fine BS, Brucker AJ, Eagle RC., Jr Pathology of human cystoid macular edema. Surv Ophthalmol. 1984;28(suppl):505–511. doi: 10.1016/0039-6257(84)90233-9. [DOI] [PubMed] [Google Scholar]
- 36.Recchia FM, Recchia CC. Foveal dysplasia evident by optical coherence tomography in patients with a history of retinopathy of prematurity. Retina. 2007;27(9):1221–1226. doi: 10.1097/IAE.0b013e318068de2e. [DOI] [PubMed] [Google Scholar]
- 37.Ota M, Tsujikawa A, Murakami T, et al. Foveal photoreceptor layer in eyes with persistent cystoid macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2008;145(2):273–280. doi: 10.1016/j.ajo.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Hammer DX, Iftimia NV, Ferguson RD, et al. Foveal fine structure in retinopathy of prematurity: an adaptive optics Fourier domain optical coherence tomography study. Invest Ophthalmol Vis Sci. 2008;49(5):2061–2070. doi: 10.1167/iovs.07-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]