Abstract
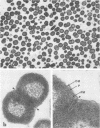
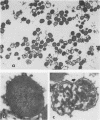
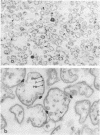
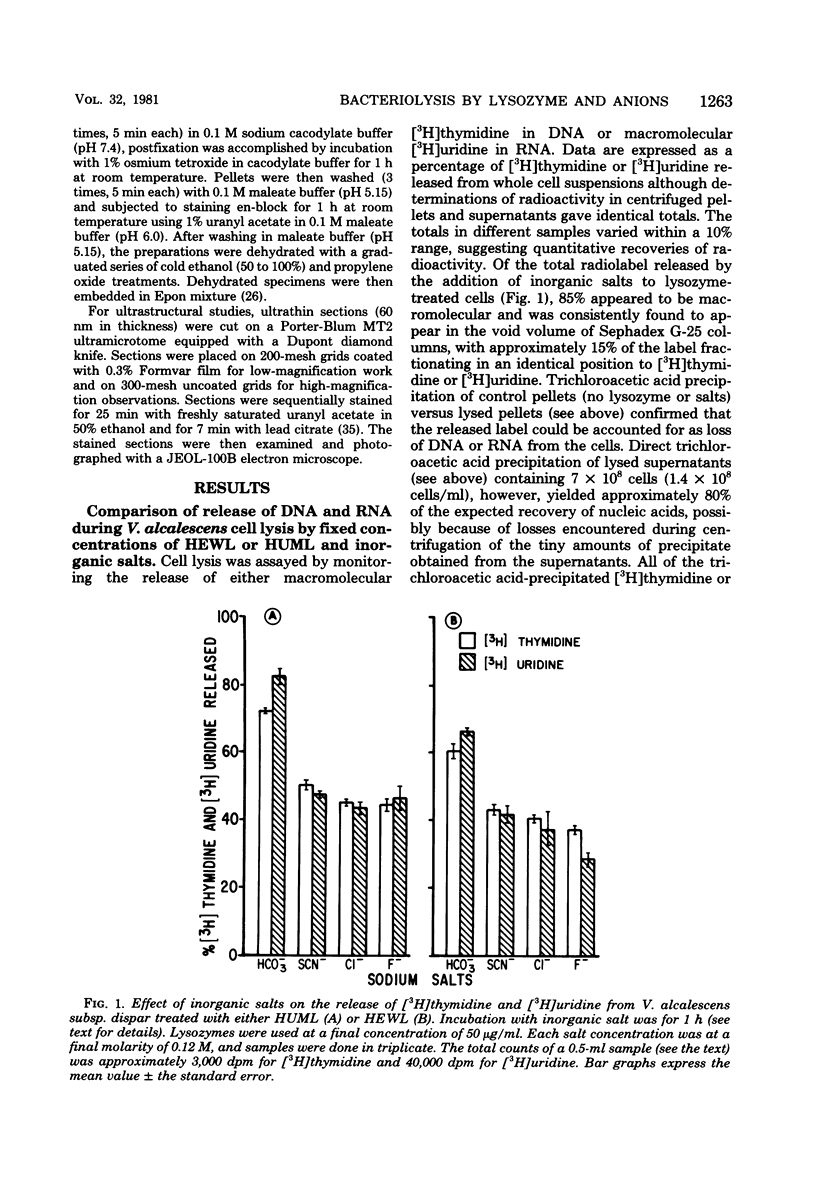
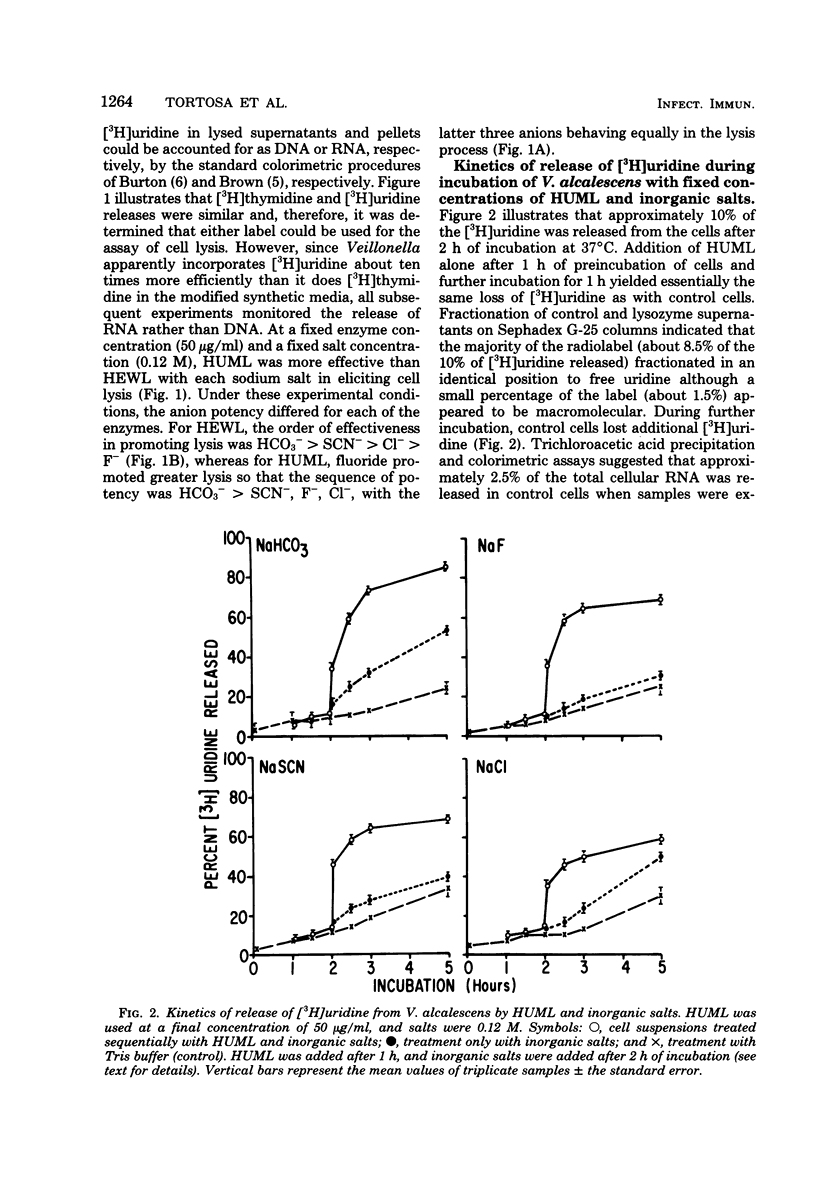
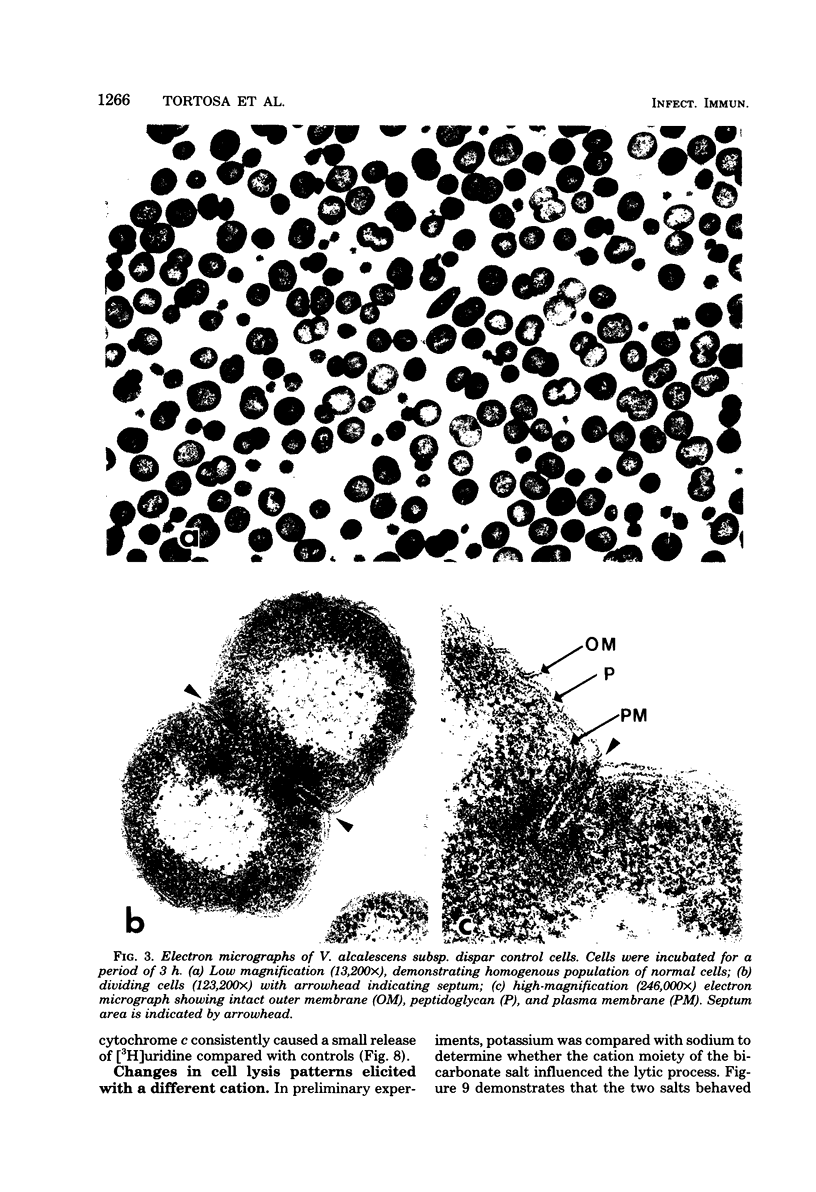
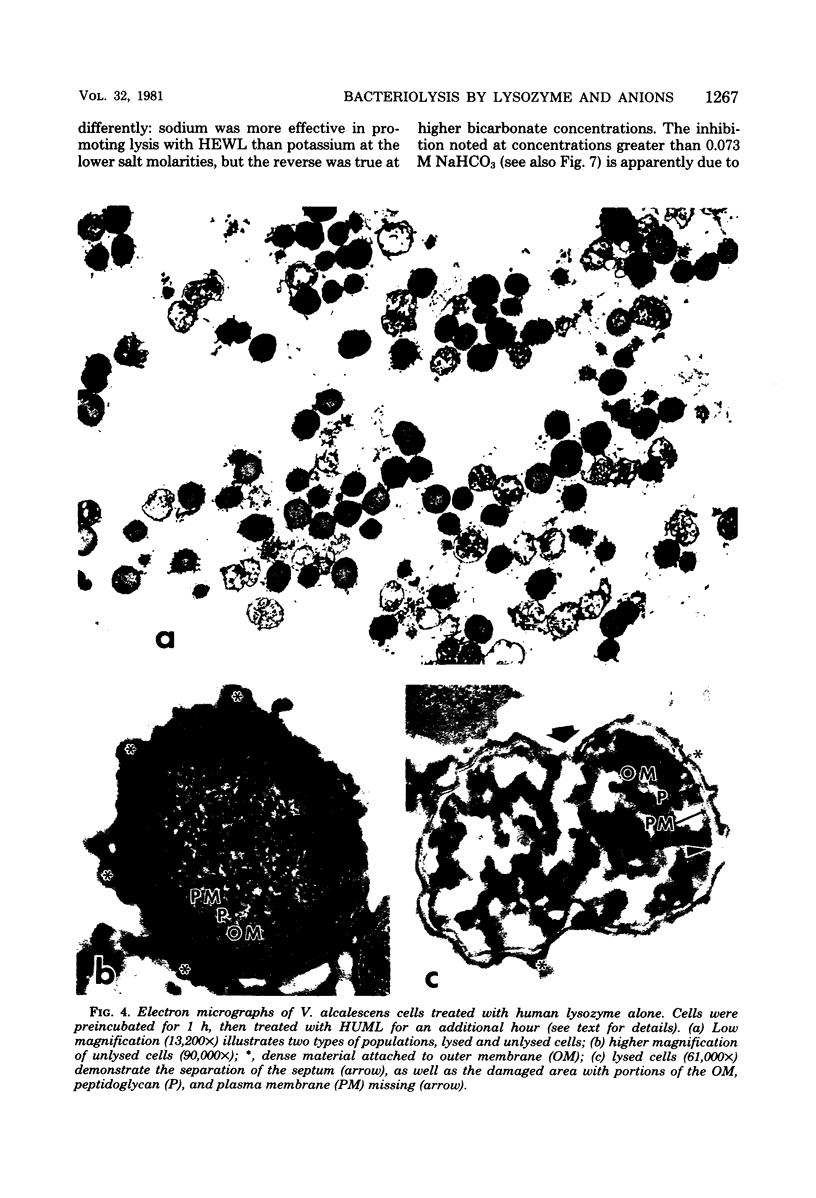
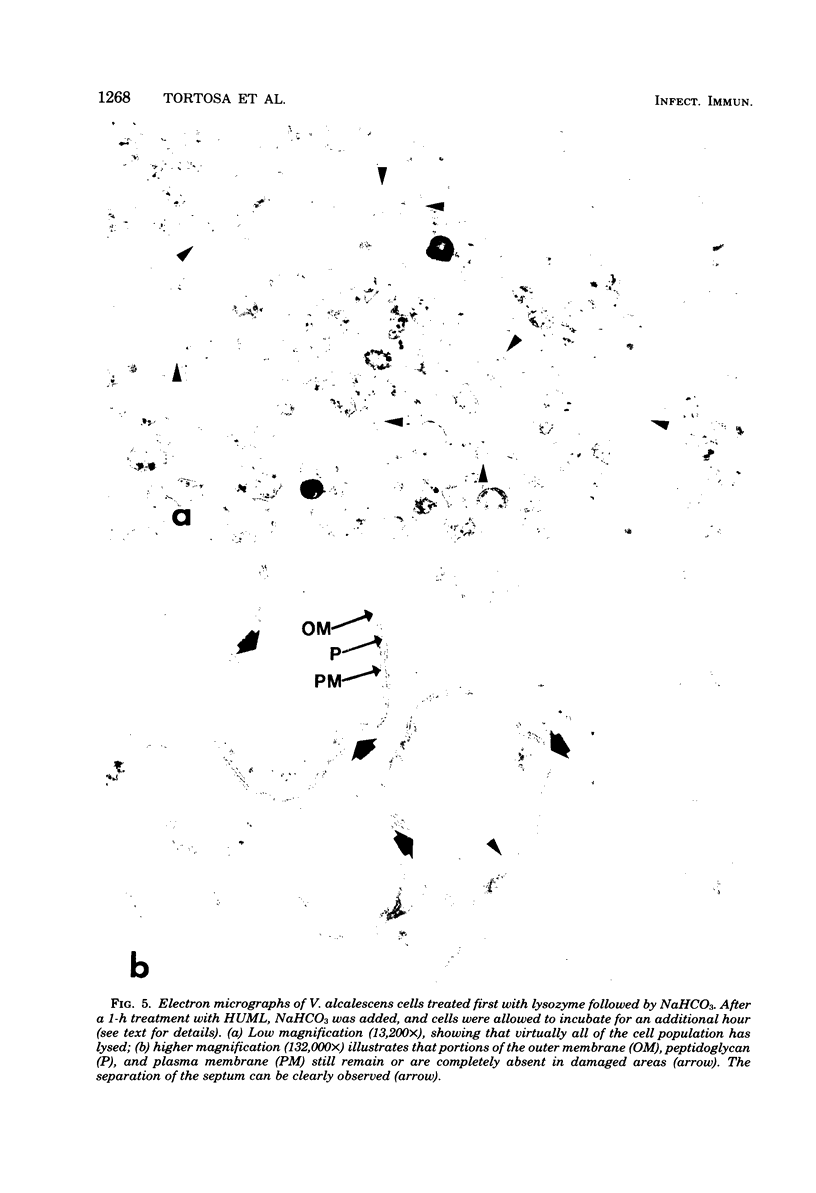
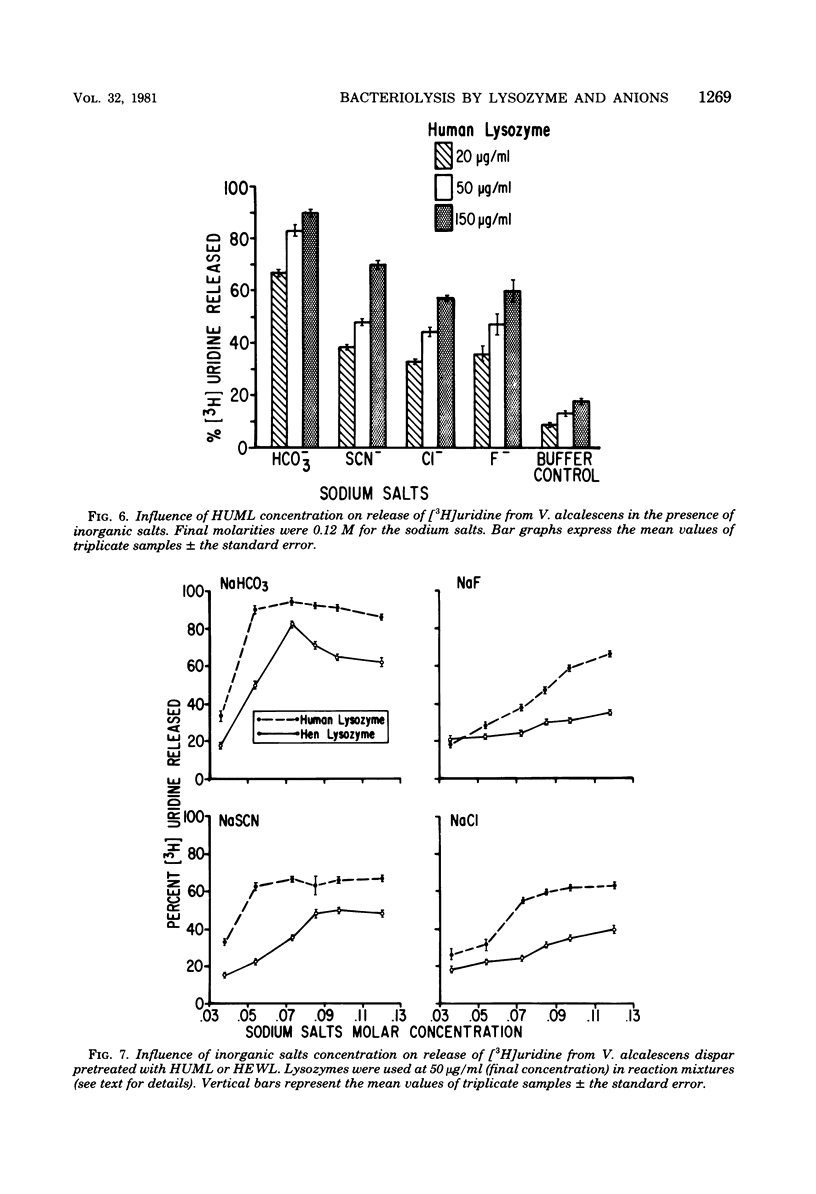
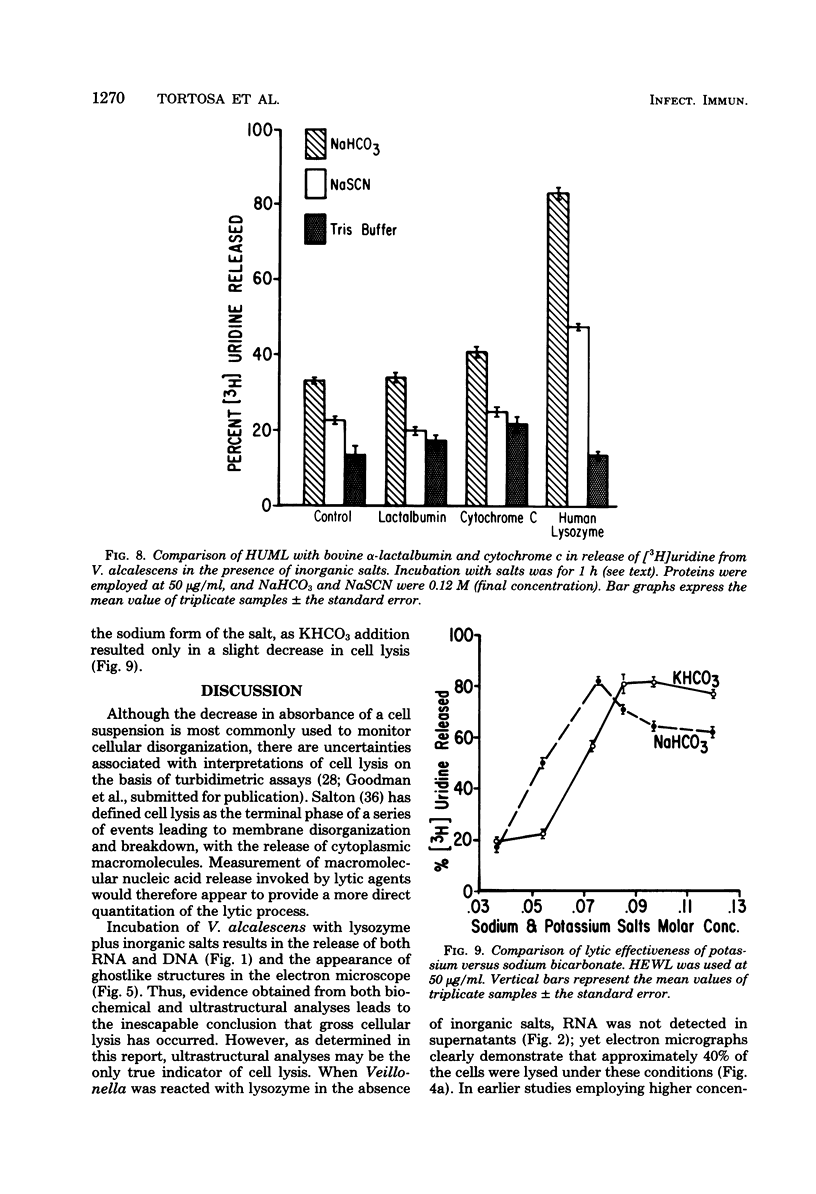
Veillonella alcalescens subsp. dispar was grown in a synthetic medium containing either radiolabeled thymidine or uridine to monitor cell lysis by assay of the release of deoxyribonucleic acid or ribonucleic acid (RNA), respectively. Biochemical analyses demonstrated that, although human or hen egg white lysozymes alone did not release deoxyribonucleic acid or RNA, the nucleic acids were liberated in equal amounts from lysozyme-treated cells by the addition of low concentrations of the sodium salts of HCO-3, SCN-, Cl-, and F-, RNA release was dependent on enzyme and anion concentration. Human lysozyme was more potent than hen egg white lysozyme, and bicarbonate was the most effective anion in promoting bacteriolysis. Surprisingly, ultrastructural analyses differed from biochemical results. Lysozyme alone caused lysis in approximately 40% of the cell population. Detailed ultrastructural examination revealed aggregated cytoplasmic components which appeared as small clumps, explaining why nucleic acids were not measurable in the biochemical assays. In reaction mixtures containing lysozyme plus inorganic salts, electron microscopy results were compatible with biochemical data. Ultrastructural studies demonstrated that the addition of inorganic salts to lysozyme-treated cells resulted in the solubilization of the protoplasmic aggregates of lysed cells, presumably freeing the complexed RNA, and in the rapid lysis of the remaining cells (approximately 60%). These data suggest that electron microscopy must be used in conjunction with biochemical assays to assess lytic damage of bacterial cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLADEN H. A., MERGENHAGEN S. E. ULTRASTRUCTURE OF VEILLONELLA AND MORPHOLOGICAL CORRELATION OF AN OUTER MEMBRANE WITH PARTICLES ASSOCIATED WITH ENDOTOXIC ACTIVITY. J Bacteriol. 1964 Nov;88:1482–1492. doi: 10.1128/jb.88.5.1482-1492.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Gewurz H., Mergenhagen S. E. Interactions of the complement system with the surface and endotoxic lipopolysaccharide of Veillonella alcalescens. J Exp Med. 1967 May 1;125(5):767–786. doi: 10.1084/jem.125.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H., Hageage G., Harr R., Pollock F. Lysis of certain organisms by the synergistic action of complement and lysozyme. J Dent Res. 1973 Mar-Apr;52(2):371–376. doi: 10.1177/00220345730520023101. [DOI] [PubMed] [Google Scholar]
- Brew K., Vanaman T. C., Hill R. L. Comparison of the amino acid sequence of bovine alpha-lactalbumin and hens egg white lysozyme. J Biol Chem. 1967 Aug 25;242(16):3747–3749. [PubMed] [Google Scholar]
- Chopra I., Howe G. B., Ball P. R. Lysozyme-promoted association of protein I molecules in the outer membrane of Escherichia coli. J Bacteriol. 1977 Nov;132(2):411–418. doi: 10.1128/jb.132.2.411-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. F., Marceau-Day M. L., Ingram J. M. Protein-lipopolysaccharide interactions. 1. The reaction of lysozyme with Pseudomonas aeruginosa LPS. Can J Microbiol. 1978 Feb;24(2):196–199. doi: 10.1139/m78-035. [DOI] [PubMed] [Google Scholar]
- GRULA E. A., HARTSELL S. E. Lysozyme in the bacteriolysis of gram-negative bacteria. I. Morphological changes during use of Nakamura's technique. Can J Microbiol. 1957 Feb;3(1):13–21. doi: 10.1139/m57-002. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., de Stoppelaar J. D., Harden L. Lysozyme insensitivity of bacteria indigenous to the oral cavity of man. J Dent Res. 1966 May-Jun;45(3):877–881. doi: 10.1177/00220345660450036201. [DOI] [PubMed] [Google Scholar]
- Goodell E. W., Lopez R., Tomasz A. Suppression of lytic effect of beta lactams on Escherichia coli and other bacteria. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3293–3297. doi: 10.1073/pnas.73.9.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie J. M., Bowden G. H. The normal microbial flora of the mouth. Soc Appl Bacteriol Symp Ser. 1974;3(0):47–83. [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Mechanism of autolysis of Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1186–1193. doi: 10.1128/jb.126.3.1186-1193.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono V. J., MacKay B. J., DiRienzo S., Pollock J. J. Selective antibacterial properties of lysozyme for oral microorganisms. Infect Immun. 1980 Aug;29(2):623–632. doi: 10.1128/iai.29.2.623-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium. Identification of proteins exposed on cell surface. Biochim Biophys Acta. 1977 Feb 4;464(3):589–601. doi: 10.1016/0005-2736(77)90033-5. [DOI] [PubMed] [Google Scholar]
- Kilian M., Thylstrup A., Fejerskov O. Predominant plaque flora of Tanzanian children exposed to high and low water fluoride concentrations. Caries Res. 1979;13(6):330–343. doi: 10.1159/000260423. [DOI] [PubMed] [Google Scholar]
- Kitano K., Tomasz A. Triggering of autolytic cell wall degradation in Escherichia coli by beta-lactam antibiotics. Antimicrob Agents Chemother. 1979 Dec;16(6):838–848. doi: 10.1128/aac.16.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu S., Ikeda K., Hamaguchi K., Miwa S., Nakashima K. Binding of N-acetyl-chitotriose to human lysozyme. J Biochem. 1975 Aug;78(2):327–333. doi: 10.1093/oxfordjournals.jbchem.a130911. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Ability of Veillonella and Neisseria species to attach to oral surfaces and their proportions present indigenously. Infect Immun. 1971 Sep;4(3):264–268. doi: 10.1128/iai.4.3.264-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Hockett R. N., Syed S. A. The predominant cultivable flora of tooth surface plaque removed from institutionalized subjects. Arch Oral Biol. 1972 Sep;17(9):1311–1325. doi: 10.1016/0003-9969(72)90164-1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A. The predominant cultivable flora of carious plaque and carious dentine. Caries Res. 1973;7(3):201–216. doi: 10.1159/000259844. [DOI] [PubMed] [Google Scholar]
- Mandel I. D. Dental caries. Am Sci. 1979 Nov-Dec;67(6):680–688. [PubMed] [Google Scholar]
- Martinez R. J., Carroll S. F. Sequential metabolic expressions of the lethal process in human serum-treated Escherichia coli: role of lysozyme. Infect Immun. 1980 Jun;28(3):735–745. doi: 10.1128/iai.28.3.735-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu L., Caron F., Luzzati V., Billecocq A. The influence of protein-lipid interactions on the order-disorder conformational transitions of the hydrocarbon chain. Biochim Biophys Acta. 1978 Mar 21;508(1):109–121. doi: 10.1016/0005-2736(78)90192-x. [DOI] [PubMed] [Google Scholar]
- Mikx F. H., van der Hoeven J. S., König K. G., Plasschaert A. J., Guggenheim B. Establishment of defined microbial ecosystems in germ-free rats. I. The effect of the interactions of streptococcus mutans or Streptococcus sanguis with Veillonella alcalescens on plaque formation and caries activity. Caries Res. 1972;6(3):211–223. doi: 10.1159/000259801. [DOI] [PubMed] [Google Scholar]
- NOLLER E. C., HARTSELL S. E. Bacteriolysis of Enterobacteriaceae. II. Pre- and co-lytic treatments potentiating the action of lysozyme. J Bacteriol. 1961 Mar;81:492–499. doi: 10.1128/jb.81.3.492-499.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER R. B., SNYDER M. L. Interactions of the oral microbiota. I. A system for the defined study of mixed cultures. Proc Soc Exp Biol Med. 1961 Dec;108:749–752. doi: 10.3181/00379727-108-27055. [DOI] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scibienski R. J. Cellular parameters of the immunological memory induced by lysozyme-LPS mixtures and complexes. Immunol Commun. 1979;8(3):325–336. doi: 10.3109/08820137909050046. [DOI] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Bacteriology of human experimental gingivitis: effect of plaque age. Infect Immun. 1978 Sep;21(3):821–829. doi: 10.1128/iai.21.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLAW A. C. The complement-dependent bacteriolytic activity of normal human serum. I. The effect of pH and ionic strength and the role of lysozyme. J Exp Med. 1962 Jun 1;115:1231–1249. doi: 10.1084/jem.115.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright P., Power D. M., Thomas E. W., Davies J. V. The interaction of cytochrome C with phospholipids. A pulse-radiolysis study. Int J Radiat Biol Relat Stud Phys Chem Med. 1978 Feb;33(2):151–162. doi: 10.1080/09553007814550041. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M. The effect of osmotic shock on the accessibility of the murein layer of exponentially growing Escherichia coli to lysozyme. Biochim Biophys Acta. 1978 Apr 4;508(2):296–305. doi: 10.1016/0005-2736(78)90332-2. [DOI] [PubMed] [Google Scholar]
- Witholt B., Heerikhuizen H. V., De Leij L. How does lysozyme penetrate through the bacterial outer membrane? Biochim Biophys Acta. 1976 Sep 7;443(3):534–544. doi: 10.1016/0005-2736(76)90471-5. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]
- van der Hoeven J. S., Toorop A. I., Mikx R. H. Symbiotic relationship of Veillonella alcalescens and Streptococcus mutans in dental plaque in gnotobiotic rats. Caries Res. 1978;12(3):142–147. doi: 10.1159/000260324. [DOI] [PubMed] [Google Scholar]