Abstract
For the first time, the phytopathogenicity of extracellular vesicles of Acholeplasma laidlawii PG8 (a ubiquitous mycoplasma that is one of the five common species of cell culture contaminants and is a causative agent for phytomycoplasmoses) in Oryza sativa L. plants was studied. Data on the ability of extracellular vesicles of Acholeplasma laidlawii PG8 to penetrate from the nutrient medium into overground parts of Oryza sativa L. through the root system and to cause alterations in ultrastructural organization of the plants were presented. As a result of the analysis of ultrathin leaf sections of plants grown in medium with A. laidlawii PG8 vesicles, we detected significant changes in tissue ultrastructure characteristic to oxidative stress in plants as well as their cultivation along with bacterial cells. The presence of nucleotide sequences of some mycoplasma genes within extracellular vesicles of Acholeplasma laidlawii PG8 allowed a possibility to use PCR (with the following sequencing) to perform differential detection of cells and bacterial vesicles in samples under study. The obtained data may suggest the ability of extracellular vesicles of the mycoplasma to display in plants the features of infection from the viewpoint of virulence criteria—invasivity, infectivity—and toxigenicity—and to favor to bacterial phytopathogenicity.
1. Introduction
Acholeplasma laidlawii is a unique mycoplasma species (class Mollicutes) from the viewpoint of its adaptation abilities. This bacterium is widely distributed in nature and is one of the five common species of cell culture contaminants and is a causative agent for phytomycoplasmoses [1–3]. Control of mycoplasma infections presents a serious problem whose resolution is connected with clarification of mechanisms of mycoplasma adaptation to the environment, formation of the “parasite-host” system, and realization of virulence. [2, 4]. Sequencing of A. laidlawii genome [5] provided an opportunity for application of postgenomic technologies for detection of molecular-genetic fundamentals of mycoplasma survival in different environments [2]. As a result of proteomic-transcriptomic analysis and nanoscopy studies, stress-reactive proteins and genes of A. laidlawii PG8 were identified, and it was presented that adaptation of the mycoplasma to unfavorable condition was connected with secretion of extracellular membrane vesicles (EMVs) [2]. It was found that EMVs of A. laidlawii, apart from a membrane, contain nucleotide sequences of some genes and display a high mutagenicity toward cells of higher eukaryotes [6]. It is known that EMVs mediate secretion in bacteria, participate in signaling, intercellular interactions, and pathogenesis [7]. It was suggested that EMVs may make a significant input into phytopathogenicity of bacteria [8–10] including mycoplasmas [2]. However, data on phytopathogenicity of bacterial EMVs are absent in the literature. Therefore, investigation of EMVs phytopathogenicity from the viewpoint of virulence criteria—invasivity, infectivity and toxigenicity—toward plants of Oryza sativa L. was the aim of the present study.
2. Materials and Methods
A. laidlawii PG8 strain obtained from the N.F. Gamaleya Institute of Epidemiology and Microbiology (Moscow) was used in this work. The mycoplasma cells were grown in a liquid modified Edward's medium (tryptose, 2% [w/v]; NaCl, 0.5% [w/v]; KCl, 0.13% [w/v]; Tris base, 0.3% [w/v]; horse serum, 10% [w/v]; yeast extract, 5% [w/v]; glucose solution, 1% [w/v]; benzylpenicillin [500,000 IE/mL], 0.2% [w/v]).
Isolation of membrane vesicles from A. laidlawii PG8 culture was performed according to Kolling and Matthews [11], with some modifications taking into account features of cell biology and cultivation of mycoplasmas [4].
Rice seeds (O. sativa L. breed “Lougovoy”) were sterilized with 0.01% solution of KMnO4 for 30 min and then washed extensively with distilled water. The plants were grown under sterile conditions [12] at 27°C (12 h : 12 h light : dark photoperiod and a light intensity 6 klux). Rice plants were infected with A. laidlawii PG8 cells and EMVs under sterile conditions as described by Chernov et al. [2] using a spontaneous infection of 10-day plant seedlings through the root system. Plant roots were incubated continuously in Murashige and Skoog medium containing cells or EMVs of A. laidlawii PG8. Control plants were incubated in the mycoplasma-free medium. Analysis of the samples was performed since 2 h to 9 days later.
Transmission electron microscopy (TEM) was done with a JEM-1200EX microscope (Japan) according to Chernov et al. [2].
To prepare samples for atomic force microscopy (AFM) studies, EMVs of A. laidlawii PG8 were placed onto the mica (Advanced Technologies Center, Moscow, Russia) with the upper layer removed. EMVs were air dried and then rinsed twice with redistilled water, and after each rinsing, the samples were air dried in both instances. AFM imaging was performed with a Solver P47H atomic force microscope (NT-MDT, Moscow, Russia) operating in the tapping mode using fpN11S cantilevers (r ≤ 10 nm, Advanced Technologies Center, Moscow, Russia). The height, Mag (signal from lock-in amplifier), RMS (signal from RMS detector), and phase (signal from the phase detector) were performed with the Nova 1.0.26 RC1 software (NT-MDT). The scan rate was 1 Hz. Image resolution was 512 × 512.
DNAs from mycoplasma cells and plant tissues were isolated according to [13]. DNA from EMVs was isolated using commercial kit “DNA-express” (“Litekh”, Moscow). Before the extraction of nucleic acids, samples of EMVs of the mycoplasma were treated with DNAse I (at 37°C for 30 min).
PCR primers were constructed in NSF “Litekh” (Moscow, Russia) using the nucleotide sequences of A. laidlawii PG8-A genes (GenBank accession number NC_010163): ftsZ (Ala1F 5′-ggtttttggatttaacgatg-3′ Ala1R 5′-gcttccgcctcttttattt-3′), pdhC (Ala9F 5′-aaagcaagaccataaggagg-3′ Ala9R 5′-tggagcctgtgtttgttga-3′), pnp (Aq1F 5′-aagcccattgcgatacctgc-3′ Aq1R 5′-ggtgctttaggagaacgtgct-3′), tufB (Aq3F 5′-ccaggtcacgctgactatgtt-3′ Aq3R 5′-acgagtttgtggcattggac-3′), rpoB (Aq6F 5′-tggcatatcttctcttggtaaa-3′ Aq6R 5′-tggcatatcttctcttggtaaa-3′), spacer 16S–23S of ribosome operon (A16LF 5′-ggaggaaggtggggatgacgtcaa-3′ A23LR 5′-ccttaggagatggtcctcctatcttcaaac-3′).
PCR was performed in the following regime: for primers Ala1, 95°C, 3 min (95°C, 20 sec; 52°C, 20 sec; 72°C, 20 sec) (30 cycles); 72°C, 10 min. For primers Ala9, 95°C, 3 min (95°C, 15 sec; 55°C, 10 sec; 72°C, 10 sec) (30 cycles); 72°C, 10 min. For primers Aq1, Aq3, Aq6, 95°C, 3 min (95°C, 5 sec; 63°C, 5 sec; 72°C, 5 sec) (35 cycles); 72°C, 5 min. For primers A23LR, 95°C, 3 min (95°C, 5 sec; 63°C, 5 sec; 72°C, 20 sec) (30 cycles); 72°C, 5 min.
Sequencing of DNA was performed using BigDyeTerminator v3.1 Cycle Sequencing Kits (“Applied Biosystems,” USA) and DNA-analyser 3130 Genetic Analyser (“Applied Biosystems,” USA). Analysis of the nucleotide sequences was done in Sequencing Analysis 5.3.1 software (“Applied Biosystems,” USA) and NCBI (National Center for Biotechnology Information, http://blast.ncbi.nlm.nih.gov/Blast.cgi) database.
3. Results and Discussion
The presence of nucleotide sequences of some mycoplasma genes within EMVs of A. laidlawii PG8 allowed a possibility to use PCR to perform differential detection of the corresponding structures of the bacterium in samples under study. Despite growth conditions, we detected sequences of genes for pdhC, rpoB, pnp, and tufB genes in EMVs, but not for ftsZ and 16S–23S rRNA genes. This allowed us to use a combination of primers for differential detection of cells and membrane vesicles in the tested samples.
Previously [2], we reported about the ability of A. laidlawii PG8 cells to enter through the root system of O. sativa L. into overground parts of plants and induce there changes in ultrastructural organization. This conclusion was based on data of TEM and PCR obtained 9 days after rice cultivation with mycoplasma cells. As a result of use of primers for amplification of the nucleotide sequences for the following genes—pdhC (codes dihydrolipoamide acetyltransferase), rpoB (codes β-subunit of RNA-polymerase), pnp (codes polyribonucleotide-nucleotidyl transferase, a global regulator of virulence in phytopathogenic bacteria [14]), and tufB (codes elongation factor Tu) permitting to detect cells as well vesicles of the mycoplasma, and primers for amplification of the nucleotide sequences of ftsZ gene (codes FtsZ, a protein for cell division), and 16S–23S rRNA gene (codes a spacer zone and flankings) permitting to detect cells but not bacterial vesicles, we found that PCR signals were presented for all indicated genes in tissues of plant leaves grown in medium with mycoplasma cells for 1–9 days (Figure 1(c)). Also, amplicon specific to pnp gene of the mycoplasma was found 2 hours since the beginning of plant incubation with mycoplasma cells (Figure 1(b)). Sequencing results confirmed the belonging of a sequence of this amplicon to A. laidlawii PG8 pnp-gene (Figure 2). The obtained data allow to suggest that (1) EMVs are able to display virulent features linked with infectivity and invasivity in plants; (2) penetration of EMVs to plant tissues precedes to plant infection with mycoplasma cells; (3) EMVs are heterogeneous on the content and functions; (4) virulent features connected with infectivity and invasivity are different in vesicles varying on the content.
Figure 1.
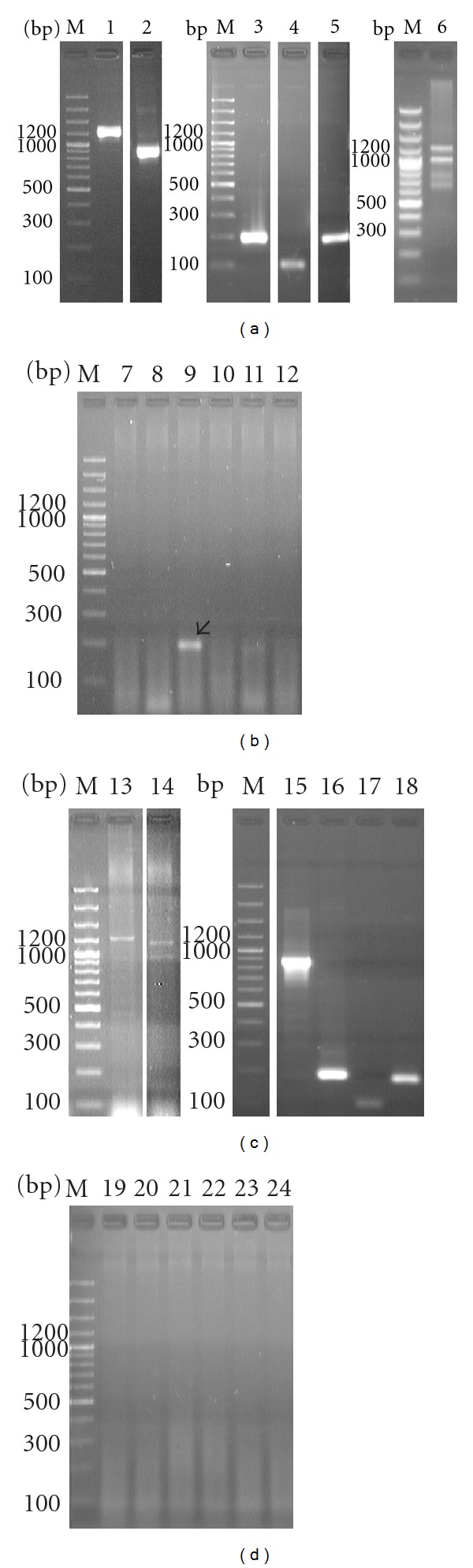
Electrophoregrams of amplification products for DNA nucleotide sequences of the following genes ftsZ: (1, 7, 13, 19), pdhC (2, 8, 15, 20), pnp (3, 9, 16, 21), tufB (4, 10, 17, 22), rpoB (5, 11, 18, 23), and 16S–23S rRNA (6, 12, 14, 24) A. laidlawii PG8 obtained at use, in PCR, total DNA (as matrix) isolated from cells of A. laidlawii PG8 (a) and plant leaves grown in medium with the mycoplasma cells (b and c—2 h and 9 days, resp.) and in the absence of the infect (d). Arrow indicates location of the corresponding PCR-signal. M—a marker of fragment lengths.
Figure 2.

Results of alignment of the nucleotide (a) and amino acid (b) sequences of the fragment of pnp-gene of A. laidlawii PG8 (1) as well as amplicon (2) obtained as a result of PCR with primers for detection of the nucleotide sequence of pnp-gene of A. laidlawii PG8 when DNA of plants grown in medium with the mycoplasma cells was used as a matrix. Sequences of forward and reverse primers are indicated with italics.
To test the ability of EMVs of A. laidlawii PG8 to penetrate from medium for O. sativa L. cultivation into tissues of overground parts of plants, special experiments were designed where rice plants grew in media with EMVs without mycoplasma cells (Figure 3), and PCR with the indicated primers was used to detect infects while TEM was used for analysis of plant ultrastructural organization.
Figure 3.
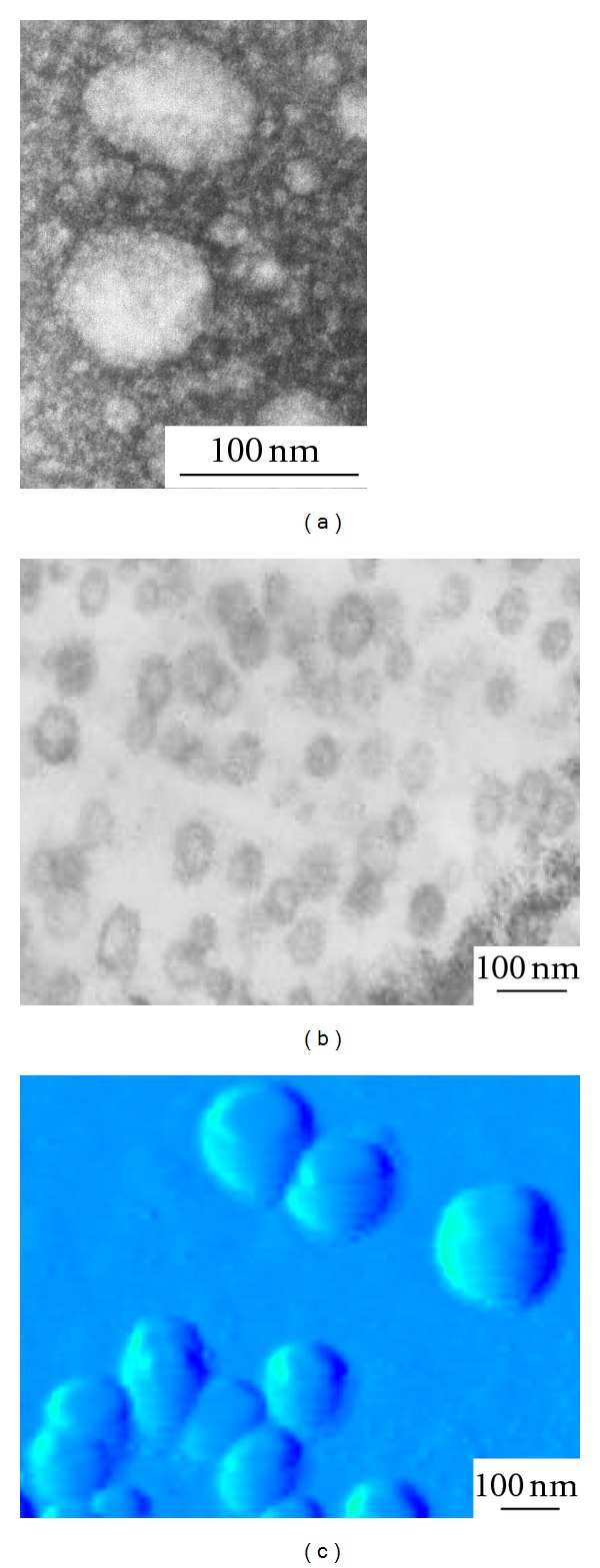
Images of EMVs of A. laidlawii PG8 obtained using transmission, (a, b) and atomic force (c) microscopy.
Results of PCR with the use of matrix DNAs from A. laidlawii PG8 cells, EMVs, and plants, grown with and without vesicles, are presented at Figure 4. As it follows from the obtained data, 2 h since the beginning of plant incubation with EMVs, PCR signals for pdhC, rpoB, pnp, tufB, ftsZ,and 16S–23S rRNA were absent in the tested samples (Figure 4(b)). However, when primers for tufB were used in plants grown for 9 days with EMVs as well as in plants cultivated with the mycoplasma cells, amplicon was registered that is similar in size to tufB in EMVs of A. laidlawii PG8 (Figure 4(c)). Results of sequencing suggest on the belonging of this amplicon sequence to tufB-gene of A. laidlawii (Figure 5). The detected nucleotide replacements are probably related to features of nucleotide sorting or/ and with subsequent DNA modifications in EMVs. However, this question should be clarified in the future.
Figure 4.

Electrophoregrams of amplification products for DNA nucleotide sequences of the following genes ftsZ: (1, 7, 15), pdhC (2, 8, 16), pnp (3, 9, 17), tufB (4, 10, 13, 14), rpoB (5, 11, 19), and 16S–23S rRNA (6, 12, 20) of A. laidlawii PG8 obtained at use, in PCR, total DNAs (as matrix) isolated from EMVs of A. laidlawii PG8 (a) and plant leaves grown in medium with EMVs (b and c—2 h and 9 days, resp.), the mycoplasma cells (d), and in the absence of the infect (e). Arrows indicate location of the corresponding PCR-signals. M—a marker of fragment lengths.
Figure 5.

Results of alignment of the nucleotide (a) and amino acid (b) sequences of the fragment of tufB-gene of cells (1) and EMVs (2) of A. laidlawii PG8 as well as amplicon (3) obtained as a result of PCR with primers for detection of the nucleotide sequence of tufB-gene of A. laidlawii PG8 when DNA of plants grown in EMVs-containing medium was used as a matrix. Sequences of forward and reverse primers are indicated with italics. (□): nucleotide and amino acid replacements; *: changes at positions 70–72 results in replacing valine by cysteine.
The obtained data may confirm the penetration of EMVs from medium to overground parts of plants. To detect the ability of mycoplasma EMVs to show virulent features linked with toxigenicity, we made analysis of plant response reactions toward EMVs persistence related with features of their ultrastructural organization. With this aim, transmission micrographies of ultrathin slices of O. sativa L. leaf tissues were investigated.
It is known that toxigenicity of A. laidlawii is significantly related with the ability of the mycoplasma to induces a chronic oxidative stress [4]. At that, in plants that are not specific host for A. laidlawii, the mycoplasmamay induce inapparent infections when clear signs of morphologic anomalies (dwarfism, development of laterals, growth delay, icterus) are not evident but transcriptome and proteome reorganization, and alterations in tissue ultrastructure are present [2].
As it was found in our studies, evident morphoses were not detected in plants grown in medium with EMVs of A. laidlawii PG8. Only in some plants, there were apical necrosis and tillering (Figure 6). However, we revealed significant alterations in tissue ultrastructure in plants grown in medium with EMVs (Figure 7): chloroplasts were located along the cell walls and did not contain amyloid grains; vacuoles of coat cells of vessels and parenchyma cells were fulfilled with soft content; mitochondria have brighten soft matrix with rare cristas. The observed alterations of ultrastructural organization of parenchyma cells in leaves of O. sativa L. are characteristic for plants infected with A. laidlawii PG8 cells [2] as well as under conditions of oxidative stress [15]. The obtained data may indicate that toxigenicity of EMVs as well the mycoplasma cells is primarily connected with the induction of oxidative stress. The detected similarity in response reactions of plants toward EMVs and the mycoplasma cells may be due to location of virulence factors at membranes of the infects [16]. The comparative analysis of the proteome profiling of membranes of cells and EMVs of the bacterium will probably be useful for justification of this statement.
Figure 6.
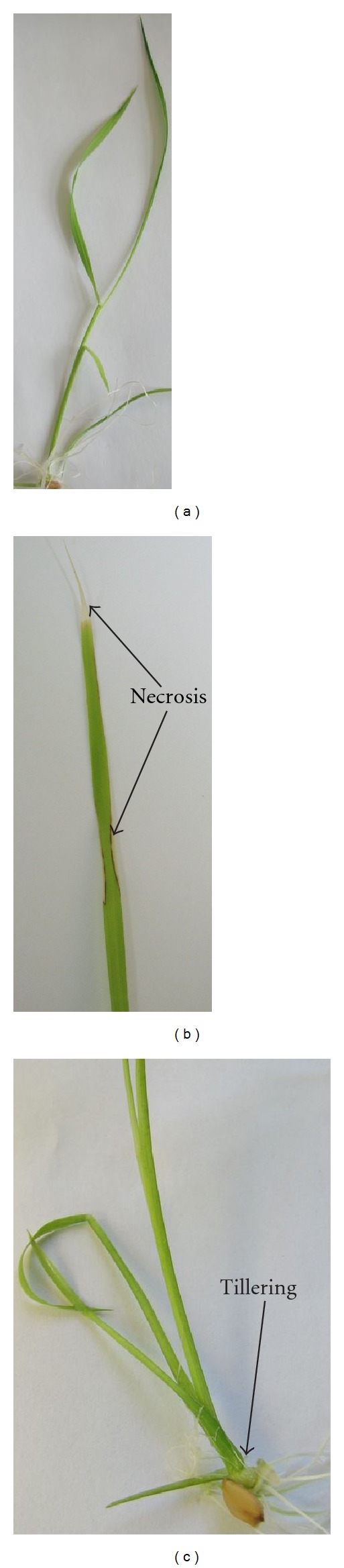
Morphologic alterations in plants (O. sativa L.) grown in medium with EMVs of A. laidlawii PG8.
Figure 7.
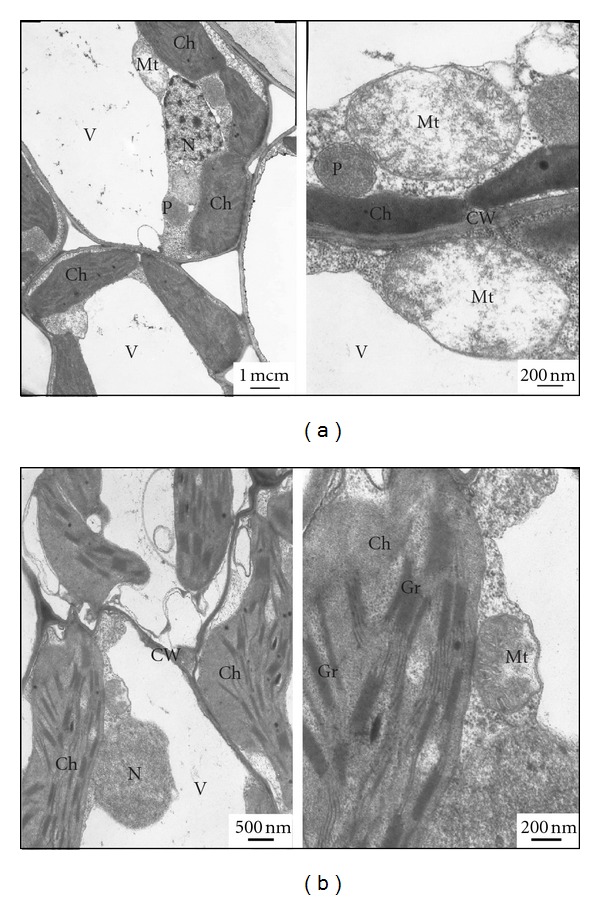
Ultrastructural organization of plant cells (O. sativa L.) grown in media with (a) and without (b) EMVs of A. laidlawii PG8. V: vacuole, Gr: grana, CW: cell wall, Mt: mitochondria, P: peroxisome, Ch: chloroplast, N: nucleus.
Thus, as a result of our studies of interaction between O. sativa L. and EMVs secreted by A. laidlawii PG8 cells, it was presented for the first time that EMVs may display in plants the features of infect from the viewpoint of virulence criteria—invasivity, infectivity and toxigenicity—and favor bacterial phytopathogenicity. In this connection, detection of full content of A. laidlawii PG8 EMVs regarding proteins, lipids, and nucleic acids in different environments and careful analysis of pathogenicity of vesicles seem very actual from the viewpoint of fundamental biological research of the smallest prokaryotes as well as practical developments of controlling mycoplasma infections and contaminations of cell cultures and vaccines.
Conflict of Interests
The authors declare no conflict of interests.
Acknowledgments
This work was supported by the grants of Russian Fund for Basic Research (Projects no. 11-04-01406a, 12-04-31396 and 12-04-01052-a), Federal Purposive Program SEC no. 8048, and Principal Scientific School no. NSH-825.2012.4.
References
- 1.Scripal IG. In: Microorganisms—Agents of the Plant Diseases. Bilay VI, editor. Kiev, Ukraine: Naukova Dumka; 1988. [Google Scholar]
- 2.Chernov VM, Chernova OA, Medvedeva ES, et al. Unadapted and adapted to starvation Acholeplasma laidlawii cells induce different responses of Oryza sativa, as determined by proteome analysis. Journal of Proteomics. 2011;74(12):2920–2936. doi: 10.1016/j.jprot.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Wilson David SA, Volokhov DV, Ye Z, Chizhikov V. Evaluation of Mycoplasma inactivation during production of biologies: ess-based viral vaccines as a model. Applied and Environmental Microbiology. 2010;76(9):2718–2728. doi: 10.1128/AEM.02776-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chernov VM, Moukhametshina NE, Gogolev YV, Nesterova TN, Trushin MV, Chernova OA. Acholeplasma laidlawii PG8 culture adapted to unfavorable growth conditions shows an expressed phytopathogenicity. The Scientific World Journal. 2007;7:1–6. doi: 10.1100/tsw.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazarev VN, Levitskii SA, Basovskii YI, et al. Complete genome and proteome of Acholeplasma laidlawii . Journal of Bacteriology. 2011;193(18):4943–4953. doi: 10.1128/JB.05059-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chernov VM, Chernova OA, Mouzykantov AA, et al. Extracellular vesicles derived from Acholeplasma laidlawii PG8. The Scientific World Journal. 2011;11:1120–1130. doi: 10.1100/tsw.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulp A, Kuehn MJ. Biological Functions and biogenesis of secreted bacterial outer membrane vesicles. Annual Review of Microbiology. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidhu VK, Vorhölter FJ, Niehaus K, Watt SA. Analysis of outer membrane vesicle associated proteins isolated from the plant pathogenic bacterium Xanthomonas campestris pv. campestris . BMC Microbiology. 2008;8, article 87 doi: 10.1186/1471-2180-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez-Martínez I, Rodríguez-Moreno L, Lambertsen L, et al. Fate of a Pseudomonas savastanoi pv. savastanoi type III secretion system mutant in olive plants (Olea europaea L.) Applied and Environmental Microbiology. 2010;76(11):3611–3619. doi: 10.1128/AEM.00133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voegel TM, Warren JG, Matsumoto A, Igo MM, Kirkpatrick BC. Localization and characterization of Xylella fastidiosa haemagglutinin adhesins. Microbiology. 2010;156(7):2172–2179. doi: 10.1099/mic.0.037564-0. [DOI] [PubMed] [Google Scholar]
- 11.Kolling GL, Matthews KR. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Applied and Environmental Microbiology. 1999;65(5):1843–1848. doi: 10.1128/aem.65.5.1843-1848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamane K, Kawasaki M, Taniguchi M, Miyake H. Correlation between chloroplast ultrastructure and chlorophyll fluorescence characteristics in the leaves of rice (Oryza sativa L.) grown under salinity. Plant Production Science. 2008;11(1):139–145. [Google Scholar]
- 13.Maniatis T, Fritsch EE, Sabrook J. Molecular Cloning. A Laboratory Manual. Long Island, NY, USA: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 14.Clements MO, Eriksson S, Thompson A, et al. Polynucleotide phosphorylase is a global regulator of virulence and persistency in Salmonella enterica . Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):8784–8789. doi: 10.1073/pnas.132047099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paakkonen E, Holopainen T, Karenlampi L. Ageing-related anatomical and ultrastructural changes in leaves of birch (Betula pendula Roth.) clones as affected by low ozone exposure. Annals of Botany. 1995;75(3):285–294. [Google Scholar]
- 16.Chernov VM, Chernova OA, Mouzykantov AA, et al. Phytopathogenicity of avian mycoplasma Mycoplasma gallisepticum S6: morphologic and ultracytostructural changes in plants infected with the vegetative forms and the viable but nonculturable forms of the bacterium. Microbiological Research. 2010;165(4):346–350. doi: 10.1016/j.micres.2009.07.001. [DOI] [PubMed] [Google Scholar]


