Abstract
Background:
MicroRNAs (miRNAs) are small noncoding RNA molecules with an essential role in regulation of gene expression. miRNA expression profiles differ between tumor and normal control tissue in many types of cancers and miRNA profiling is seen as a promising field for finding new diagnostic and prognostic tools.
Materials and Methods:
In this study, we have analyzed expression of three miRNAs, miR-21, miR-125b, and miR-203, and their potential target proteins p53 and p63, known to be deregulated in squamous cell carcinoma of the head and neck (SCCHN), in two distinct and one mixed subsite in squamous cell carcinoma in the oral cavity.
Results:
We demonstrate that levels of miRNA differ between tumors of different subsites with tongue tumors showing significant deregulation of all three miRNAs, whereas gingival tumors only showed significant downregulation of miR-125b and the mixed group of tumors in tongue/floor of the mouth showed significant deregulation of miR-21 and miR-125b. In the whole group of oral squamous cell carcinoma (SCC), a significant negative correlation was seen between miR-125b and p53 as well as a significant correlation between TP53 mutation status and miR-125b.
Conclusion:
The present data once again emphasize the need to take subsite into consideration when analyzing oral SCC and clearly show that data from in vitro studies cannot be transferred directly to the in vivo situation.
Keywords: MicroRNA, oral squamous cell carcinoma, p53, p63
BACKGROUND
Squamous cell carcinoma of the head and neck (SCCHN) is the 6th most common malignancy worldwide and has a poor prognosis which has not improved over the last 25-30 years.[1] Like many other human tumor types, approximately 50% of the SCCHN tumors show mutations in the well-known tumor suppressor gene TP53. The prognostic significance of TP53 mutations in SCCHN varies between different studies.[2] So far, factors of proven prognostic significance for SCCHN tumors are presence of regional lymph node metastasis (N-status) and amplification of chromosome 3q, where the TP53 homolog, p63, is located.[3] The six members of the p63 family, which is crucial for formation of the oral mucosa,[4,5] are deregulated in SCCHN[6–9] and overexpression of p63 has been correlated to aggressive disease and poor outcome in this tumor type.[10] We have also shown that repressing p63 in SCCHN cell lines decreases their survival and sensitizes them to chemotherapy and radiotherapy.[11] SCCHN is a very heterogeneous group of tumors and looking at only intraorally located SCC; we recently showed that p63 status differs between tumors of different subsites within this limited area. Tongue tumors, in contrast to gingival and tongue/floor of the mouth tumors, showed downregulation of p63 compared to corresponding normal tissue.[12] This indicates that apart from being an important player in SCCHN tumors, p63's role differs depending on subsite of the tumor. Although the downstream pathways of p63 are the focus of many studies, pathways regulating p63 are not well characterized. One way of regulating gene expression is through microRNAs (miRNAs), noncoding RNAs that can regulate protein coding genes by inducing mRNA degradation or by interfering with mRNA translation.[13,14] Results from mouse and human cell lines also indicate a role for miRNAs in activation of translation.[15,16] One of the first miRNAs detected in the human genome[17] was miR-21, a miRNA found to be overexpressed in different tumor types including SCCHN.[18–23] Transfection with miR-21 mimics in SCCHN cell lines caused statistically significant increased cell growth[24] and overexpressing miR-21 enhanced tumor cell survival in tongue SCC.[25] In glioblastoma cells, miR-21 targets the full length p63 isoforms, TAp63, and can also indirectly affect the function of its relative, p53.[26] A miRNA directly regulating p53 is miR-125b[27] and overexpression of miR-125b caused repression of the p53 protein in human neuroblastoma cells and human lung fibroblasts, while knockdown of miR-125b increased the levels of p53 protein in lung fibroblasts.[27] Levels of miR- 125b are downregulated in SCCHN[28,29] and transfection of miR-125b into OSCC cells reduced cell proliferation.[30] A miRNA identified to target ΔNp63 isoforms, at least in vitro in mouse keratinocytes, is miR- 203,[31] and miR-203 regulates ΔNp63 levels in SCCHN upon genotoxic damage.[31] miR-203 is expressed in suprabasal layers of stratified epithelia[32] with the primary role to suppress the proliferative capacity of epithelial cells upon differentiation.[33] Only a few studies have mapped miR-203 status in SCCHN, showing downregulation in SCCHN cell lines[34] and oral SCC compared to non-cancerous oral mucosa.[23,35] In our previous analysis of 836 miRNAs in formalin fixed paraffin-embedded samples from tongue SCC, we identified 54 miRNAs to be differentially expressed. Among these, miR- 21 was the second highest upregulated, and miR-203 among the most downregulated miRNAs.[23]
In this study, we have mapped expression of three miRNAs and the levels of two of their potential target proteins in tumor samples from patients with SCCHN. In accordance with our previous data, we also clearly show differential expression for miRNAs based on tumor subsite. Furthermore, the correlations previously seen between miRNAs and their target proteins in vitro were not as clear-cut in tumors in vivo.
MATERIALS AND METHODS
Patient material
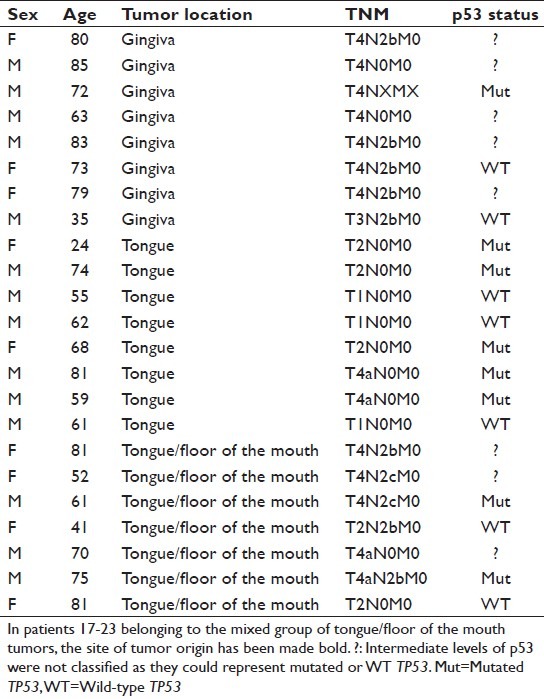
After obtaining informed consent, tumor biopsies were collected from 23 patients with squamous cell carcinoma in the oral cavity. For patient data, see Table 1. Tumors were divided into three groups based on subsite, with one group of gingival tumors, one group of tongue tumors, and one mixed group comprising four tumors originating in the tongue with overgrowth into the floor of the mouth and three tumors originating in the floor of the mouth with overgrowth into the tongue. A biopsy was also taken from clinically normal tissue of the same subsite as the tumor from all patients. From the mixed group, clinically normal tissue was taken from the tongue in six patients and from the floor of the mouth in one patient. Tissue samples were snap-frozen in liquid nitrogen and stored at −80°C until RNA and protein were extracted. The project was approved by the local Ethical Committee (dnr 08-003M).
Table 1.
Patient material
miRNA extraction and quantitative RT-PCR
One part of each biopsy was homogenized in trizol using a Precellys (Bertin Technologies, Aix-en-Provence, France) and total RNA including miRNA extracted using chloroform. After dilution in water, RNA concentration was measured with a nanodrop (Thermoscientific). For the cDNA reaction, 20 ng of total RNA was used with the mercury LNA™ Universal cDNA synthesis kit according to the manufacturer's protocol (Exiqon, Vedbaek, Denmark), total volume of each reaction was 20 μl. The expression levels of miR-21, miR-125b, miR-203, and the reference gene U48 were analyzed with real-time PCR amplification for individual assays using LNA™ primer sets for miRNA (Exiqon).
Protein extraction and Western blot of miRNA target proteins
Protein was extracted from the other half of each biopsy using a Micro-dismembrator (B. Braun. Biotech International, Melsungen, Germany) pulverizing the sample. One hundred microliters of lysis buffer containing 0.5% NP-40, 0.5% Na-doc, 0.1% SDS, 150 mM NaCl, 50 mM Tris pH 7.5, 1 mM EDTA, 1 mM NaF, and protease inhibitor cocktail (Sigma-Aldrich) were then added and samples were homogenized. Protein concentration was determined using BCA Protein Assay Kit (Pierce, Rockford, USA). For protein analysis, 30 μg protein extract was run on 10% Clear PAGE, SDS-polyacrylamide mini-gels (VWR International, Radnor, USA). Proteins were transferred to a PVDF membrane (Millipore) and stained with Ponceau red for evaluation of transfer efficiency and loading. Primary monoclonal antibodies used were 4A4 anti-p63 (Abcam ab3239) diluted 1/500, p53 (Abcam ab80645) diluted 1/1000, and actin (Chemicon International MAB1501R, Temecula, USA) diluted 1/5000. Secondary antibody was peroxidase-labeled rabbit anti-mouse (DakoCytometric) diluted 1/50,000. For chemiluminescence detection, Chemidoc XRS (Bio-Rad) was used in combination with ECL, Electrochemiluminescence, advance (GE Healthcare, Uppsala, Sweden). Twenty-one of the 23 samples included had previously been analyzed for expression of p63.[12]
Statistical tests
For all statistical analyses, SPSS version 18.0 was used. Data were not normally distributed and nonparametric methods therefore used. Wilcoxon-signed rank test was used for calculation of differences between tumor and normal samples and Spearman's rank correlation coefficient for correlation analysis.
RESULTS
Altered expression of miR-21, miR-125b, and miR- 203 in oral SCC
Three selected miRNAs, miR-21, miR-125b, and miR-203, previously reported to be connected to two important factors in SCCHN, p53 and p63, were analyzed in 23 patients with oral SCC and corresponding clinically normal tissue from each patient used as control. Levels of miRNA were measured using qRT-PCR and results showed that all miRNAs examined were significantly altered in tumor tissue compared to corresponding normal tissue. In general, there were relatively large interindividual variations in miRNA expression. In accordance with previous studies,[25] miR- 21 was significantly upregulated in tumors compared to normal tissue in 20/23 patients [Figure 1a] (P < 0.001). On the other hand, miR-125b and miR-203 were significantly downregulated in 22/23 and 18/23 patients, respectively, [Figures 1b and c] (P < 0.001), also in agreement with previous data.[25,30]
Figure 1.
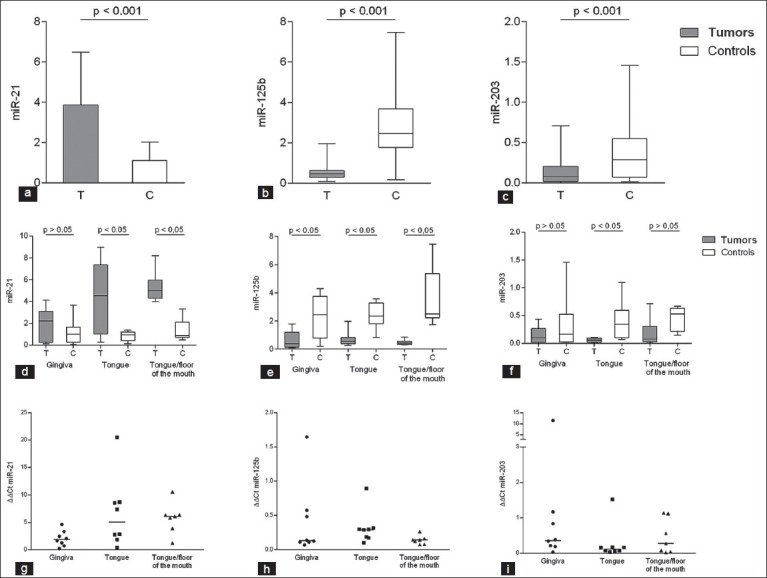
Altered microRNA (miRNA) expression in oral squamous cell carcinoma (SCC). To analyze differences in expression of miR-21, miR-125b, and miR-203 in oral SCC tumors compared to corresponding clinically normal tissue, samples were analyzed with qRT-PCR. miR-21 expression was significantly upregulated (P < 0.001) (T = tumors, C = controls) (a), and miR-125b and miR-203 significantly downregulated in tumors compared to corresponding normal tissue (P < 0.001 and P < 0.001, respectively) (b and c). Dividing tumors into different subsites showed miR-21 to be significantly upregulated in tongue and tongue/floor of the mouth tumors but not in gingival tumors (d). miR-125b showed significant downregulation in tumor samples compared to controls regardless of subsite (e). miR-203 showed downregulation in all three subsites, but significant only in tongue tumors (f). Whiskers represent minimum to maximum values. The miR-21 ratio is calculated as expression in tumor divided to expression in corresponding normal control (ΔΔCt) (g). ΔΔCt for miR-125b in the different locations (h) and ΔΔCt for miR-203 (i)
Subsite specific alterations of miRNAs
When dividing tumors into different subsites, results showed that expression of individual miRNAs was only significantly altered in specific tumor sites. Thus, miR-21 was not significantly altered in gingival tumors but significantly upregulated in both tongue and tongue/floor of the mouth tumors [Figure 1d]. For miR-125b, tumors in all three subsites showed significant downregulation compared to clinically normal tissue of the same subsite [Figure 1e], whereas miR-203 was neither significantly altered in gingival nor in tongue/floor of the mouth tumors but significantly downregulated in tumors in the tongue [Figure 1f]. Analyzing data at the individual level by calculating a ratio between miRNA expression in each tumor divided by its corresponding normal control showed similar pattern as when analyzing samples group wise [Figures 1g–i].
Expression of the miRNA target proteins, p53 and p63
miRNAs can have effect both through destabilization of mRNA and through blocking of protein translation,[36,37] but whichever way regulation occurs the effect will be seen at protein levels. Protein levels of the two potential targets, p53 and p63, were therefore measured in the same samples. Results showed p53 to be significantly upregulated in 18/23 samples [Figure 2a] (P < 0.01). Looking at protein expression based on subsite, p53 was significantly increased in the group of tongue/floor of the mouth tumors but not in gingival and tongue tumors [Figure 2b]. It is well known that missense mutations in TP53 occur in about 50% of tumors including SCCHN and lead to a dramatic stabilization of p53 protein, although other mechanisms may also induce protein stabilization of wild-type (WT) p53.[2] Thus, very high levels of p53 equate to missense mutations and low levels of p53 equate to WT status, whereas moderately increased levels of p53 are seen in roughly equal proportions of tumors containing WT and mutant p53.[38] In order to evaluate the mutational status of p53, the specimens were divided into the three groups based on intensity of Western blot bands, where very high-level expression was classified as mutated p53 (Mut) and weak expression represented WT p53, whereas intermediate expression levels were not classified since they may represent mutated or WT TP53.[38] Results showed that eight samples had mutated p53, seven WT p53, and eight of the samples could not be determined [Table 1]. When analyzing miRNA expression based on p53 mutation status, WT-p53 tumors had higher levels of miR-125b compared to mutated p53 tumors (P = 0.021), whereas expression of miR- 21 and miR-203 was not significantly altered between tumors with WT or mutated p53.
Figure 2.
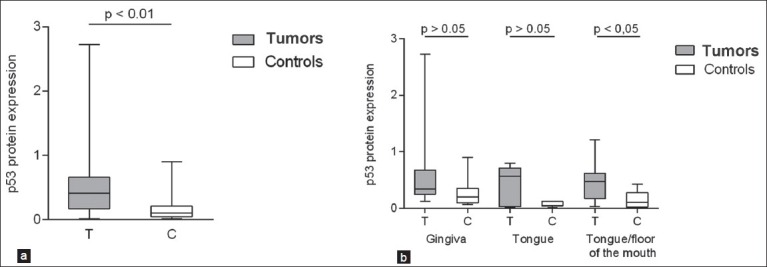
MicroRNA (miRNA) target protein expression in oral squamous cell carcinoma (SCC). By using Western blot, levels of the miRNA target p53 were analyzed in the same tumors that were analyzed for miRNA expression. p53 protein expression was significantly upregulated in tumor tissue (P < 0.01) (T = tumors, C = controls) (a). Taking subsite into consideration, only tongue/ floor of the mouth tumors showed significant upregulation of p53 protein (b) even if both gingival and tongue tumors showed a trend toward upregulation of p53 protein
Protein levels of p63 in 21 of the samples have previously been reported.[12] The addition of two tumors and corresponding clinically normal tissue did not alter results, showing p63 to be significantly downregulated in tumors compared to corresponding normal samples (P < 0.01). When dividing tumors into different subsites, significantly lower expression of p63 was seen in tongue tumors, but not in the other two groups, in accordance with previous results.[12]
Correlation of miRNA expression with potential target proteins
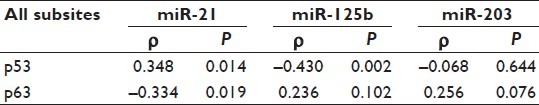
Whatever miRNAs that are changed in tumors, the important task is to map the effect of these changes at protein levels of their potential targets in the in vivo situation. In order to do this, correlation analysis was performed. When comparing all samples as one group, miR-21 showed significant correlation with both p53 (positive) and p63 (negative) [Table 2], whereas miR-125b showed significant negative correlation with p53 only [Table 2]. miR-203 in turn did not show any correlation with either p53 or p63 [Table 2]. As the number of tumors in each subsite was limited, no correlation analysis based on subsites was performed.
Table 2.
Correlation analysis between miRNAs and their targets
DISCUSSION
SCCHN is a disease with poor prognosis and a survival rate that has not improved much over the last decades. Lately, much research has focused on the expression of miRNAs and their role in tumor progression, and numerous studies have identified altered miRNA expression profiles in different tumor types. MiRNA genes represent about 1% of the genome but are estimated to regulate up to 30% of human genes[39] and single miRNAs may target up to 200 different mRNAs.[40] Therefore, it is important not only to study the expression of miRNAs but also to map their effect on expression of putative targets. However, few studies have so far attempted to connect miRNA expression with expression of target proteins in vivo. Therefore, we set out to analyze expression of selected miRNAs in correlation to their suggested targets in the p53 family in tissue samples from patients in one subgroup of SCCHN, SCC in the oral cavity. Based on our recent results showing differences in p63 expression between different oral subsites,[12] site of origin of the tumors was also taken into consideration in these analyses.
There were large interindividual variations in miRNA expression, which have previously been shown in normal and psoriatic skin.[41] Our results clearly showed expression of all three miRNAs studied to be altered in tumor tissue compared to clinically normal tissue from the same patients. In accordance with previous studies, miR-21 was significantly upregulated in tumors compared to control samples, whereas miR-125b and miR-203 showed significantly decreased expression in tumor samples compared to controls, indicative of a role for all these miRNAs in this tumor type. Taking subsite into consideration, tongue tumors showed significant changes in expression of all three miRNAs, gingival tumors showed significant changes in miR-125b, and tongue/floor of the mouth tumors showed significant changes in miR-21 and miR-125b expressions. This subsite-based difference in miRNA expression highlights the importance of taking subsite of tumors into consideration when analyzing oral SCC. In contrast to our data, however, a previous study of miR-21 did not report any difference in expression among tumors in oropharynx, oral cavity, larynx, and hypopharynx.[24] In that study, however, no division of intraoral tumors based on subsite was made.
For p63 expression, the significant decrease in p63 protein in tongue tumors, the slight increase in tongue/floor of the mouth tumors, and the unaffected expression in gingival tumors have previously been reported for 21 of the 23 samples analyzed here.[12] The addition of two tumors did not change this result. Significantly increased expression of p53 protein (irrespective of mutation status) was seen in tongue/floor of the mouth tumors but not in tongue and gingival tumors. In that study, however, no division of intraoral tumors based on subsite was made.
When correlating miRNA levels to expression of their potential target proteins, significant positive correlation was seen between miR-21 and p53 and significant negative correlation between this miRNA and p63. As miRNAs are known to have both repressive and activating functions,[16] this could indicate a dual role for miR-21 on these target proteins. As many of the previous results are derived from tissue other than SCCHN, a tissue-specific effect of miR-21 in oral SCC could also be suggested.
A significant negative correlation between miR-125b and p53, in accordance with previous results in lung fibroblasts, was also observed.[27] However, as we analyzed whole biopsies we cannot say whether the miR-125 expression is in the tumor cells or in the stromal cells. As the activity of the p53 tumor suppressor is commonly lost in tumors by missense mutations, we also analyzed the expression of miRNAs in WT and mutant tumors. The finding that tumors with mutant p53 had low levels of miR-125b is therefore in keeping with a role for this miRNA in transcriptional inhibition as an alternative to gene mutation for inactivating p53 tumor-suppressor function in these tumors. Of the miRNAs included in this study, both miR-21 and miR-203 have previously been correlated with p63.[31] We could, however, not see any correlation between miR-203 and p63 in the tumors analyzed and only a moderate, but significant, correlation between miR-21 and p63, once again emphasizing the fact that results from studies on tumor cell lines cannot be directly translated to tumors in the in vivo situation.
To further evaluate the role of the miRNAs in SCCHN, it would be interesting to study the underlying mechanisms for the differential expression of miRNA in SCCHN. Several mechanisms have been suggested in regulation of miRNA expression, for example structural genetic alterations such as chromosomal abnormalities and mutations, defects in the miRNA biogenesis machinery, regulation by other miRNAs, and epigenetic changes such as methylation and histone deacetylase inhibition.[42]
In summary, we show that miR-21, miR-125b, and miR-203 are significantly altered in oral cavity SCC, and that, in accordance with previous data,[12] tumor subsite is an important factor that must be taken into consideration when interpreting results. Data also indicate that there may be a tumor type specific adverse effect of different miRNAs on their target proteins. An important thing to keep in mind is also that miRNA regulation is a network of signaling with many factors working together, and that one miRNA is thus not the single regulator of a protein. Studying overexpression or inhibition of a specific miRNA in vitro is therefore unlikely to accurately reflect the complex regulatory networks that exist in vivo, and such data require confirmation in primary material.
CONCLUSIONS
The present data once again emphasize the need to take subsite into consideration when analyzing oral SCC and clearly show that data from in vitro studies cannot be transferred directly to the in vivo situation.
AUTHOR'S PROFILE
Prof. Karin Nylander, Department of Medical Biosciences/Pathology Umeå University, Building 6M, 2nd floor, SE – 901 85 Umeå, Sweden. Email: karin.nylander@medbio.umu.se
Dr. Linda Boldrup, Department of Medical Biosciences/Pathology Umeå University, Building 6M, 2nd floor, SE – 901 85 Umeå, Sweden. Email: linda.boldrup@medbio.umu.se
Dr. Philip John Coates, Division of Medical Sciences, University of Dundee, Ninewells Hospital and Medical School, Dundee DD1 9SY, UK. Email: p.j.coates@dundee.ac.uk
Dr. Magnus Wahlgren, Department of Clinical Sciences/ENT, Umeå University, SE – 901 85 Umeå, Sweden. Email: magnus.wahlgren@vll.se
Prof. Göran Laurell, Department of Clinical Sciences/ENT, Umeå University, SE – 901 85 Umeå, Sweden. Email: goran.laurell@ent.umu.se
ACKNOWLEDGMENTS
This study was supported by grants from Lion's Cancer Research Foundation, Umeε University, the Swedish Cancer Society Contract number 11 0651 and Vδsterbotten County Council.
Footnotes
Source of Support: This study was supported by grants from Lion's Cancer Research Foundation, Umeå University, the Swedish Cancer Society Contract number 11 0651 and Västerbotten County Council
Conflict of Interest: None declared.
REFERENCES
- 1.Lam L, Logan RM, Luke C, Rees GL. Retrospective study of survival and treatment pattern in a cohort of patients with oral and oropharyngeal tongue cancers from 1987 to 2004. Oral Oncol. 2007;43:150–8. doi: 10.1016/j.oraloncology.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 2.Nylander K, Dabelsteen E, Hall PA. The p53 molecule and its prognostic role in squamous cell carcinomas of the head and neck. J Oral Pathol Med. 2000;29:413–25. doi: 10.1034/j.1600-0714.2000.290901.x. [DOI] [PubMed] [Google Scholar]
- 3.Bockmühl U, Schwendel A, Dietel M, Petersen I. Distinct patterns of chromosomal alterations in high- and low-grade head and neck squamous cell carcinomas. Cancer Res. 1996;56:5325–9. [PubMed] [Google Scholar]
- 4.Yang A, McKeon F. P63 and P73: P53 mimics, menaces and more. Nat Rev Mol Cell Biol. 2000;1:199–207. doi: 10.1038/35043127. [DOI] [PubMed] [Google Scholar]
- 5.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dötsch V, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–16. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 6.Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE, et al. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci U S A. 2000;97:5462–7. doi: 10.1073/pnas.97.10.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sniezek JC, Matheny KE, Westfall MD, Pietenpol JA. Dominant negative p63 isoform expression in head and neck squamous cell carcinoma. Laryngoscope. 2004;114:2063–72. doi: 10.1097/01.mlg.0000149437.35855.4b. [DOI] [PubMed] [Google Scholar]
- 8.Thurfjell N, Coates PJ, Uusitalo T, Mahani D, Dabelsteen E, Dahlqvist A, et al. Complex p63 mRNA isoform expression patterns in squamous cell carcinoma of the head and neck. Int J Oncol. 2004;25:27–35. [PubMed] [Google Scholar]
- 9.Weber A, Bellmann U, Bootz F, Wittekind C, Tannapfel A. Expression of p53 and its homologues in primary and recurrent squamous cell carcinomas of the head and neck. Int J Cancer. 2002;99:22–8. doi: 10.1002/ijc.10296. [DOI] [PubMed] [Google Scholar]
- 10.Lo Muzio L, Santarelli A, Caltabiano R, Rubini C, Pieramici T, Trevisiol L, et al. p63 overexpression associates with poor prognosis in head and neck squamous cell carcinoma. Hum Pathol. 2005;36:187–94. doi: 10.1016/j.humpath.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Thurfjell N, Coates PJ, Vojtesek B, Benham-Motlagh P, Eisold M, Nylander K. Endogenous p63 acts as a survival factor for tumour cells of SCCHN origin. Int J Mol Med. 2005;16:1065–70. [PubMed] [Google Scholar]
- 12.Boldrup L, Coates PJ, Laurell G, Nylander K. Differences in p63 expression in SCCHN tumours of different sub-sites within the oral cavity. Oral Oncol. 2011;47:861–5. doi: 10.1016/j.oraloncology.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 15.Huang V, Place RF, Portnoy V, Wang J, Qi Z, Jia Z, et al. Upregulation of cyclin B1 by miRNA and its implications in cancer. Nucleic Acids Res. 2012;40:1695–707. doi: 10.1093/nar/gkr934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: MicroRNAs can up-regulate translation. Science. 2007;318:1931–4. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 17.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–66. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–33. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 19.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–70. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 20.Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672–5. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 21.Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14:2690–5. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- 22.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rentoft M, Fahlén J, Coates PJ, Laurell G, Sjöström B, Rydén P, et al. miRNA analysis of formalin-fixed squamous cell carcinomas of the tongue is affected by age of the samples. Int J Oncol. 2011;38:61–9. [PubMed] [Google Scholar]
- 24.Chang SS, Jiang WW, Smith I, Poeta LM, Begum S, Glazer C, et al. MicroRNA alterations in head and neck squamous cell carcinoma. Int J Cancer. 2008;123:2791–7. doi: 10.1002/ijc.23831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Huang H, Sun L, Yang M, Pan C, Chen W, et al. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res. 2009;15:3998–4008. doi: 10.1158/1078-0432.CCR-08-3053. [DOI] [PubMed] [Google Scholar]
- 26.Papagiannakopoulos T, Shapiro A, Kosik KS. MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008;68:8164–72. doi: 10.1158/0008-5472.CAN-08-1305. [DOI] [PubMed] [Google Scholar]
- 27.Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B, Korzh V, et al. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009;23:862–76. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui AB, Lenarduzzi M, Krushel T, Waldron L, Pintilie M, Shi W, et al. Comprehensive MicroRNA profiling for head and neck squamous cell carcinomas. Clin Cancer Res. 2010;16:1129–39. doi: 10.1158/1078-0432.CCR-09-2166. [DOI] [PubMed] [Google Scholar]
- 29.Ramdas L, Giri U, Ashorn CL, Coombes KR, El-Naggar A, Ang KK, et al. miRNA expression profiles in head and neck squamous cell carcinoma and adjacent normal tissue. Head Neck. 2009;31:642–54. doi: 10.1002/hed.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henson BJ, Bhattacharjee S, O’Dee DM, Feingold E, Gollin SM. Decreased expression of miR-125b and miR-100 in oral cancer cells contributes to malignancy. Genes Chromosomes Cancer. 2009;48:569–82. doi: 10.1002/gcc.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lena AM, Shalom-Feuerstein R, Rivetti di Val Cervo P, Aberdam D, Knight RA, Melino G, et al. miR-203 represses ‘stemness’ by repressing DeltaNp63. Cell Death Differ. 2008;15:1187–95. doi: 10.1038/cdd.2008.69. [DOI] [PubMed] [Google Scholar]
- 32.Sonkoly E, Wei T, Janson PC, Sääf A, Lundeberg L, Tengvall-Linder M, et al. MicroRNAs: Novel regulators involved in the pathogenesis of psoriasis? PLoS One. 2007;2:E610. doi: 10.1371/journal.pone.0000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452:225–9. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Jiang L, Wang A, Yu J, Shi F, Zhou X. MicroRNA-138 suppresses invasion and promotes apoptosis in head and neck squamous cell carcinoma cell lines. Cancer Lett. 2009;286:217–22. doi: 10.1016/j.canlet.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozaki K, Imoto I, Mogi S, Omura K, Inazawa J. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in oral cancer. Cancer Res. 2008;68:2094–105. doi: 10.1158/0008-5472.CAN-07-5194. [DOI] [PubMed] [Google Scholar]
- 36.Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–40. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nenutil R, Smardova J, Pavlova S, Hanzelkova Z, Muller P, Fabian P, et al. Discriminating functional and non-functional p53 in human tumours by p53 and MDM2 immunohistochemistry. J Pathol. 2005;207:251–9. doi: 10.1002/path.1838. [DOI] [PubMed] [Google Scholar]
- 39.Rajewsky N. MicroRNA target predictions in animals. Nat Genet. 2006;38:S8–13. doi: 10.1038/ng1798. [DOI] [PubMed] [Google Scholar]
- 40.Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 41.Gu X, Nylander E, Coates PJ, Nylander K. Effect of narrow-band ultraviolet B phototherapy on p63 and microRNA (miR-21 and miR-125b) expression in psoriatic epidermis. Acta Derm Venereol. 2011;91:392–7. doi: 10.2340/00015555-1086. [DOI] [PubMed] [Google Scholar]
- 42.Iorio MV, Croce CM. Causes and consequences of microRNA dysregulation. Cancer J. 2012;18:215–2. doi: 10.1097/PPO.0b013e318250c001. [DOI] [PMC free article] [PubMed] [Google Scholar]


