Abstract
The aim of this study is to assess the role of ultrasound (US) in Peyronie's Disease (PD). PD is a psychologically and physically devastating disorder that manifests in middle-aged men. Fibrous inelastic plaques in the tunica albuginea, result in palpable penile scar in the flaccid condition and cause painful erections and penile deformity, including penile curvature, hinging, narrowing, and shortening of penis. Penile deformity is the most common (52%) first symptom of PD and is present in 94% of affected men. US is the primary imaging modality of choice due to its easy availability, low risk, and ability to image and quantify both calcified and soft tissue elements of PD. US provides identification of smaller and non-palpable lesions and shows the extent of fibrosis. Detection of calcifications within the plaque suggests stabilization of the disease and provides information useful to select patients for appropriate treatment.
Keywords: Penile deformity, peyronie's disease, ultrasound
INTRODUCTION
Kiriaki Kalokairinou.

Peyronie's disease (PD) is a psychologically and physically devastating disorder that arises due to growth of a fibrous inelastic plaque in the tunica albuginea. The plaques result in palpable penile scar in the flaccid condition and cause painful erections and penile deformity, including penile curvature, hinging, narrowing, and shortening. The role of ultrasound in the investigation of penile pathology is well established.[1] High resolution gray-scale imaging, alone or in combination with color and pulsed - wave Doppler, forms the basis of modern ultrasound evaluation.
DEFINITION, ETIOLOGY, AND PREVALENCE
PD is a benign condition, characterized by the formation of fibrous tissue plaques within the tunica albuginea, usually causing penile deformity. Over 250 years after the first description, the etiology of the disorder still remains obscure. The most widely accepted hypothesis is that the initiation of PD is caused by a micro trauma to the erect penis with subsequent aberrant wound healing and scar formation.[1–5] Normal wound healing can be divided into three distinct phases based on biochemical activity: The acute phase, characterized by hemostasis and inflammation, the proliferative phase, characterized by fibroblast and epithelial growth, and the remodeling phase, characterized by collagen breakdown and reorganization. Fibrin deposition is one of the initial consequences of microvascular injury. Some years after the development of the disease, fibrin has been localized in the tunica's tissue.[6] Perivascular round cell infiltration has been seen in tissue adjacent to diseased tunica in PD. Plaques consist of dense, immature type 3 collagen with reduced and fragmented elastic fibers. Infection, autoimmune origin, local manifestation of a general fibromatosis and part of generalized arterial disease, have been suggested as probablee causes of PD.[7–10] A family history of PD in 2% of patients and an association with Dupuytren's palmar fibromatosis in 20%, which is a known inherited autosomal dominant disease, appear to suggest that patients may have an inherited predisposition to this disease.[8] In addition to a genetic element, an autoimmune component may be present, as evidenced by the finding of abnormal serologic tests in 785 men with PD[11] and the finding of elevated antielastin antibodies in the sera of men with the disease.[12] It has been hypothesized that susceptible men respond to mechanical stress or microvascular trauma with a genetically aberrant wound healing process that involves the expression of growth factors and cytokines. It can be associated also with Ledderhose disease, diabetes mellitus, and gout.[13] Dupuytren contracture is a genetically inherited disorder that primarily involves the palmar fascia, whereas Ledderhose disease involves retraction of the plantar aponeurosis, known as fibromatosis plantaris.
A current thinking regarding the etiology of PD is that a trauma to the tunica allows intravasation of fibrin from the blood into the tunica's tissue. It appears that the fibrin is responsible for initiating the release of the profibrotic compound Transforming growth factor beta (TGF-β1) within the tunica, which induces the formation of reactive oxygen species (ROS), and it is ROS that leads to the pathologic hallmarks of PD (i.e. increased collagen deposition, disorganization of the newly deposited collagen, decrease in the breakdown of the newly deposited collagen, and calcification of the plaque). Although first observed in 1561 by Fallopius and Vesalius, it was not until 1743 that the disease was fully described by Francois Gigot De la Peyronie.[14] Penile pain and deformity on erection associated with a variable degree of erectile dysfunction are the most common primary symptoms.[15,16]
Despite the well-described histology and variety of deformities, the epidemiology remains relatively vague. The epidemiological data on PD are quite inconsistent and vary widely. Polkey[17] reported on 550 case reports worldwide up to 1928, while an Italian publication, published in 1966, described 3600 affected patients. In 1968, Ludvik and Wasserburger[18] established a rate of 0.3–0.7% in all male patients who were examined in their private urological practice. Lindsey et al.,[19] in their study done in 1991, postulated a prevalence of 388.6 cases of PD per 100,000 male patients in Rochester, Minnesota. Sommer et al.,[20] analyzing over 4000 respondents to a questionnaire, reported a prevalence of PD, defined as a palpable plaque, in 3.2% of his cases. The limitation of these studies is that they were based on questionnaires. More recently, Mulhall et al.,[21] based his findings on physician examination, identifying penile plaques, during a screening program for prostate cancer. In this study PD, defined as a palpable penile plaque, was found in 9%, while penile deformity was absent in 1 / 3 of them. The high prevalence found in this study may result from the fact that the cohort screened consisted of older men compared with other studies and a physician performed the exams, thus discovering masses that the patients may not be aware of them. In the literature review of more than 1500 patients with PD, the mean reported age at disease presentation was 53.5 years. Thus it seems that PD is a disease of middle-aged men.[12,19,22,23] In Table 1, we present a literature review of the age distribution of PD and in Table 2, we present the factors predisposing to Peyronie's disease.
Table 1.
The age distribution of Peyronie's disease
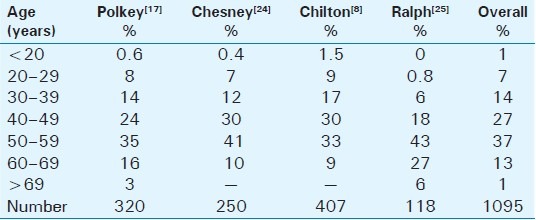
Table 2.
Main factors predisposing to Peyronie's disease

CLINICAL EVALUATION
The medical history of a patient with PD, additionally to standard general and sexual history, should include the time of onset, the duration of the disease, and the presence or absence (or resolution) of pain. Questions regarding family history, presence of associated conditions, infections, and instrumentation are of interest but do not have any bearing on treatment. The most important information to obtain is how the disease impacts the quality of life of the patient and his partner and the patient's expectations of therapy.
Physical exam should establish the degree and the orientation of the penile curvature and the presence of penile shortening or an hourglass-type indentation. The number and location of plaques must be identified, as well. Detection of the plaque on clinical examination is facilitated by stretching the penis with one hand and gently compressing the penile shaft between the fingers and thumb of the other hand. Photographs of the erect penis, in Figures 1 and 2, which demonstrate the degree and the angle of the defect, are helpful for following up the disease and they are crucial for the surgical planning.
Figure 1.
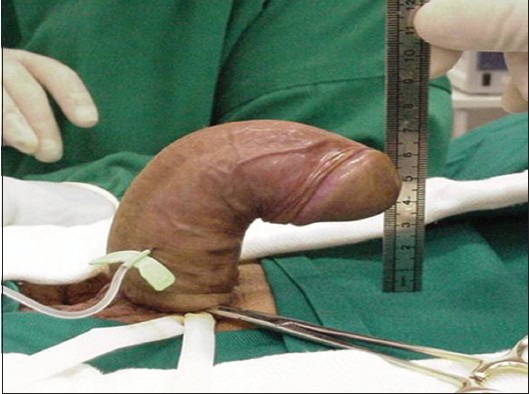
Penile curvature due to Peyronie's disease. A 47-year-old man with excessive dorsal penile curvature at the middle of the penile shaft. The image shows the deformity after artificial erection prior to reconstructive operation
Figure 2.
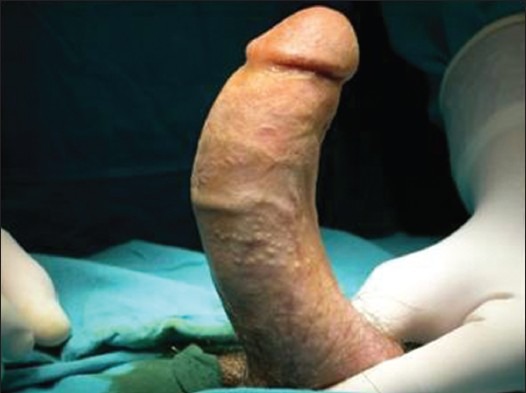
Penile deformity due to Peyronie's disease. A 56-year-old man with ventral penile curvature. The image shows the curvature after artificial erection at the beginning of reconstructive surgery
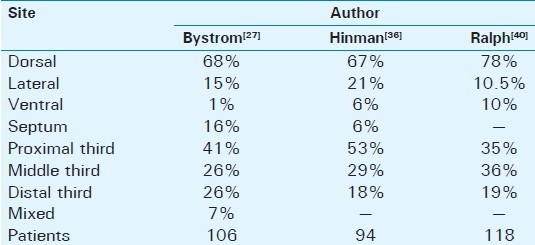
Penile deformity is the most common (52%) first symptom of PD and is present in 94% of affected men.[25] Another study[26] reports as initial symptom penile pain in 27%, penile curvature in 49%, and a palpable plaque in 39% of the patients. Twenty percent of men were able to recall a specific event that they attributed the onset of PD. Ability to achieve coitus was found in 25%, and painful intercourse was reported in 14%. A family history of PD was found in 2%.[26] The bend is usually toward the left side at first but by the time of admission it is usually toward the belly (dorsally). The degree and the orientation of curvature are proportional to the difficulty in vaginal penetration. A ventral curvature makes for greater difficulty of penetration than a dorsal one. In flaccid condition there is no deformity. Some men complain of wasting of the penis at the site of the plaque and others of distal narrowing of the shaft. In some cases complex curves may be present in more than one direction. The shape of the erect penis depends on the extent of the Peyronie's plaques. According to other studies, a palpable plaque is present 67% of the patients. Considering plaque location, distal position is reported in 30%, at the midshaft in 42%, and proximally in 28%. Penile curvature is reported in about 87% of patients. The direction of curvature is lateral in 20%, dorsal in 77%, and ventral in 9%. A septal plaque is reported in 15% while Dupuytren's contracture on physical examination is reported in 10% of PD patients.[27–34] Table 3demonstrates the location of the plaques in PD. Most of them are located dorsally through the entire length of the shaft. It is rare to find a plaque in the perineum. The consistency of the plaques vary between the fleshy plaque which is found in the early stages of the disease and the calcified or even ossified one which is found later on the disease progression. The plaque becomes firmer and harder as the disease progress and stabilizes. Most patients are anxious that the lump in the penis may be a malignant tumor and need to be reassured about the benign nature of PD. Primary or secondary tumors are rare and are seldom confused with PD.
Table 3.
Location of plaque in the penis
Pain is the next most common symptom of Peyronie's disease is seldom severe and rare when the penis is flaccid. Pain usually occurs during erection in the inflammatory stage of the disease and the absence of pain is often taken as an indication that the disease has stabilized. The incidence of pain varies between 20% and 70% in the literature.[26,33–39] Pain rarely persists for more than 12 months.[28] Plaque biopsy in 12 men with painful PD showed an inflammatory infiltrate to be present in 8 of them.[35]
Sexual dysfunction in PD may be multifactorial. Excessive deformity may disturb intercourse. Occasionally there are some patients suffering from troublesome pain that they avoid having an erection and intercourse. Additionally, there is always a psychological element associated with PD, which gives rise to performance anxiety. This acts as an additional factor leading to erectile dysfunction. Cavernous fibrosis often circumferentially, may be responsible for localized damage of veno-occlusive mechanism during erection, causing venous escape syndrome. Another factor for erectile dysfunction has to do with insufficient blood supply as the fibrosis may impair the arterial inflow.[41,42]
Often, the disease state may be divided into an acute (or inflammatory) phase and a chronic phase. During the former, there may be penile pain, even when flaccid, and there are often dynamic changes of the penile malformation. During the latter, pain (at least without intromission) resolves, and the malformation becomes stable in its characteristics. Contemporary series have reported a disappointing 13% or less rate of spontaneous regression without intervention.[37,43]
DIAGNOSTIC TOOLS IN PD
The diagnosis can be established by medical history and physical examination (plaque palpation) while it can be verified by X-ray mammography, computed tomography (CT), magnetic resonance imaging (MRI), and color Doppler ultrasound.
The calcification of the plaque can be detected with X-ray mammography and CT, while noncalcified plaques are not radiologically visible.[44] In contrast, MRI shows high sensitivity in assessing noncalcified plaques and the use of gadolinium can detect perifocal contrast enhancement, which is associated with inflammatory reactions in and around the plaque.[45]
Ultrasonography
Gray scale ultrasonography
Patients presenting with circumscribed or diffuse penile induration are commonly encountered in urological practice. The majority of them have PD; however, a differential diagnosis that includes several benign and malignant entities must be considered. US is the primary imaging modality in patients with penile disease. The penis should be scanned along its ventral surface with high-frequency linear probe, using longitudinal and transverse views(>10 MhZ) In general, as higher the ultrasound frequencies are used, better diagnostic images are obtained. The use of real time spatial compounding and adaptive image-processing technique is useful to reduce artifacts. Additionally, dynamic enhancement of the margins improves the visualization of tissue conspicuity and increases the diagnostic confidence. Evaluation should be carried out while the penis is flaccid. At US the corpora cavernosa and corpus spongiosum appear as homogenous cylindrical structures. The tunica albuginea and Buck fascia are stuck together and appear as a thin echogenic line surrounding the corpora, [Figure 3]. On transverse scans, the cavernosal arteries appear as linear or narrow tubular structures. The dorsal vessels are visible in the dorsal aspect of the shaft as anechoic structures.[46] Ultrasonography is performed due to its easy availability, low risk, and ability to image and quantify both calcified and soft tissue elements of PD. Additionally, the vascular status can be assessed if it is indicated. It also provides identification of smaller and non-palpable lesions and evaluates the extent of fibrosis. This information has clinical relevance because sonography can show lesions that precede the formation of classic plaques. Careful sonographic evaluation is needed in order to identify subtle forms of the condition. Whenever possible, the radiologist should correlate the findings with the physical examination in cooperation with the urologist. When confronted with a clinically palpable lesion but negative sonographic findings, a new examination with pharmacologic induction of an erection should be performed. This is necessary as hypoechoic and isoechoic lesions maybe detectable with distension or retraction of the corpora cavernosa. The circumferential narrowing of the corpora cavernosa results in an “hourglass appearance” in the erect penis. This alteration is not necessarily associated with a calcified plaque but can be the outcome of a circular albuginea lesion. This lesion usually appears slightly echogenic or even hypoechoic and is better identified after pharmacologic induction of an erection. Lateral penile deviations indicate that the thickening should be sonographically interrogated on the lateral aspect of the corpora cavernosa, where the cicatricial retraction is located, or even on the contralateral side, opposite from the site of the penile lesion. The isolated thickening and fibrosis of the septum represent the most challenging aspect of the disease, from a technical point of view, because it is more difficult to be identified sonographically. This is due to the orthogonal orientation of the septum, which naturally produces acoustic attenuation in both axial and sagittal planes. When the fibrous tissue is well circumscribed, an echogenic nodule can be observed in the septal region. When it is more diffuse, however, the examination of the penis should be performed in the longitudinal plane with the surface of the transducer parallel to the septum. In this case, the fibrosis may have a “veil-like” appearance, as described by Broderick and Arger.[47] This “veil-like” acoustic attenuation which is produced by dense septal fibrosis may also be found in some isoechoic lesions of the tunica albuginea of penis.[47] The incidence of the acoustic beam should be perpendicular to the septal fibers so the slight septal fibrosis can be imaged more easily. The evaluation must include the measurement of the plaque in three dimensions, the presence or absence of calcification and the precise topography of the plaque or the thickening of the tunica albuginea. Plaques that show only slight acoustic shadowing may not be calcified. In addition, sonography is sensitive enough to identify subtle lesions that are not calcified. As PD can present with lesions which stabilize quickly or are regressed (either partially or completely), sonography appears to represent an ideal method for the follow-up of these patients. In addition, sonography can also distinguish lesions that lie outside the echogenic tunica albuginea. To enhance the communication between physicians, it is essential to map the US findings which should include the topography and dimensions of the lesions.[48] Other associated disorders (e.g., Dupuytren's contractures or vascular disease) and inciting events (e.g., trauma or genitourinary instrumentation) should be evaluated, as well.
Figure 3.
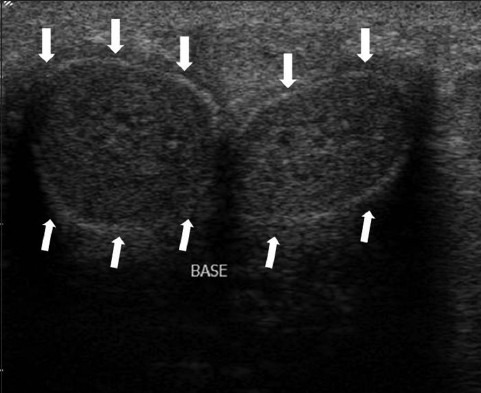
US imaging of a normal penis in transverse view of a 55-year-old man. The corpora cavernosa appear as homogenous cylindrical structures. The tunica albuginea and Buck fascia are stuck together and appear as a thin echogenic line surrounding the corpora (white arrows).
Echogenicity of the plaques
The normal tunica albuginea appears as a thin hyperechoic line covering the corpora cavernosa. The echoes from the tunica albuginea are specular reflections and thus are demonstrated with efficiency only when the ultrasound beam is perpendicular to them. Perpendicular insonation, in particular, is of paramount importance to evaluate the echogenicity of the plaques. Penile plaques are usually seen as focal hyperechoic thickening of the tunica albuginea, exhibiting strong echogenicity with substantial attenuation of the acoustic beam [Figures 4–6]. The vast majority of noncalcified plaques are isoechoic or slightly hyperechoic compared with the surrounding tunica albuginea. Hypoechoic plaques are rare and characterized by focal thickening of the pericavernous tissue [Figure 7]. This form of presentation is found in the initial stages of the disease when the fibrosis is limited and the interstitial edema predominates.[13] Echogenic plaques might appear falsely as hypoechoic due to incorrect insonation in patients with insufficient penile turgidity or due to artifacts produced by the penile septum or by extensive albugineal fibrotic changes. In fact, in contrast to the common belief, in our experience hypoechoic appearance of the plaque is usually a consequence of beam attenuation in patients with stabilized disease rather than a sign of inflammation in the active state of the disease. Gray- scale ultrasonography allows the recognition of plaque involvement with the penile septum. In this case, the normal ultrasonographic feature of the septum is replaced by inhomogeneous tissue with echogenicity similar to the adjacent plaque. Small and large calcifications may be present. This pathological situation is most often observed in patients with large dorsal plaques, but can occur also in patients with plaques in the ventral site. The relationship between the plaques and the penile vasculature should be evaluated as well. In particular, in patients with extensive dorsal plaques encasement of the neurovascular bundle may occur.[49] This condition must be identified before surgical correction of the curvature in order to minimize the risk of postoperative penile numbness. Encasement of the cavernosal arteries is rare,[47] but if it is present must be identified as a cause of arteriogenic erectile dysfunction. The focal lack of the tunica albuginea is quite rare. To our knowledge the only reference of this condition was made by Padro[13] who reported two cases. At the first case, this focal lack was observed without any other concomitant findings and it was coincident with the area of the focal pain on the clinical examination. In the second patient, a focal disappearance of the echogenic line representing the tunica albuginea was identified in an area adjacent to a small echogenic plaque.[13]
Figure 4.
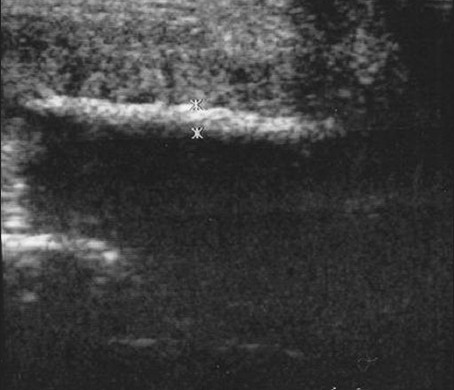
Penile plaques due to Peyronie's disease. A 39-year-old man with palpable mass at dorsal-right area of the penile shaft. Longitudinal sonogram demonstrates the length of the plaque. The thickness of the lesion (1.2 mm) is measured by the crosses
Figure 6.
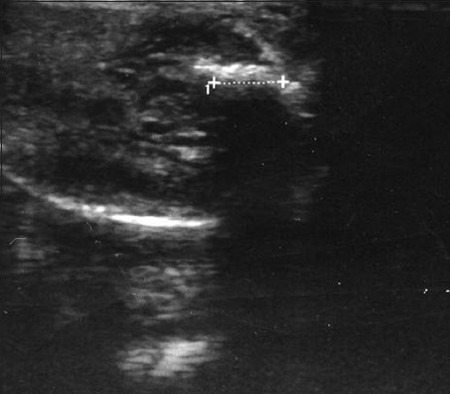
Small echogenic Peyronie's plaque. A 43-year-old man suffering from lateral curvature. The scan shows the length of the plaque as defined by the crosses (1.2 cm).
Figure 7.
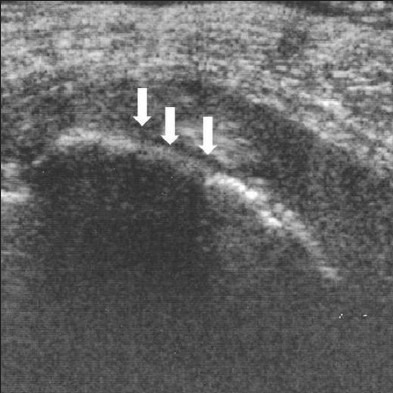
Hypoechoic lesion of the tunica albuginea. A 61-year-old man with left-dorsal curvature and painful erection. The scan shows hypoechoic lesion marked by white arrows
Figure 5.
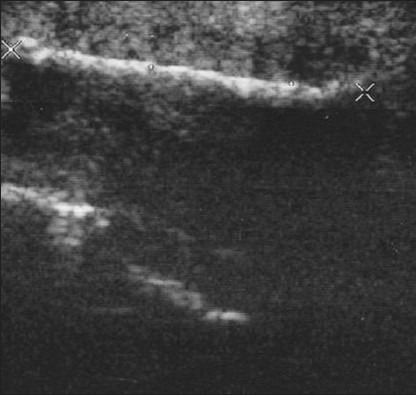
Large calcified penile plaques with acoustic shadow due to Peyronie's disease (Same patient shown in Figure 3). Longitudinal sonogram evaluates the length of the plaque, by the large crosses (4.2 cm).
Calcifications
Detection of plaque calcifications is associated with stabilization of the disease and provides information useful to select patients for lithotripsy therapy.[50] US shows 100% sensitivity in detecting and measuring gross calcifications of the plaques. The detection rate of microcalcifications increases when the highest frequency probes and real time spatial compounding are used [Figure 8]. Microcalcifications can be occasionally identified also in regions in which the tunica albuginea does not appear definitely thickened. Acoustic shadowing produced by extensive calcification of the plaques can reduce visibility of associated pathological changes of the corpora cavernosa.
Figure 8.
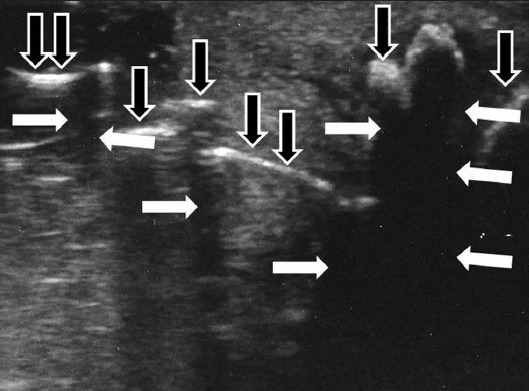
A 49-year-old man with Peyronie's disease presenting with hourglass deformity. The scan shows acoustic shadows produced by the calcifications (white arrows) and multiple calcifications of the tunica albuginea (black arrows).
Position, extent, and morphology of the plaques
Peyronie's plaques are more often located on the dorsal aspect of the penis, but they can also be found ventrally or, less frequently, in other positions. Circumscribed plaques present with focal thickening of the tunica albuginea, which correspond to the site of the penile bending. Annular plaques produce hourglass deformity, so the girth of the corpora cavernosa is reduced, at the site of the plaque, compared with the more proximal and distal regions of the shaft. Measurement of the size of the corpora cavernosa in different portions of the penis while erect is useful to assess the severity of the deformity.
Doppler ultrasonography
If coevaluation of erectile function is indicated, examination must be performed after intracavernosal injection of vasoactive drugs.[49,51,52] After plaque assessment, Doppler interrogation of all visible vessels should be done, and the erectile response of the patient should be evaluated. Doppler spectra are recorded on both cavernosal arteries under the guidance of color signal measuring peak systolic velocity and end-diastolic velocity for at least 30 min after vasoactive drug injection.[53,54]
Anatomical vascular variations, arterial vascular communications, and leakage pathways should be identified along the penile shaft, with specific notice to vessels adjacent to the plaques.[55] Color Doppler ultrasonography of the cavernosal arteries provides information on penile arterial inflow and venous outflow, which is useful in planning surgical or medical intervention. In particular, in patients with PD there is a higher incidence of venous leakage than in the age-matched control population[43] While in normal erection venules draining the corpora cavernosa are passively compressed between the expanding corporeal tissue and the tunica albuginea, in patients with PD corporeal compliance is decreased, preventing venous compression.[53] This mechanism is particularly evident adjacent to large circumscribed plaques producing severe penile bending or hourglass deformity and can result in widespread venous occlusive dysfunction or in localized venous leakage at the site of fibrotic plaque. Duplex Doppler interrogation of cavernosal arteries allows diagnosis of widespread venous occlusive dysfunction when high end-diastolic velocity and low resistance flows are recorded.[53] In patients with plaque-related leakage, cavernosal waveforms alternations are usually recorded. Moreover, there is Doppler evidence that in patients with severe PD cavernosal-spongiosal communications near the plaques remain patent. In this case, a higher peak systolic velocity and a lower resistance index is detected, compared with the other cavernosal-spongiosal communications, supporting the hypothesis that blood leakage can occur also through these vessels.[48] Although alteration of the venous occlusive mechanism has been claimed to be present in a high percent of Peyronie's patients with erectile dysfunction, the role of arterial inflow must be investigated. In fact, color Doppler ultrasonography shows associated arterial insufficiency in 30–50% of these patients.[43] As mentioned before, plaque encasement of the cavernosal arteries may be considered as a possible cause of arteriogenic erectile dysfunction. A clinically relevant reduction of penile arterial inflow is caused by bilateral cavernosal artery encasement or by unilateral encasement in men with widespread arterial disease. Doppler interrogation of the involved cavernosal artery distal to the plaque usually shows absence of flows or abrupt reduction of the peak systolic velocity.[35,56] Refilling from distal collaterals can present with reversal cavernosal artery flow.
In case of grafting reconstruction, evaluation of the perforating collateral vessels and their relationships with the plaque should be performed. In fact, shortening surgical procedures are preferred to correct dorsal curvature in patients with arterial perforating collaterals between the dorsal and the cavernosal arteries in order to preserve these vessels. If vascular injury occurs, postoperative erectile dysfunction may be present.[43] Some investigators claim that in patients with PD, Doppler analysis can reveal hyperperfusion around the plaques as a sign of inflammation in the active state of the disease, while absence of color signals around the plaques should be considered as a sign of disease stabilization.[57,58] Other investigators, however, failed to confirm these findings. In fact, the presence or absence of flow in microvascular structures cannot be determined with Doppler techniques. Other theories support that the detection of color signals around the plaques results from the patency of leakage pathways, associated with emissary veins passing through the plaque, or from twinkling artifacts produced by calcifications.[58]
DIFFERENTIAL DIAGNOSIS
During ultrasound evaluation of patients referred for PD, other possible causes of penile bending and induration must be considered. The differential diagnoses includes the following conditions: congenital curvature of the penis, chordee with or without hypospadia, dorsal vein thrombosis,[15] albugineal scar and cavernosal fibrosis secondary to local trauma, chronic inflammation, scleroderma,[59] benign or malignant primary or secondary tumors. In particular, epithelioid sarcoma of the penis is a rare, slowly growing mesenchymal neoplasm, that may manifest as focal induration and can mimic PD.[60,61] Although rare, this lesion should be considered in the differential diagnosis of growing plaques. Isolated fibrosis of the penile septum[62] and ventral curvature secondary to fibrosis and scarring of the corpus spongiosum, should be considered, as it can be the result of urethral instrumentation.[63] Subjectively, the tunica albuginea of patients with PD is perceived as globally thickened and more echogenic than in most of the patients with other penile pathologies, even in locations in which no circumscribed plaques are identified.
DIAGNOSTIC ROLE OF OTHER IMAGING MODALITIES
Mammography technique and CT are able to detect calcifications of Peyronie's plaques and to determine the degree of plaque calcification.[64] Noncalcified plaques, however, cannot be shown accurately. The limited information gained by these methods does not support the extent of ionizing radiation used for this purpose.[58]
Dynamic infusion cavernosometry and cavernosography have been used in the past to evaluate erectile function in patients with PD.[63] This procedure is an effective means to obtain objective information on the presence of abnormal venous drainage at the site of the plaques, but is no longer performed because of its invasiveness and potential risk of severe complications. An excellent correlation was found, for the diagnosis of veno-occlusive dysfunction, between cavernosometry/cavernosography and duplex Doppler interrogation of the cavernosal arteries.[64] In fact, erectile function in patients with PD can be evaluated sufficiently by noninvasive Doppler techniques.
MRI is at least as sensitive as ultrasonography to determine the extent of plaque formation and to assess whether the corpora cavernosa and the penile septum are involved.[65] Moreover, MRI is more sensitive than gray-scale ultrasonography in assessing noncalcified plaques at the penile base[66] and can be useful during the follow-up of patients undergoing conservative pharmacologic treatment as an objective measure of therapeutic response. Plaques appear as thickened and irregular low signal intensity areas, on T1- and T2-weighted images in and around the tunica albuginea, with optimal imaging on T2-weighted images.[67] A diffuse, irregular plaque-like thickening of the tunica albuginea can be recognized as well. The invasion of the plaque into the corpora cavernosa or penile septum is seen as areas with irregular dark signal intensity. Several authors suggest that after intravenous gadolinium administration the enhancement of the plaque, offers information regarding the presence of active inflammation within or around the plaque. MRI could therefore be useful in guiding appropriate therapeutic strategy and timing by depicting the stage of the disease. Correlation between plaque enhancement characteristics, pain, and histologically proved inflammation, has been demonstrated by Andresen et al.,[62] while other investigators do not confirm this finding.[68] The signal from microvessels may be difficult to distinguish from enhancement of larger vessels such as dorsal arteries and veins, especially when the plaque is located dorsally. The diffusion of gadolinium within fibrotic plaques may result in artifactual enhancement. Evaluation of contrast enhancement curves after gadolinium administration might be useful to differentiate plaque vascularity from late enhancement of fibrous tissue. Nevertheless, MRI is an expensive imaging modality and is not commonly used in our clinical practice to evaluate patients with PD.
MANAGEMENT OF THE DISEASE
As already mentioned the disease has two phases, an acute inflammatory phase and a latter phase where the symptoms have stabilized, usually in a course of 12 - 18 months. Although spontaneous improvement of the disease has been reported in 3 - 13% of the cases, the symptoms worsen in 30 - 50% of the patients over time while stabilizing in the rest of the patients[69] and usually require an active treatment.
The treatment in the early (acute) phase of the disease is mainly conservative[69] leaving the surgical interventions for the latter, stable phase of the disease in which the role of conservative treatment is not yet well defined. The conservative treatment of PD includes oral pharmacotherapy, intralesional injection therapies, and other topical treatments.[70]
While oral treatments traditionally have been used in the early management of PD, only potassium para-aminobenzoate (Potaba), which is thought to exert an antifibrotic effect, has been classified by the Food and Drug Administration (FDA) as possibly effective in the treatment of PD. Other oral medication include the use of Vitamin E, tamoxifen, colchicine, acetyl esters of carnitine, pentoxifylline, and PDE5 inhibitors, used in the treatment of sexual dysfunction. Injection of active agents directly into the penile plaques represents another conservative option including intralesional injection of steroids, verapamil, clostridial collagenase, and interferon. Other topical treatments that have been used in the management of PD and can also be considered conservative include topical application of verapamil cream, iontophoresis, extracorporeal shockwave lithotripsy of the plaque and the use of traction and vacuum devices in small uncontrolled studies.
The surgical management of the disease is usually kept for a latter phase during the course of the disease when the disease has stabilized. The timing for surgical intervention by some authors is considered to be at 3 months while other authors prolong this period to 6-12 months.[71] The surgical procedures include two major categories, the penile shortening procedures where the main procedure usually applied the Nesbit technique but also other plication techniques,, and the penile lengthening procedures which create a tunical defect on the short (concave) side of the penis, which is covered by various types of graft.[72] Finally in patients with PD and erectile dysfunction not responding to medical treatments the use of either of the aforementioned surgical techniques with concomitant implantation of a penile prosthesis can be considered.[73]
CONCLUSION
In the diagnosis of PD, US has advantages compared to X-ray/CT and MRI. It shows 100% sensitivity in detecting calcified plaques. Even noncalcified plaques of fibrosis can be detected. Sonography is easy to perform, painless, noninvasive, easily repeatable, and has no negative side effects. Exact measurement of plaque size makes it a perfect tool for following up patients either under conservative treatment or after surgery. MRI is still expensive and not widely available imaging modality so it is not suitable for follow-up. Inflammation in and around the plaques, may be detected by the plaque enhancement after gadolinium administration in MRI or by the hypeperfusion in power Doppler investigation. Nevertheless inflammation causes pain and the clinical examination and medical history provide us with all the necessary information so that a costly imaging modality rarely is needed, for further assessment.
Another advantage of sonography and the color duplex technique is the proper assessment of the penile vessel status, as erectile dysfunction occurs in about one-third of the patients. This is important in order to plan the surgical strategy, especially if a penile prosthesis has to be implanted. Sonography provides the best cost effectiveness compared with CT and MRI and it can also be used to follow-up patients who have undergone clinical or surgical treatment and can verify regression of the plaques.[74,75]
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/63/103053
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.King BF. The penis. In: Rumack CM, Wilson SR, Charboneau JW, editors. Diagnostic Ultrasound. St Louis: Mosby - Year Book; 1997. pp. 823–42. [Google Scholar]
- 2.Devine CJ, Jr, Somers KD, Jordan SG, Schlossberg SM. Proposal trauma as the cause of the Peyronie's lesion. J Urol. 1997;157:285–90. doi: 10.1016/s0022-5347(01)65361-8. [DOI] [PubMed] [Google Scholar]
- 3.Jarow JP, Lowe FC. Penile trauma: An etiologic factor in Peyronie's disease and erectile dysfunction. J Urol. 1997;158:1388–90. doi: 10.1016/s0022-5347(01)64222-8. [DOI] [PubMed] [Google Scholar]
- 4.Wahl SM. Inflammation and growth factors. J Urol. 1997;157:303–5. [PubMed] [Google Scholar]
- 5.Gur S, Limin M, Hellstrom WJ. Current status and new developments in Peyronie's disease: Medical, minimally invasive and surgical treatment options. Expert Opin Pharmacother. 2011;12:931–44. doi: 10.1517/14656566.2011.544252. [DOI] [PubMed] [Google Scholar]
- 6.Somers KD, Dawson DM. Fibrin deposition in Peyronie's disease plaque. J Urol. 1997;157:311–5. [PubMed] [Google Scholar]
- 7.Ralph DJ, Schwartz G, Moore W, Pryor JP, Ebringer A, Bottazzo GF. The genetic and bacteriological aspects of Peyronie's disease. J Urol. 1997;157:291–4. [PubMed] [Google Scholar]
- 8.Chilton CP, Castle WM, Westwood CA, Pryor JP. Factors associated in the etiology of Peyronie's disease. Br J Urol. 1982;54:748–50. doi: 10.1111/j.1464-410x.1982.tb13640.x. [DOI] [PubMed] [Google Scholar]
- 9.Carrieri MP, Serraino D, Palmiotto F, Nucci G, Sasso F. A case - control study on risk factors for Peyronie's disease. J Clin Epidemiol. 1998;51:511–5. doi: 10.1016/s0895-4356(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 10.Schneider HJ, Rugendorff EW, Röhrborn C. Pathogenesis, diagnosis and therapy of Induratio penis plastica (IPP) Int Urol Nephrol. 1985;17:235–44. doi: 10.1007/BF02085410. [DOI] [PubMed] [Google Scholar]
- 11.Schiavino D, Sasso F, Nucera E, Alcini E, Gulino G, Milani A, et al. Immunologic findings in Peyronie's disease: A controlled study. Urology. 1997;50:764–8. doi: 10.1016/S0090-4295(97)00333-6. [DOI] [PubMed] [Google Scholar]
- 12.Stewart S, Malto M, Sandberg L, Colburn KK. Increased serum levels of anti-elastin antibodies in patients with Peyronie's disease. J Urol. 1994;152:105–6. doi: 10.1016/s0022-5347(17)32828-8. [DOI] [PubMed] [Google Scholar]
- 13.Prando D. New sonographic aspects of Peyronie Disease. J Ultrasound Med. 2009;28:217–32. doi: 10.7863/jum.2009.28.2.217. [DOI] [PubMed] [Google Scholar]
- 14.De la Peyronie F. Sur quelques obstacles quis opposental ejaculation naturelle de la semence. Mem Acad R Chir. 1 17;3:425–34. [Google Scholar]
- 15.Hakim LS. Peyronies disease: Un update -the role of diagnostics. Int J Impot Res. 2002;14:321–3. doi: 10.1038/sj.ijir.3900871. [DOI] [PubMed] [Google Scholar]
- 16.Pryor JP, Ralph DJ. Clinical presentations of Peyronie Disease. Int J Impot Res. 2002;14:414–7. doi: 10.1038/sj.ijir.3900877. [DOI] [PubMed] [Google Scholar]
- 17.Polkey HJ. Induratio penis plastica. Urol Cut Rev. 1928;32:287–308. [Google Scholar]
- 18.Ludvik W, Wasserburger K. Die Radiumbehandlung der Induratio penis plastica. Z Urol Nephrol. 1968;61:319–25. [PubMed] [Google Scholar]
- 19.Lindsay MB, Schain DM, Grambsch P, Benson RC, Beard CM, Kurland LT. The incidence of Peyronie's disease in Rochester, Minnesota, 1950 through 1984. J Urol. 1991;146:1007–9. doi: 10.1016/s0022-5347(17)37988-0. [DOI] [PubMed] [Google Scholar]
- 20.Sommer F, Schwarzer U, Wassmer G, Bloch W, Braun M, Klotz T, Engelmann U. Epidemiology of Peyronie's disease. Int J Impot Res. 2002;14:379–83. doi: 10.1038/sj.ijir.3900863. [DOI] [PubMed] [Google Scholar]
- 21.Mulhall JP, Creech SD, Boorjian SA, Ghaly S, Kim ED, Moty A, et al. Subjective and objective analysis of the prevalence of Peyronie's disease in a population of men presenting for prostate cancer screening. J Urol. 2004;171:2350–3. doi: 10.1097/01.ju.0000127744.18878.f1. [DOI] [PubMed] [Google Scholar]
- 22.Devine CJ., Jr Editorial International conference on Peyronie's disease. J Urol. 1997;157:272–5. [PubMed] [Google Scholar]
- 23.Darling CA, Davidson JK, Sr, Conway-Welch C. Female ejaculation: Perceived origins, the Grafenberg spot area, and sexual responsiveness. Arch Sex Behav. 1990;19:29–47. doi: 10.1007/BF01541824. [DOI] [PubMed] [Google Scholar]
- 24.Levine LA, Estrada CR, Storm DW, Matkov TG. Peyronie Disease in younger men characteristics and Treatment Results. J Androl. 2003;24:27–32. [PubMed] [Google Scholar]
- 25.Scardino PL, Scott WW, Ant M. The use of tocopherols in the treatment of Peyronie's disease. Ann NY Acad Sci. 1949;52:390–2. [Google Scholar]
- 26.Williams JL, Thomas GG. The natural history of Peyronie's disease. J Urol. 1970;103:75–6. doi: 10.1016/s0022-5347(17)61894-9. [DOI] [PubMed] [Google Scholar]
- 27.Bystrom J, Rubio C. Induratio penis plastica (Peyronie's disease): Clinical features and etiology. Scand J Urol Nephrol. 1976;10:12–20. doi: 10.3109/00365597609179648. [DOI] [PubMed] [Google Scholar]
- 28.Pryor JP, Fitzpatrick JM. A new approach to the correction of the penile deformity in Peyronie's disease. J Urol. 1979;122:622–3. doi: 10.1016/s0022-5347(17)56530-1. [DOI] [PubMed] [Google Scholar]
- 29.Wild RM, Devine CJ, Jr, Horton CE. Dermal graft repair of Peyronie's disease: Survey of 50 patients. J Urol. 1979;121:47–50. doi: 10.1016/s0022-5347(17)56657-4. [DOI] [PubMed] [Google Scholar]
- 30.Palomar JM, Halikiopoulos H, Thomas R. Evaluation of the surgical management of Peyronie's disease. J Urol. 1980;123:680–2. doi: 10.1016/s0022-5347(17)56090-5. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein M, Laungani G, Abrahams J, Waterhouse K. Correction of adult penile curvature with a Nesbit operation. J Urol. 1984;131:56–8. doi: 10.1016/s0022-5347(17)50199-8. [DOI] [PubMed] [Google Scholar]
- 32.Riedl CR, Plas E, Engelhardt P, Daha K, Pfluger H. Iontophoresis for treatment of Peyronie's disease. J Urol. 2000;163:95–9. [PubMed] [Google Scholar]
- 33.Furlow WL, Swenson HE, Lee RE. Peyronie's disease: A study of its natural history and treatment with orthovoltage radiotherapy. J Urol. 1975;114:69–71. doi: 10.1016/s0022-5347(17)66945-3. [DOI] [PubMed] [Google Scholar]
- 34.Hinman F., Jr Etiologic factors in Peyronie's disease. Urol Int. 1980;35:407–13. doi: 10.1159/000280358. [DOI] [PubMed] [Google Scholar]
- 35.Ralph DJ, Brooks MJ, Bottazzo GF, Pryor JP. The treatment of Peyronie's disease with tamoxifen. Br J Urol. 1992;70:648–51. doi: 10.1111/j.1464-410x.1992.tb15836.x. [DOI] [PubMed] [Google Scholar]
- 36.Burford CE, Glen JE, Burfrod EW. Fibrous cavernositis: Further observations with 31 additional cases. J Urol. 1946;56:118–20. doi: 10.1016/S0022-5347(17)69782-9. [DOI] [PubMed] [Google Scholar]
- 37.Gelbard MK, Dorey F, James K. The natural history of Peyronie's disease. J Urol. 1990;144:1376–9. doi: 10.1016/s0022-5347(17)39746-x. [DOI] [PubMed] [Google Scholar]
- 38.Andrews OH, Adeniyi AA, Palumbo F, Pryor JP, Ralph DJ. Atypical Peyronie's Disease. BJU Int. 1999;84:537–8. doi: 10.1046/j.1464-410x.1999.00241.x. [DOI] [PubMed] [Google Scholar]
- 39.Chesney J. Peyronie's disease. Br J Urol. 1975;47:209–18. doi: 10.1111/j.1464-410x.1975.tb03950.x. [DOI] [PubMed] [Google Scholar]
- 40.Ralph DJ. Pathogenesis of Peyronie's disease. MS Thesis, University of London; 1996. [Google Scholar]
- 41.Pryor JP. Peyronie's disease and impotence. Acta Urol Belg. 1988;56:317–21. [PubMed] [Google Scholar]
- 42.Gasior BL, Levine FJ, Howannesian A, Krane RJ, Goldstein I. Plaque associated c orporal veno occlusive dysfunction in idiopathic Peyronie's disease: A pharmacocavernosometric and pharmacocavernosographic study. World J Urol. 1990;8:90–6. [Google Scholar]
- 43.Kadioglu A, Tefekli A, Erol B, Oktar T, Tunc M, Tellaloglu S. A retrospective review of 307 men with Peyronie's disease. J Urol. 2002;168:1075–9. doi: 10.1016/S0022-5347(05)64578-8. [DOI] [PubMed] [Google Scholar]
- 44.Helweg G. The value of imaging procedures in the diagnosis and therapy follow-up of induratio penis plastica. Urologe A. 1992;31:19–23. [PubMed] [Google Scholar]
- 45.Tunuguntla HS. Management of Peyronie's disease – a review. World J Urol. 2001;19:244–50. doi: 10.1007/s003450100209. [DOI] [PubMed] [Google Scholar]
- 46.Bertolotto M, Pavlica P, Serafini G, Quaia E, Zappeti R. Painful penile induration: Imaging findings and management. Radiographics. 2009;29:477–93. doi: 10.1148/rg.292085117. [DOI] [PubMed] [Google Scholar]
- 47.Broderick GA, Arger P. Duplex Doppler ultrasonography: Non invasive assessment of penile anatomy and function. Semin Roentgenol. 1993;28:43–56. doi: 10.1016/s0037-198x(05)80112-9. [DOI] [PubMed] [Google Scholar]
- 48.Bertolotto M, Coss M, Neumaier C. U/S evaluation of patients with Peyronie disease. In: Bertolotto M, editor. Color Doppler US of the penis. Berlin Germany: Springer-Verlag; 2008. pp. 61–9. [Google Scholar]
- 49.Cormio L, Bettocchi C, Ricapito V, Zizzi V, Traficante A, Selvaggi FP. Resistance index as a prognostic factor for prolonged erection after penile dynamic colour Doppler ultrasonography. Eur Urol. 1998;33:94–7. doi: 10.1159/000019518. [DOI] [PubMed] [Google Scholar]
- 50.Kim SC, Seo KK, Park BD, Lee SW. Risk factors for an early increase in dose of vasoactive agents for intracavernous pharmacotherapy. Urol Int. 2000;65:204–7. doi: 10.1159/000064877. [DOI] [PubMed] [Google Scholar]
- 51.Patel U, Amin Z, Friedman E, Vale J, Kirby RW, Lees WR. Colour flow and spectral Doppler imaging after papaverine –induced penile erection in 220 impotent men: Study of temporal patterns and the importance of repeated sampling, velocity asymmetry and the vascular anomalies. Clin Radiol. 1993;48:18–24. doi: 10.1016/s0009-9260(05)80101-1. [DOI] [PubMed] [Google Scholar]
- 52.Montorsi F, Guazzoni G, Bergamaschi F, Consonni P, Rigatti P, Pizzini G, et al. Vascular abnormalities in Peyronie's disease: The role of color Doppler sonography. J Urol. 1994;151:373–5. doi: 10.1016/s0022-5347(17)34952-2. [DOI] [PubMed] [Google Scholar]
- 53.Wilkins CJ, Sriprasad S, Sidhu PS. Colour Doppler ultrasound of the penis. Clin Radiol. 2003;58:514–23. doi: 10.1016/s0009-9260(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 54.Amin Z, Patel U, Friedman EP, Vale JA, Kirby R, Lees WR. Colour Doppler and duplex ultrasound assessment of Peyronie's disease in impotent men. Br J Radiol. 1993;66:398–402. doi: 10.1259/0007-1285-66-785-398. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed M, Chilton CP, Munson KW, Williams JH, Pallan JH, Turner G. The role of colour Doppler imaging in the management of Peyronie's disease. Br J Urol. 1998;81:604–6. [PubMed] [Google Scholar]
- 56.Carbone M, Rossi E, Iurassich S, Amodio F, Gatta G, Brunese L, et al. Assessment of microvascularization around the plaques in Peyronie's disease with Doppler color ultrasonography, power Doppler and ultrasonography contrast media. Radiol Med. 1999;97:66–9. [PubMed] [Google Scholar]
- 57.Fornara P, Gerbershagen HP. Ultrasound in patients affected with Peyronie's disease. World J Urol. 2004;22:365–7. doi: 10.1007/s00345-004-0424-x. [DOI] [PubMed] [Google Scholar]
- 58.Chen TY, Zahran AR, Carrier S. Penile curvature associated with scleroderma. Urology. 2001;58:282. doi: 10.1016/s0090-4295(01)01161-x. [DOI] [PubMed] [Google Scholar]
- 59.Sirikci A, Bayram M, Demirci M, Bakir K, Sarica K. Penile epithelioid sarcoma: MR imaging findings. Eur Radiol. 1999;9:1593–5. doi: 10.1007/s003300050891. [DOI] [PubMed] [Google Scholar]
- 60.Usta MF, Adams DM, Zhang JW, Davis R, Hellstrom WJ. Penile epithelioid sarcoma and the case for a histopathological diagnosis in Peyronie's disease. BJU Int. 2003;91:519–21. doi: 10.1046/j.1464-410x.2003.04137.x. [DOI] [PubMed] [Google Scholar]
- 61.Brant WO, Bella AJ, Garcia MM, Tantiwongse K, Dean RC, Lue TF. Isolated septal fibrosis or hematoma–atypical Peyronie's disease? J Urol. 2007;177:179–82. doi: 10.1016/j.juro.2006.08.065. discussion 183. [DOI] [PubMed] [Google Scholar]
- 62.Afsar H, Sozduyar N. Urethral manipulation syndrome (Kelamy syndrome): Acquired ventral penile deviation. Arch Ital Urol Nefrol Androl. 1992;64:349–51. [PubMed] [Google Scholar]
- 63.Andresen R, Wegner HE, Miller K, Banzer D. Imaging modalities in Peyronie's disease. An intrapersonal comparison of ultrasound sonography, X-ray in mammography technique, computerized tomography, and nuclear magnetic resonance in 20 patients. Eur Urol. 1998;34:128–34. doi: 10.1159/000019698. discussion 135. [DOI] [PubMed] [Google Scholar]
- 64.Jordan GH, Angermeier KW. Preoperative evaluation of erectile function with dynamic infusion cavernosometry/cavernosography in patients undergoing surgery for Peyronie's disease: Correlation with postoperative results. J Urol. 1993;150:1138–42. doi: 10.1016/s0022-5347(17)35708-7. [DOI] [PubMed] [Google Scholar]
- 65.Lopez JA, Jarow JP. Penile vascular evaluation of men with Peyronie's disease. J Urol. 1993;149:53–5. doi: 10.1016/s0022-5347(17)35997-9. [DOI] [PubMed] [Google Scholar]
- 66.Vossough A, Pretorius ES, Siegelman ES, Ramchandani P, Banner MP. Magnetic resonance imaging of the penis. Abdom Imaging. 2002;27:640–59. doi: 10.1007/s00261-001-0136-2. [DOI] [PubMed] [Google Scholar]
- 67.Hauck EW, Hackstein N, Vosshenrich R, Diemer T, Schmelz HU, Bschleipfer T, et al. Diagnostic value of magnetic resonance imaging in Peyronie's disease – a comparison both with palpation and ultrasound in the evaluation of plaque formation. Eur Urol. 2003;43:293–9. doi: 10.1016/s0302-2838(03)00003-4. discussion 299-300. [DOI] [PubMed] [Google Scholar]
- 68.Pretorius ES, Siegelman ES, Ramchandani P, Banner MP. MR imaging of the penis. Radiographics. 2001;21 Spec No:S283–98. doi: 10.1148/radiographics.21.suppl_1.g01oc24s283. discussion S298-9. [DOI] [PubMed] [Google Scholar]
- 69.Vosshenrich R, Schroeder-Printzen I, Weidner W, Fischer U, Funke M, Ringert RH. Value of magnetic resonance imaging in patients with penile induration (Peyronie's disease) J Urol. 1995;153:1122–5. [PubMed] [Google Scholar]
- 70.Mulhall JP, Schiff J, Guhring P. An analysis of the natural history of Peyronie's disease. J Urol. 2006;175:2115–8. doi: 10.1016/S0022-5347(06)00270-9. discussion 2118. [DOI] [PubMed] [Google Scholar]
- 71.Hellstrom WJ, Bivalacqua TJ. Peyronie's disease: Etiology, medical, and surgical therapy. J Androl. 2000;21:347–54. [PubMed] [Google Scholar]
- 72.Kendirci M, Hellstrom WJ. Critical analysis of surgery for Peyronie's disease. Curr Opin Urol. 2004;14:381–8. doi: 10.1097/00042307-200411000-00015. [DOI] [PubMed] [Google Scholar]
- 73.Langston JP, Carson CC., 3rd Peyronie disease: Plication or grafting. Urol Clin North Am. 2011;38:207–16. doi: 10.1016/j.ucl.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 74.Mulhall J, Anderson M, Parker M. A surgical algorithm for men with Combined Peyronie's disease and erectile dysfunction: Functional and satisfaction outcomes. J Sex Med. 2005;2:132–8. doi: 10.1111/j.1743-6109.2005.20113.x. [DOI] [PubMed] [Google Scholar]
- 75.King BF. The penis. In: Rumack CM, Wilson SR, Charboneau JW, editors. Diagnostic Ultrasound. 1st ed. Vol. 1. New York NY: Mosby-Year Book; 1991. pp. 591–607. [Google Scholar]


