Abstract
Objective:
To study the effect of antiretroviral therapy (ART) on clinical, immunologic, and nutritional progression of disease in human immunodeficiency virus (HIV)-infected children for 1 year.
Materials and Methods:
The study included 54 children aged 1.5–15 years who registered at the ART center, Surat, from August 2007 to August 2009. During the study period, the children were followed-up at 6 monthly intervals up to 1 year after starting ART. World Health Organization (WHO) clinical staging and CD4 cell count as per national guidelines, and nutritional status were used to measure clinical and immunologic progression of disease up to 1 year.
Results:
Out of 54 children, mother-to-child transmission was reported in 96.2% children; for 74% of the children, both parents were HIV positive. All the children were classified according to WHO clinical staging into 4 stages and as per CD4 cell count (%), followed up at 6 and 12 months and the benefits with ART reported. At 12 months follow-up, 15% of the study group children had died. Both mean CD4 count and a relative percentage showed significant increase (P < 0.01) in the study group 1 year after ART.
Conclusion:
The present study reports benefits of ART in terms of clinical and immunologic progression of disease, nutritional status of HIV-infected children after 1 year of ART.
Keywords: ART, CD4 cell count, HIV-positive children, nutrition, WHO clinical staging
INTRODUCTION
An estimated 33.2 million people, including 2.5 million children < 15 years of age were infected with the human immunodeficiency virus (HIV) in 2007.[1] In infants and young children, immune system immaturity and high viral loads lead to a high risk of rapid disease progression.[2] Among children and adolescents infected with HIV, low CD4 T-lymphocyte counts have been associated with the failure of weight growth[3] and mortality.[3,4] Mortality in HIV-infected children living in low-income countries compared with high-income countries is still high.[5,6] The course of HIV disease in children has been changed with Highly Active Antiretroviral Therapy (HAART). Various studies in developed countries have shown that rates of mortality, morbidity, and hospitalization decrease significantly in HIV-infected children who receive HAART.[7–9] National AIDS Control Organization (NACO), India, started pediatric antiretroviral therapy (ART) on World AIDS Day, 2006, based on the clinical profile and immunologic status of seropositive children.[10]
A prospective study was conducted from August 2007 to August 2009 in HIV-positive children aged 1.5–15 years at ART center, New Civil Hospital, Surat, India. The present study was planned with the following objectives: To study the epidemiologic and clinical profile of pediatric HIV/acquired immunodeficiency syndrome (AIDS) patients enrolled in the ART center; to study the effect of ART on clinical staging, on sequential CD4 cell count and on the nutritional status at 6 months intervals up to 1 year.
MATERIALS AND METHODS
The study was conducted in HIV-positive children aged 1.5–15 years who had registered at the ART center, New Civil Hospital, Surat, from August 2007 to August 2009. The children in the study age group who came to the ART center for antiretroviral therapy, and had been referred from pediatric outpatient department, pediatric indoor wards, Integrated Counseling and Testing Centre (ICTC), tuberculosis clinics, and other private practitioners of Surat district were enrolled during the study period and were included in the study. The status of HIV positive was confirmed by Enzyme-linked immunosorbent assay test with 3 different kits. The study was approved by ethics committee of the Government Medical College, Surat, in accordance with the Helsinki declaration. Prior permission and written consent had been taken from patients who were enrolled in the study, their parents or caregivers. Confidentiality about the seropositive status and other important details of identified patients was strictly maintained.
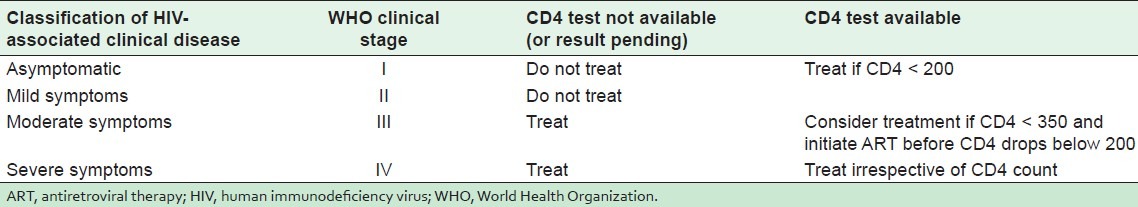
Children were enrolled in the study on the following inclusion criteria: Between 1.5 and 15 years of age, patients eligible to start ART, patients on ART navirapine. The exclusion criteria were as follows: <1.5 years and >15 years of age, transferred to another center, lost to follow up, stopped treatment, not eligible for ART, already registered and on ART before the start of the study. Criteria for initiating ART was based on CD4 count and WHO clinical staging recommended by NACO[11] as indicated in Table 1.
Table 1.
Initiation of ART based on CD4 count and WHO clinical staging
Different ART regimens recommended by NACO,[11] used for pediatric patients include the following: (1) preferred first-line regimen—zidovudine (AZT) + lamivudine (3TC) + nevirapine (NVP), (2) alternative first-line regimen—ziduvudine (AZT) + lamivudine (3TC) + efavirenz (EFV), (3) other regimens—tenofavir (TDF) + lamivudine (3TC) + navirapine (NVP)/efavirenz (EFV) or zidovudine (AZT) + lamivudine (3TC) + tenofavir (TDF).
During the study period, a total of 75 patients were enrolled in the ART center. Of the 75 enrolled patients, 21 dropped out of study because of transfer to another center or migration to another state. The remaining 54 children were enrolled during the study period and followed up at 6-month intervals for 1 year after starting ART. All enrolled patients at the ART center participated in the study.
The purpose and objectives of study were explained to the patients/parents/caregivers. A detailed proforma was completed with information from the parents of the children, and clinicoepidemiologic details collected, including age, sex, age at the time of HIV diagnosis, WHO clinical stage, details on available CD4 count and viral load, any opportunistic infections, birth history, breastfeeding history, anthropometric details, developmental history, vaccination details, sibling history, parent history, including seropositive status for HIV, whether on ART or not at the time of first visit. The nutritional status was graded (weight for age) according to the classification of the Indian Academy of Pediatrics.[12] In the follow-up visits at 6-month intervals, a detailed physical examination was done, with routine investigations as well as CD4 count (%), WHO clinical stage, anthropometric measurements, and any side effects of ART. For the measurement of CD4 count, PARTEC CD4 easy count kit provided by NACO was used.
WHO clinical staging[13] of HIV for infants and children as recommended by NACO was used to classify the children into 4 stages: (1) Stage I—Asymptomatic, (2) Stage II–Mild, (3) Stage III—Moderate, and (4) Stage IV—Severe. Similarly, WHO-defined criteria were used for age-related CD4 value classification[14] as per HIV-associated immunodeficiency. In this study, CD4 values are classified as CD4% instead of actual numbers.
The study data were entered and analyzed by using Epi Info software (version 3.5.1).[15] The analysis was done using Chi-square test or Fisher's exact test considering P value < 0.05 as significant with 95% confidence interval. A comparison was made between different variables for mode of transmission of HIV infection, age group-wise CD4 increase at the beginning and after 6 months of ART, and mean CD4 count at the beginning and 12 months after starting ART for all WHO clinical stages.
RESULTS
Baseline characteristics of the 54 HIV-positive children enrolled in the ART center is shown in Table 2. Out of 54 children, 15% of the children died during the follow up. More than half of the (55.6%) children were male, 46% of the children belonged to the 6–10 years age group. The probable mode of HIV transmission was from mother to child (96.2%), one child reported infection through blood transfusion. HIV–tuberculosis co-infection was reported in 17% children at the beginning of ART. Both parents of 74% of the children were HIV positive, whereas for 26%, only the mother was positive. Both parents were alive for 41% of the children, whereas one quarter of the children had lost both parents. A majority (75%) of the 8 expired children [Table 3], were younger than 5 years, 62.5% were male and had died within 6 months of starting ART.
Table 2.
Baseline characteristics of 54 pediatric patients (1.5–15 years) on antiretroviral therapy
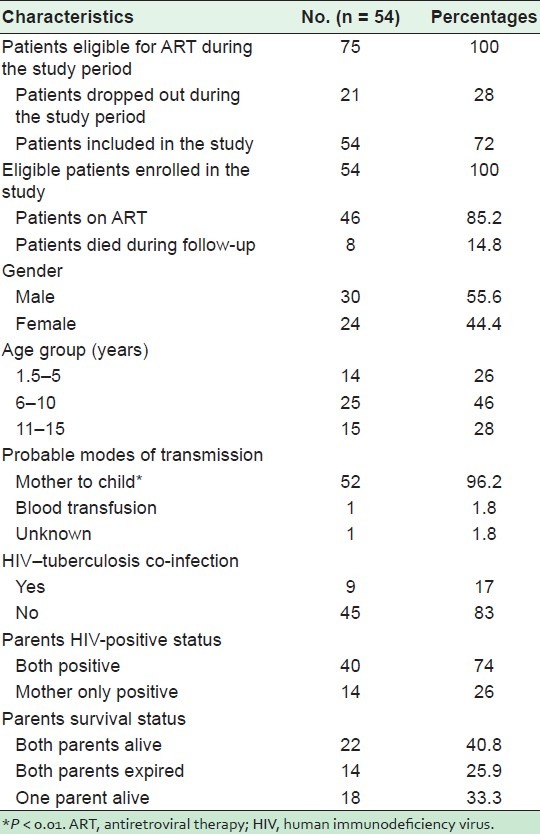
Table 3.
Demographic characteristics of 8 expired patients on antiretroviral therapy
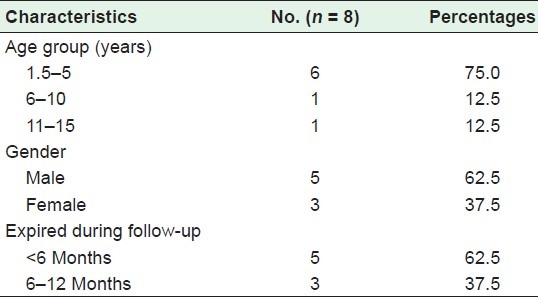
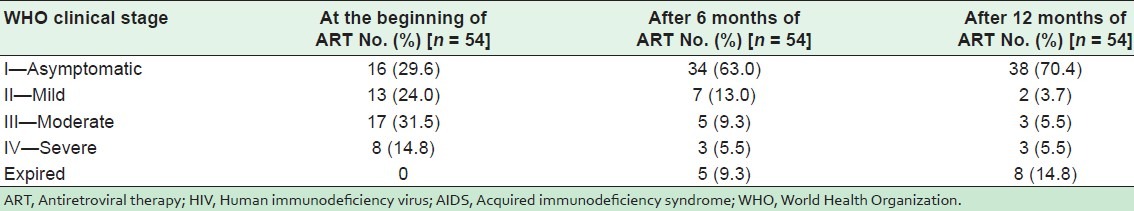
Table 4 shows WHO clinical staging of HIV/AIDS of the 54 children. At the beginning of ART, 29.6% of the children were asymptomatic (stage I), 24% had mild disease (stage II), 31.5% had moderate disease (stage III), and 14.8% had severe disease (stage IV). After 6 months of ART, stage IV cases had decreased from 14.8% to 5.5%, stage III from 31.5% to 9.3% and stage II from 24% to 13%. The number of asymptomatic cases increased from 29.6% to 63% (stage I) after 6 months of ART, and to 70.4% after 12 months of ART.
Table 4.
WHO clinical staging of HIV/AIDS of 54 pediatric patients on ART
Nutritional status was normal grade in only 14.8% of the children at the beginning of ART, but had improved to 45.7% after 12 months of ART [Table 5]. Similarly, the nutritional status of the children in grades 2, 3, and 4 improved after 12 months of antiretroviral therapy, thus increasing the number of children in grade 1.
Table 5.
Nutritional status of 54 HIV-positive pediatric patients on ART
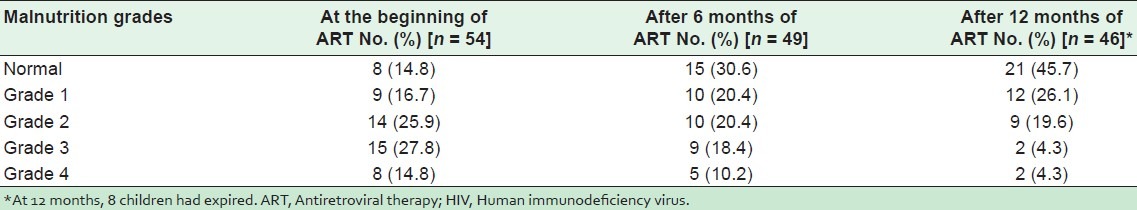
Age group-wise relative CD4% increase as per WHO clinical staging is shown in Table 6. The patients were grouped into 2 in order to compare CD4% with WHO clinical stage as follows: (1) age group 1.5–5 years and (2) age group 5–15 years. In the children aged from 1.5 to 5 years, CD4% increased in WHO clinical stages I, II, and III after 6 and 12 months of ART. After 12 months of ART, in clinical stage I, CD4% increased from 15.25% to 35.36%. But in clinical stage IV in this study, the CD4% remained same even after 12 months of ART. When a comparison was made in the children aged between 5 and 15 years, an increase in CD4% was reported in all 4 clinical stages. The CD4% increase was reported after 6 and 12 months of ART in all children, including stage IV (severe disease).
Table 6.
Age group-wise relative CD4 percentage increase with WHO clinical stages in 54 HIV-positive pediatric patients on ART
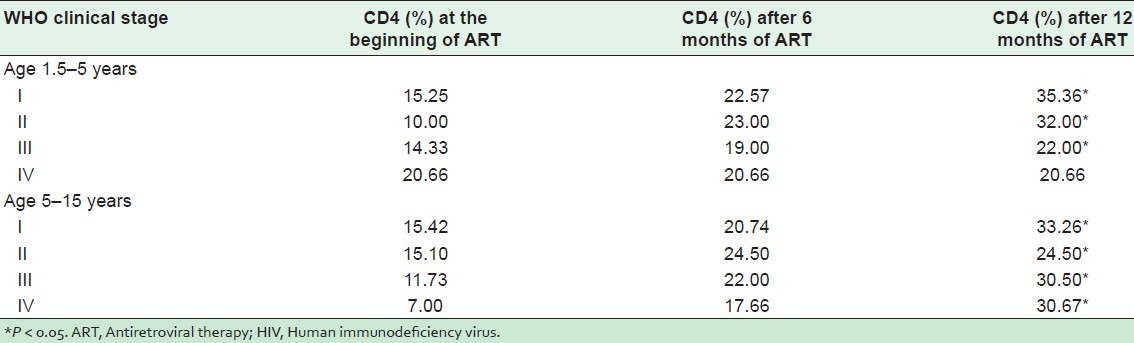
Table 7 shows mean CD4 count and percent improvement for all WHO clinical stages at 12 months of ART in the 2 age groups. Both mean CD4 count and relative percent show a significant increase (P < 0.01) in both age groups 1 year after antiretroviral therapy. Almost all of the 54 children tolerated ART very well with the exception of 2 who developed tuberculosis after 1 year of ART. No other side effects were reported of any child.
Table 7.
Mean CD4 count and percentage of 54 HIV positive children on ART*
DISCUSSION
During the study period, a total of 75 HIV-positive children were eligible for the ART, but 21 (28%) children were excluded for dropping out. The remaining 54 children who had been started on ART were included in the study. Of these 54 children, 8 (14.8%) had expired by the end of 1 year of follow up. Male children (55.6%) were more than females, which was similar to other studies.[16,17] The majority (74%) of the children were above 5 years age in contrast to Agrawal et al.'s[16] and Merchant et al.'s[18] studies.
In this study, the probable mode of transmission of HIV infection was from mother to children 52/54 (96.2%). Only one child was reported to have been infected through blood transfusion. Similar findings were reported by various authors[18,19] in India. Tuberculosis (17%) was the only opportunistic infection reported among study children, unlike other studies on HIV in children.[18,19] High prevalence of tuberculosis in HIV-infected adults leads to an increased risk of this infection in their HIV-infected children.[20] Therefore, it is essential to monitor these children carefully and institute treatment early, since response to treatment is good. As the tuberculin test may be negative in a significant proportion of these children, chest radiographs must be obtained when tuberculosis is suspected. In contrast to the current study, other authors have reported various other opportunistic infections, such as candidiasis, herpes zoster, and pneumocystis carinii pneumonia. Almost three fourths (74%) of the parents of seropositive children had already been diagnosed positive for HIV, whereas for one quarter (26%) of the children, only the mother had been diagnosed as HIV positive. One quarter of the study children had already lost both parents, whereas both parents were still alive for 40.8% of the children.
At the beginning of ART, 29.6% children were asymptomatic (stage I), 24% had mild disease (stage II), 31.5% had moderate disease (stage III), and 14.8% had severe disease (stage IV). Reitz et al.[21] reported more children in stage III (51.2%) and stage IV (29.1%) at the initiation of ART in South Africa compared with present study. After 6 months of ART, stage IV cases had decreased from 14.8% to 5.5%, stage III had decreased from 31.5% to 9.3% and stage II from 24% to 13%. The number of asymptomatic cases increased from 29.6% to 63% (stage I) after 6 months of ART, and up to 70.4% after 12 months of ART. Similar findings have also been reported in South Africa[21] after 39 weeks of ART. Significant reduction in clinical stages from severe to mild/normal clinical stage were reported by Patel et al.[22] and Newell et al.[23] in their respective studies. We report 14.8% mortality at the end of 1 year follow-up after starting ART, which is similar to the Reitz et al.[21] study, which reported 14% mortality after 39 weeks of ART.
The nutritional status was graded according to the classification of the Indian Academy of Pediatrics.[12] At the beginning of ART, 15% children had normal nutrition, 25.9% children in grade 2, 27.8% children in grade 3, and 14.8% children in grade 4 protein energy malnutrition (PEM). Daga et al.[20] in their study at Mumbai reported more pediatric cases with grades 2, 3, and 4 PEM at the time of diagnosis of HIV. The nutritional status was normal in only 14.8% of the children at the beginning of ART, but improved to 45.7% after 12 months of ART. Similarly, the nutritional status of children in grades 2, 3, and 4 improved after 12 months of antiretroviral therapy, consequently increasing the number of children in grade 1. Many studies have reported benefits of ART with respect to both clinical and immunologic progression of disease in HIV-infected children.[24–26]
Two groups formed for comparison of CD4% with WHO clinical stage were (1) age group 1.5–5 years and (2) age group 5–15 years. In the group of children aged 1.5–5 years, CD4% increased in WHO clinical stages I, II, and III after 6 and 12 months of ART. Similar findings were reported in a European collaborative study.[23] After 12 months of ART in clinical stage I, CD4% significantly (P < 0.05) increased from 15.25% to 35.36%, but in clinical stage IV in the present study, the CD4% remained the same even after 12 months of ART. In the comparison made among the group of children aged 5–15 years, CD4% increase was reported in all 4 clinical stages. The significant (P < 0.05) CD4% increase was reported after 6 and 12 months of ART in all children, including stage 4 (severe disease). The mean CD4 count and % improved for all WHO clinical stages at 12 months of ART in the 2 age groups. Both mean CD4 count and relative percent shows a significant increase (P < 0.01) in both age groups 1 year after initiation of antiretroviral therapy. Almost all 54 children tolerated ART very well with the exception of the 2 who developed the tuberculosis after 1 year of ART. No other side effects were reported from any child.
Limitations
Frequent CD4 estimation at 3 months interval will help to track CD4 cell count more effectively, but CD4 estimation was done at 6 months intervals as per NACO guidelines. Viral load estimation was not available in the present study. A longer follow-up is required to observe adverse reactions and any treatment failure. There was no group to provide a better comparison and interpretation of results in the study. Besides, the small sample size may have negatively affected the validity of the study. A larger sample size and a group for comparison would have given the study a much stronger conclusion.
CONCLUSION
The present study reports the benefits of ART in terms of clinical and immunologic disease progression in HIV-infected children after 1 year of treatment. The nutritional status of children on ART also showed an improvement after a year of ART.
ACKNOWLEDGMENT
The authors are thankful to the parents and their children for participating in the study. Our gratitude also goes to the nursing staff of the New Civil Hospital, Surat, for their support.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
REFERENCES
- 1.Geneva, Switzerland: WHO; 2007. Joint United Nations program on HIV/AIDS and World Health Organization (WHO). AIDS epidemic update. Vol. UNAIDS/07.27E/JC1322E. [Google Scholar]
- 2.Obimbo EM, Wamalwa D, Richardson B, Mbori-Ngacha D, Overbaugh J, Emery S, et al. Pediatric HIV-1 in Kenya: Pattern and correlates of viral load and association with mortality. J Acquir Immune Defic Syndr. 2009;51:209–15. doi: 10.1097/qai.0b013e31819c16d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindsey JC, Hughes MD, McKinney RE, Cowles MK, Englund JA, Baker CJ, et al. Treatment mediated changes in human immunodeficiency virus (HIV) type 1 RNA and CD4 cell counts as predictors of weight growth failure, cognitive decline, and survival in HIV infected children. J Infect Dis. 2000;182:1385–93. doi: 10.1086/315865. [DOI] [PubMed] [Google Scholar]
- 4.Seage GR, 3rd, Buchacz K, Weinberg GA, Patel K, McIntosh K, Dankner WM. The pediatric AIDS Severity Score (PASS): A multidimensional AIDS severity adjustment for pediatric HIV infection. J Acquir Immune Defic Syndr. 2006;43:603–10. doi: 10.1097/01.qai.0000242453.20521.4f. [DOI] [PubMed] [Google Scholar]
- 5.Brahmbhatt H, Kigozi G, Wabwire-Mangen F, Serwadda D, Lutalo T, Nalugoda F, et al. Mortality in HIV infected and uninfected children of HIV infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr. 2006;41:504–8. doi: 10.1097/01.qai.0000188122.15493.0a. [DOI] [PubMed] [Google Scholar]
- 6.Villamor E, Misegades L, Fataki MR, Mbise RL, Fawzi WW. Child mortality in relation to HIV infection, nutritional status, and socio-economic background. Int J Epidemiol. 2005;34:61–8. doi: 10.1093/ije/dyh378. [DOI] [PubMed] [Google Scholar]
- 7.Gibb DM, Duong T, Tookey PA, Sharland M, Tudor-Williams G, Novelli V, et al. National Study of HIV in Pregnancy and Childhood Collaborative HIV Paediatric Study. Decline in mortality, AIDS and hospital admissions in perinatally HIV 1 infected children in the United Kingdom and Ireland. BMJ. 2003;327:1019–24. doi: 10.1136/bmj.327.7422.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viani RM, Araneta MR, Deville JG, Spector SA. Decrease in hospitalization and mortality rates among children with perinatally acquired HIV type 1 infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39:725–31. doi: 10.1086/423178. [DOI] [PubMed] [Google Scholar]
- 9.Puthanakit T, Aurpibul L, Oberdorfer P, Akarathum N, Kanjananit S, Wannarit P, et al. Hospitalization and mortality among HIV infected children after receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;44:599–604. doi: 10.1086/510489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National AIDS Control Organization (NACO). National Pediatric HIV/AIDS initiative launched. [Last accessed on 2010 Oct1];NACO News. 2006 2:2–7. Available from: http://www.nacoonline.org/upload/naco%20newsletters/Oct-Dec-4.pdf . [Google Scholar]
- 11.National AIDS Control Organization (NACO). Antiretroviral therapy guidelines for HIV infected adults and adolescents including post exposure prophylaxis. 2007. May, [Last accessed on 2010 Oct 8]. Available from: http://www.nacoonline.org/upload/Policies%20&%20Guidelines/1.%20Antiretroviral%20Therapy%20Guidelines%20for%20HIVInfected%20Adults%20and%20Adolescents%20Including%20Postexposure.pdf .
- 12.Nutrition Sub-Committee of the Indian Academy of Pediatrics. Report of Convener. Indian Pediatr. 1972;7:360. [Google Scholar]
- 13.World Health Organization (WHO). WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV related disease in children younger than 15 years of age. Regional office for South East Asia. WHO. 2006. [Last accessed on 2010 Oct 6]. Available from: http://www.searo.who.int/LinkFiles/Publications_StagingCardsChild.pdf .
- 14.World Health Organization (WHO). HIV staging in children using clinical and immunological criteria. WHO. 2006. [Last accessed on 2010 Oct 6]. Available from: http://www.searo.who.int/LinkFiles/Publications_7.hiv.pdf .
- 15.Center for Disease Control & Prevention (CDC). Epi Info version 3.5.1. [Last accessed on 2008 Aug 12]. Available from: http://www.cdc.gov/epiinfo/
- 16.Agrawal M, Koppikar GV, Ghildiyal R, Chavarkar M, Joshi SM, Lahiri KR. Seropositivity rate for HIV infection in hospitalized children on selective screening. Indian Pediatr. 2001;38:267–71. [PubMed] [Google Scholar]
- 17.Verghese VP, Cherian T, Cherian AJ, Babu PG, John TJ, Kirubakaran C, et al. Clinical manifestations of HIV-1 infection. Indian Pediatr. 2002;39:57–63. [PubMed] [Google Scholar]
- 18.Merchant RH, Oswal JS, Bhagwat RV, Karkare J. Clinical profile of HIV infection. Indian Pediatr. 2001;38:239–46. [PubMed] [Google Scholar]
- 19.Dhurat R, Manglani M, Sharma R, Shah NK. Clinical spectrum of HIV infection. Indian Pediatr. 2000;37:831–6. [PubMed] [Google Scholar]
- 20.Daga SR, Verma B, Gosavi DV. HIV infection in children: Indian experience. Indian Pediatr. 1999;36:1250–3. [PubMed] [Google Scholar]
- 21.Reitz C, Coovadia A, Ko S, Meyers T, Strehalu R, Sherman G, et al. Initial response to protease inhibitor based antiretroviral therapy among children less than 2 years of age in South Africa: Effect of treatment for tuberculosis. J Infect Dis. 2010;201:1121–31. doi: 10.1086/651454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel K, Hernan MA, Williams PL, Seeger JD, McIntosh K, Van Dyke RB, et al. Pediatric AIDS Clinical Trials Group 219/219C Study Team.Long term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: A 10 year follow up study. Clin Infect Dis. 2008;46:507–15. doi: 10.1086/526524. [DOI] [PubMed] [Google Scholar]
- 23.Newell ML, Patel D, Goetghebuer T, Thorne C. European Collaborative Study. CD4 cell response to antiretroviral therapy in children with vertically acquired HIV infection: Is it associated with age at initiation? J Infect Dis. 2006;193:954–62. doi: 10.1086/500842. [DOI] [PubMed] [Google Scholar]
- 24.Luzuriaga K, Bryson Y, Krogstad P, Robinson J, Stechenberg B, Lamson M, et al. Combination treatment with zidovudine, didanosine, and nevirapine in infants with human immunodeficiency virus type I infection. N Engl J Med. 1997;336:1343–49. doi: 10.1056/NEJM199705083361902. [DOI] [PubMed] [Google Scholar]
- 25.Luzuriaga K, McManus M, Mofenson LM, Britto P, Graham BS, Sullivan JL. PACTG 356 Investigators. A trial of three antiretroviral regimens in HIV-1-infected children. N Engl J Med. 2004;350:2471–80. doi: 10.1056/NEJMoa032706. [DOI] [PubMed] [Google Scholar]
- 26.Soh CH, Oleske JM, Brady MT, Spector SA, Borkowsky W, Burchett SK, et al. Long term effect of protease-inhibitor-based combination therapy on CD4 T-cell recovery in HIV-1-infected children and adolescents. Lancet. 2003;362:2045–51. doi: 10.1016/s0140-6736(03)15098-2. [DOI] [PubMed] [Google Scholar]


