Abstract
Background: The large majority of patients with multiple myeloma develop bone lesions and typically receive bisphosphonates to maintain bone health and prevent/delay skeletal-related events. Recent clinical data show that the newer-generation bisphosphonate, zoledronic acid, may confer a survival benefit when combined with antimyeloma therapy. However, clinical data describing the combination of zoledronic acid with newer antimyeloma regimens are limited. Design and Methods: This retrospective study analyzed efficacy and safety outcomes in patients with multiple myeloma receiving first- and second-line treatment with bortezomib, lenalidomide, or thalidomide, with or without zoledronic acid. Results: Records data from 94 patients with Durie-Salmon stage 3A/B multiple myeloma were collated. Most patients (~80%) had bone lesions at study entry. Almost all patients received zoledronic acid at some time during their treatment. Adding zoledronic acid was associated with a numerical, but statistically nonsignificant, benefit in the 1-year progression-free survival rate in both the first- and second-line setting. A similar benefit was observed on the 2-year skeletal-related event rate. Notably, combining zoledronic acid with newer antimyeloma agents was feasible, tolerable, and did not affect the duration of antimyeloma treatment. Three cases of osteonecrosis of the jaw were reported; there were no reports of acute renal failure. Conclusions: This retrospective analysis suggests that extended treatment with zoledronic acid in combination with bortezomib, lenalidomide, or thalidomide is safe and tolerable in patients receiving these therapies as first- or second-line treatment. The addition of zoledronic acid may improve both myeloma and skeletal-related outcomes.
Keywords: Multiple myeloma, novel agents, progression-free survival, skeletal-related events, zoledronic acid
Introduction
In patients with multiple myeloma (MM), clonal expansion of terminally differentiated plasma cells (B cells) in the bone marrow results in the excessive production of monoclonal protein, which leads to anemia, renal failure, and immunodeficiency [1,2]. The increased population of plasma cells also profoundly disrupts the homeostasis within the bone marrow microenvironment. Interactions between myeloma cells and bone marrow stromal cells directly increase growth and survival of myeloma cells through the dysregulation of growth factors and cytokines [3-6] and accelerate the destruction of the bone. Thus, 70% to 95% of MM patients develop osteolytic bone lesions, which are often associated with bone pain and skeletal-related events (SREs) that include spinal cord compression, pathologic fractures, or the need for surgery or radiation to the bone [7].
The bisphosphonates (BPs) pamidronate, zoledronic acid (ZA), and clodronate (approved in the European Union but not in the United States) are employed for the treatment of patients with osteolytic lesions from MM for the prevention of SREs. In-vitro evidence suggests that, in addition to inhibiting malignant osteolysis by their effects on osteoclasts, BPs may have antimyeloma activity [8-13]. Bisphosphonates also may synergize with anticancer agents used in the treatment of myeloma, including dexamethasone, thalidomide, and bortezomib [10,12,14]. Retrospective analyses of data from early clinical trials evaluating the efficacy of BPs to prevent SREs in patients with MM have suggested that BPs may also provide antimyeloma benefits in specific high-risk patient subsets [15-18]. More recently, the results of the Medical Research Council (MRC) Myeloma IX trial in patients with newly diagnosed MM show that patients receiving ZA concurrently with antimyeloma treatment derived a survival advantage compared with patients receiving clodronate [19]. In the overall study population, ZA significantly prolonged median overall survival by 5.5 months (50 months, versus 44.5 months for clodronate; hazard ratio=0.87, p=0.04). Moreover, in an exploratory analysis adjusted for SREs, the effect of ZA on overall survival remained significant (hazard ratio= 0.850, p=0.018), showing that the survival benefit is independent of the effect on SREs. However, patients in the MRC Myeloma IX trial were not treated with antimyeloma therapies based on newer agents such as bortezomib and lenalidomide in their primary or maintenance treatment phases.
This retrospective study evaluated the safety and efficacy of combining ZA with first- and/or second-line antimyeloma therapy, which included both bortezomib and immunomodulatory agents (IMIDs) such as lenalidomide.
Materials and methods
Patients
The records of patients who had received treatment at the Department of Biomedical Science and Human Oncology of the University of Bari Medical School for MM (Durie-Salmon stage 3A or 3B) were analyzed. All patients had clinical records from before initial therapy to a follow-up of at least 2 years. Bone lesions were evaluated by skeletal survey and spinal magnetic resonance imaging (MRI) according to the 2009 International Myeloma Working Group consensus statement [20].
Study design
The analysis presented herein was based on retrospective chart review. Evaluation of MM disease and response to treatment were assessed by standard criteria [21]. Patient treatment followed the standard practice at the participating institution. After initial treatment, patients received maintenance therapy with bortezomib or IMIDs with or without ZA. At relapse, patients were given second-line antimyeloma treatment with or without ZA, as deemed appropriate by the treating physicians. Zoledronic acid was administered as a monthly intravenous infusion at a dose adjusted for renal function, per the prescribing guidelines [22].
The primary endpoint was progression-free survival (PFS), defined as the time interval between initiating antimyeloma therapy and disease progression. Moreover, the effect of the combination of ZA with antimyeloma therapy was evaluated in subsets of patients based on the time that ZA was added to the treatment program: at diagnosis, at relapse, or throughout antimyeloma therapy. Secondary endpoints included safety and the 2-year SRE rate. Data were collated from chart reviews of treatment received, disease assessments, physical examination notes, adverse event (AE) reports, vital signs, and standard laboratory evaluations.
Statistical analysis
Progression-free survival was summarized using the Kaplan-Meier estimation. Descriptive statistics were used for reporting patient characteristics and the incidence of AEs.
Results
Patients
The records of 94 patients treated between 2001 and 2010 were included in this retrospective, single-institution study. Data were reviewed for patient demographics, baseline disease characteristics, and treatments received during the period of observation (Table 1). Approximately 50% of patients had International Staging System stage 1 disease and ~80% of patients had bone lesions at study entry.
Table 1.
Patient demographics, disease characteristics, and treatments received during observation period
| ZA at diagnosis (n = 76) | No ZA at diagnosis (n = 18) | |||
|---|---|---|---|---|
| ZA at relapse (n = 48) | No ZA at relapse (n = 28) | ZA at relapse (n = 15) | No ZA at relapse (n = 3) | |
| Median age, years (range) | 62 (37-84) | 66 (41-86) | 61 (40-88) | 62 (68-90) |
| Male sex, n (%) | 26 (54) | 14 (50) | 8 (53) | 2 (66) |
| ISS stage | ||||
| 1 | 24 (50) | 13 (46) | 8 (53) | 2 (67) |
| 2 | 14 (29) | 7 (25) | 5 (33) | 1 (33) |
| 3 | 10 (21) | 8 (29) | 2 (13) | 0 |
| Durie-Salmon stage 3, n (%) | 48 (100) | 28 (100) | 15 (100) | 3 (100) |
| Renal impairment at relapse, n (%) | 11 (23) | 5 (17) | 3 (20) | 0 (0) |
| Prior ASCT | 16 | 9 | 11 | 3 |
| Paraprotein type, n (%) | ||||
| IgG | 28 (58) | 19 (68) | 8 (53) | 3 (100) |
| IgA | 11 (23) | 5 (18) | 5 (33) | 0 |
| k | 5 (10) | 1 (4) | 2 (13) | 0 |
| λ | 4 (8) | 3 (11) | 0 | 0 |
| Bone metastases at diagnosis | ||||
| Yes/No | 43/51 | 19/92 | 12/33 | 0/34 |
| Median time to first relapse, months (range) | 56.3 (9.5-74.2) | 52.4 (11.2-68.3) | 59.1 (10.8-71.6) | 48.2 (18.2-84.5) |
| Median time to second relapse, months (range) | 23.6 (2.7-34.3) | 14.3 (2.8-29.9) | 19.6 (2.7-34.3) | 9.6 (4.1-12.7) |
| Duration of ZA therapy at diagnosis, months (range) | 28 (8-56) | 32 (12-60) | 0 | 0 |
| Duration of ZA therapy at relapse, months (range) | 16 (8-26) | 0 | 21 (12-72) | 0 |
| Therapy at diagnosis, n (%) | ||||
| Bortezomib | 24 (50) | 17 (61) | 4 (27) | 1 (33) |
| Lenalidomide | 6 (13) | 2 (7) | 4 (27) | 2 (67) |
| Thalidomide | 18 (38) | 9 (32) | 7 (47) | 0 |
| Therapy at relapse, n (%) | ||||
| Bortezomib | 9 (19) | 9 (32) | 9 (60) | 0 |
| Lenalidomide | 28 (58) | 15 (53) | 6 (40) | 2 (67) |
| Thalidomide | 11 (23) | 4 (14) | 0 | 1 (33) |
ISS = International Staging System; ASCT = autologous stem cell transplant; Ig = immunoglobulin.
All 5 patients without bone metastases presented with osteopenia at diagnosis.
All 9 patients without bone metastases presented with osteopenia at diagnosis.
8 patients presented with osteopenia at diagnosis.
2 of 3 patients presented with osteopenia at diagnosis.
Treatment
The majority of patients (81%) received ZA therapy concurrently with first-line treatment, and almost all patients (97%) received ZA at some time during the study. The proportions of patients receiving IMIDs versus bortezomib-based therapies at initial diagnosis were similar. However, a greater proportion of patients received IMIDs at relapse compared with bortezomib (71% vs 29%).
The mean duration of exposure to ZA for patients who received it concurrently with first-line antimyeloma therapy was 19 months versus 15 months for patients who received ZA concurrently with second-line treatment. The mean duration of first-line antimyeloma treatment was 9.2 months in patients who received ZA and 9.4 months in patients who did not receive ZA. The mean duration of second-line antimyeloma treatment was 8.1 months in patients who received ZA and 7.6 months in patients who did not receive ZA.
A subset of patients (n=48) included in this study received ZA throughout (ie, at diagnosis and at relapse) for a mean of 34 months (range, 18 to 52 months). It should be noted that this group included 32 patients who received maintenance ZA once every 3 months after the standard monthly dosing during the first 24 months of treatment.
Progression-free survival
In the analysis of 1-year PFS based exclusively on ZA treatment received, the benefit was greatest among patients who received ZA throughout the treatment period (p = NS; log-rank test for trend < 0.05) (Table 2; Figure 1). The 1-year PFS rate was higher for trend in patients who received ZA at diagnosis compared with patients who did not (Figure 1B). Only 3 patients never received ZA (they did not have bone lesions at diagnosis): the first 2 patients were progression-free for 5 and 8.8 months, and the third patient was progression-free after 13 months’ follow-up.
Table 2.
Progression-free survival rate at 1 year in patient subsets grouped by ZA treatment status and/or antimyeloma therapy
| N | PFS at 1 year, n (%) | Discontinuation, n (%) | p value | |
|---|---|---|---|---|
| All patients | 94 | NS | ||
| ZA at relapse | 63 | 42 (67) | 12 (19) | |
| No ZA at relapse | 31 | 17 (55) | ||
| ZA at diagnosis | 76 | NS | ||
| ZA at relapse | 48 | 34 (71) | 10 (21) | |
| No ZA at relapse | 28 | 16 (58) | ||
| No ZA at diagnosis | 18 | NS | ||
| ZA at relapse | 15 | 8 (54) | 2 (14) | |
| No ZA at relapse | 3 | 1 (44) | ||
| Bortezomib at relapse | 27 | <0.05 | ||
| ZA at relapse | 18 | 15 (82) | 3 (17) | |
| No ZA at relapse | 9 | 5 (55) | ||
| IMIDs at relapse | 67 | NS | ||
| ZA at relapse | 45 | 27 (60) | 9 (20) | |
| No ZA at relapse | 22 | 12 (55) |
NS = not significant.
A.
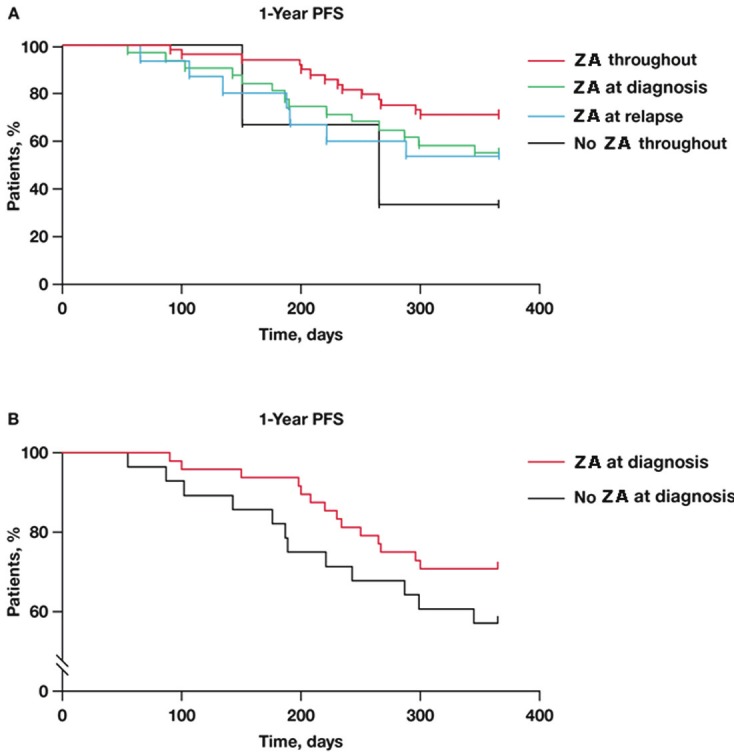
Kaplan-Meier estimate of PFS in patients who received ZA throughout antimyeloma treatment, at diagnosis only, at relapse only, or never received ZA (p = NS; log-rank test for trend < 0.05). (B) Kaplan-Meier estimate of PFS in patients who received ZA at diagnosis compared with patients who did not (p = NS; hazard ratio = 0.5801). NS = not significant; PFS = progression-free survival; ZA = zoledronic acid.
The benefit of ZA was most apparent and significant (p<0.05) in patients receiving bortezomib at relapse (Figure 2A). A similar effect was observed in patients treated with bortezomib plus ZA at diagnosis (Figure 2B).
A.
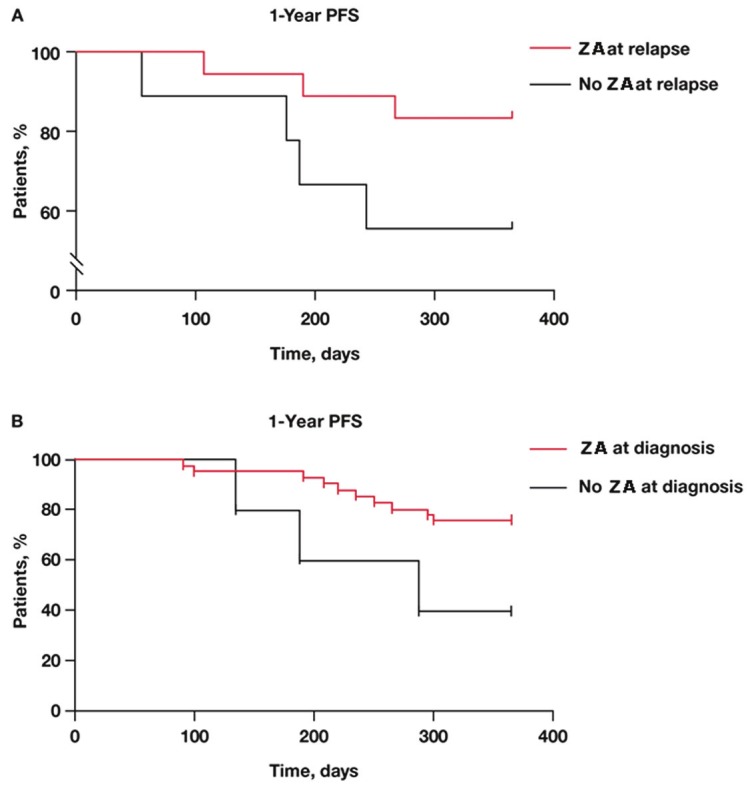
Kaplan-Meier estimate of PFS in patients who received bortezomib at relapse with or without ZA (p = NS; hazard ratio = 0.2517). (B) Kaplan-Meier estimate of PFS in patients who received bortezomib at diagnosis with or without ZA (p = NS; hazard ratio = 0.1436). NS = not significant; PFS = progression-free survival; ZA = zoledronic acid.
Concurrent treatment with ZA improved PFS in patients who received IMIDs at diagnosis compared with patients who received IMIDs alone. In contrast, ZA did not increase PFS after second-line therapy in patients who received IMIDs.
Skeletal-related event rates
The 2-year SRE rates among patient subsets grouped exclusively by ZA treatment received were significantly lower among patients who received ZA at any time during their treatment (Table 3). The most common SREs were new bone lesions detected at skeletal survey and/or new spine fractures at MRI.
Table 3.
Incidence of skeletal-related events at 2 years in patient subsets grouped by ZA treatment status and/or antimyeloma therapy
| N | SRE at 2 years, n (%) | p value | |
|---|---|---|---|
| All patients | 94 | ||
| ZA at relapse | 63 | 35 (56) | <0.05 |
| No ZA at relapse | 31 | 23 (74) | |
| ZA at diagnosis | 76 | ||
| ZA at relapse | 48 | 27 (57) | <0.05 |
| No ZA at relapse | 28 | 21 (75) | |
| No ZA at diagnosis | 18 | ||
| ZA at relapse | 15 | 8 (53) | NS |
| No ZA at relapse | 3 | 2 (66) | |
| Bortezomib at relapse | 27 | ||
| ZA at relapse | 18 | 8 (46) | <0.05 |
| No ZA at relapse | 9 | 6 (66) | |
| IMIDs at relapse | 67 | ||
| ZA at relapse | 45 | 27 (60) | NS |
| No ZA at relapse | 22 | 17 (76) |
NS = not significant.
Safety
The most commonly reported AEs included peripheral neuropathy, thrombocytopenia, neutropenia, constipation, and nausea. All of these events were attributed to antimyeloma therapy. All observed AEs attributable to ZA treatment (Table 4) were grade 1/2. No grade 3/4 AEs related to ZA treatment have been observed. All patients treated with ZA received calcium and vitamin D support.
Table 4.
Adverse events (all grades) occurring in more than 5% of patients (safety population) receiving ZA
| Patients | (n = 91a) |
|---|---|
| Adverse event, n (%) | |
| Any | 54 (59) |
| Fatigue | 13 (14) |
| Bone pain | 10 (11) |
| Asthenia | 8 (9) |
| Asthenia | 8 (9) |
| Pyrexia | 7 (8) |
| Hot flush | 6 (7) |
| Influenza-like illness | 6 (7) |
Three patients never received ZA and are excluded from this table.
All patients had a dental visit, mouth examination, and any dental care deemed appropriate before initiating BP therapy. Three cases of osteonecrosis of the jaw (ONJ) were recorded among patients treated with ZA at diagnosis. These patients received thalidomide (2 patients) and bortezomib (1 patient) as antimyeloma therapy. They were not administered ZA at relapse. No case of ONJ or acute renal insufficiency related to ZA was recorded in patients receiving ZA at relapse.
Discussion
The data from the MRC Myeloma IX trial support the safety and antimyeloma benefit of ZA in patients with newly diagnosed MM across treatment pathways (ie, intensive and non-intensive) in conjunction with the then-prevailing standard antimyeloma regimens [19]. However, at the time that the MRC Myeloma IX trial was conducted, antimyeloma regimens based on bortezomib and newer-generation IMIDs were still in clinical development. Thus, as noted earlier, data on the safety and efficacy of combining ZA with these newer agents are needed. The current retrospective analysis suggests that ZA therapy may be safely combined with these new agents in patients with newly diagnosed MM and at relapse.
Patients who received ZA therapy first-line or throughout treatment had longer disease-free intervals compared with patients who received ZA therapy later or not at all, consistent with the early benefits reported in the MRC Myeloma IX trial, and the reported antimyeloma effects of ZA with bortezomib [12]. Overall, adding ZA to either first- or second-line treatment numerically improved the 1-year PFS rate regardless of the type of antimyeloma therapy received at relapse. Moreover, patients who received ZA throughout the study period had a better 1-year PFS rate (71%) compared with patients who received ZA only at diagnosis or at relapse (58% and 54%, respectively). In addition, patients receiving ZA plus bortezomib had fewer SREs than any other treatment group, which is not surprising given previous clinical evidence of increased bone mineral density in a subset of patients with relapsed MM who received the combination of bortezomib, dexamethasone, and ZA [23]. These effects are consistent with preclinical data showing that bortezomib promotes bone formation in myelomatous and non-myelomatous bone tissue by simultaneously inhibiting osteoclastogenesis and stimulating osteoblastogenesis, thus having an overall anabolic effect on bone [24]. Further, translational data show that bortezomib and ZA have distinct and synergistic inhibitory effects on cell proliferation, adhesion, migration, and expression of angiogenic cytokines (ie, VEGF, bFGF, HGF, and PDGF), suggesting that combined therapy may improve MM-related outcomes [12]. Taken together, these data suggest that ZA may delay disease progression and improve skeletal health in patients with symptomatic MM in clinical practice and in the context of the newer antimyeloma regimens (eg, those including bortezomib and/or lenalidomide). These data are consistent with the survival advantage associated with ZA versus clodronate demonstrated in the MRC Myeloma IX trial [19] and with the earlier data from the phase 3 head-to-head comparison of denosumab versus ZA, wherein it was shown that patients treated with ZA had longer overall survival compared with patients who were treated with denosumab (a monoclonal antibody that neutralizes RANKL) [25].
During the course of the current study, 3 cases of ONJ were reported. This event rate (~3%) is not unexpected, as this patient population appears to be more susceptible to ONJ compared with patients receiving complex anticancer treatments for other solid tumors [26]. However, the assessment of the true incidence of ONJ in retrospective analyses is limited by the confounding effects of the use of different diagnostic criteria and the availability of records for review. It should be noted that the implementation of recommendations for prevention of ONJ [27] has resulted in a reduction of the incidence of ONJ [28], which is supported by recent prospective trials [19,25]. Further, the use of novel (non-BP) antiresorptives in patients with cancer is also associated with a risk for ONJ. Indeed, in a recent phase 3 randomized study that compared the efficacy and safety of denosumab versus those of ZA, the incidence of ONJ in patients with skeletal involvement from advanced cancer, including MM, was similar in the ZA (1.3%) and denosumab (1.1%) arms (p=1.00) [25].
In addition, there were no reports of acute renal failure, despite the fact that approximately 20% of patients had renal impairment at diagnosis. This is of particular relevance in patients with MM who receive ZA because renal (glomerular) filtration must clear their serum of both ZA and the excessive immunoglobulins; these could, in combination, increase the risk of renal dysfunction.
Based on the data from the MRC Myeloma IX trial, recent guidelines of the National Comprehensive Cancer Network have been amended to recommend ZA in all patients with MM [29]. Similarly, the 2011 update to the United Kingdom MM treatment guidelines and a Canadian perspective paper recommend BP therapy for all patients with MM (with or without bone lesions) [30,31]. Taken together with the clinical practice data reported herein, it is reasonable to suggest that ZA may be safely combined with a diverse array of antimyeloma agents and regimens. Moreover, rates of ONJ occurrence were low, and events were generally low grade.
Patients without bone lesions at diagnosis received ZA as treatment for reduced bone mineral density. The mean time to development of a first bone lesion in these patients was 19 months (range, 8 to 56 months). Because of the small number of patients without bone lesions at diagnosis in the current study, the benefit of ZA in this population needs further evaluation.
As with all retrospective studies, this analysis had several limitations. First, there is a lack of controlled comparisons as seen in randomized trials. Second, there were imbalances in the proportion of patients receiving specific antimyeloma treatments, which are known to differ in efficacy. Moreover, the effects of primary antimyeloma treatments on bone and PFS cannot be evaluated in these analyses. Third, there may have been imbalances in disease characteristics and skeletal involvement among patient subgroups during the course of treatment, which may be further confounded by differences in imaging assessments of skeletal disease and interpretation of data. Finally, treatment initiation/continuation decisions could be based by clinical practice preferences of individual physicians.
Nevertheless, the overall analysis suggests that early initiation of ZA treatment delayed disease progression in patients with MM in clinical practice, and that ZA was well tolerated with a broad array of antimyeloma agents and regimens. In addition, the study suggests that ZA and bortezomib may act synergistically to improve skeletal- and cancer-related outcomes. Future prospective studies will provide further insight on the effects of combining ZA with bortezomib and newer-generation IMIDs.
Acknowledgments
We thank Jerome F Sah, PhD, ProEd Communications, Inc.®, for his medical editorial assistance with this manuscript. This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), Investigator Grant and Special Program Molecular Clinical Oncology 5 per thousand (number 9965), Milan, the EU Multiple Myeloma Program FP7 OVER-MyR HEALTH.2011.2.4.1-2. and the Ministry of Health (Progetto PRIN 2009), Rome, Italy. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation.
References
- 1.Kumar SK, Mikhael JR, Buadi FK, Dingli D, Dispenzieri A, Fonseca R, Gertz MA, Greipp PR, Hayman SR, Kyle RA, Lacy MQ, Lust JA, Reeder CB, Roy V, Russell SJ, Short KE, Stewart AK, Witzig TE, Zeldenrust SR, Dalton RJ, Rajkumar SV, Bergsagel PL. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009;84:1095–1110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324–339. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- 4.Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, Anderson KC. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- 5.Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC. Advances in biology of multiple myeloma: clinical applications. Blood. 2004;104:607–618. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]
- 6.Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host interactions. Nat Rev Cancer. 2002;2:175–187. doi: 10.1038/nrc746. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 8.Shipman CM, Rogers MJ, Apperley JF, Russell RG, Croucher PI. Bisphosphonates induce apoptosis in human myeloma cell lines: a novel anti-tumour activity. Br J Haematol. 1997;98:665–672. doi: 10.1046/j.1365-2141.1997.2713086.x. [DOI] [PubMed] [Google Scholar]
- 9.Tassone P, Forciniti S, Galea E, Morrone G, Turco MC, Martinelli V, Tagliaferri P, Venuta S. Growth inhibition and synergistic induction of apoptosis by zoledronate and dexamethasone in human myeloma cell lines. Leukemia. 2000;14:841–844. doi: 10.1038/sj.leu.2401770. [DOI] [PubMed] [Google Scholar]
- 10.Ural AU, Yilmaz MI, Avcu F, Pekel A, Zerman M, Nevruz O, Sengul A, Yalcin A. The bisphosphonate zoledronic acid induces cytotoxicity in human myeloma cell lines with enhancing effects of dexamethasone and thalidomide. Int J Hematol. 2003;78:443–449. doi: 10.1007/BF02983818. [DOI] [PubMed] [Google Scholar]
- 11.Corso A, Ferretti E, Lunghi M, Zappasodi P, Mangiacavalli S, De Amici M, Rusconi C, Varettoni M, Lazzarino M. Zoledronic acid down-regulates adhesion molecules of bone marrow stromal cells in multiple myeloma: a possible mechanism for its antitumor effect. Cancer. 2005;104:118–125. doi: 10.1002/cncr.21104. [DOI] [PubMed] [Google Scholar]
- 12.Moschetta M, Di Pietro G, Ria R, Gnoni A, Mangialardi G, Guarini A, Ditonno P, Musto P, D'Auria F, Ricciardi MR, Dammacco F, Ribatti D, Vacca A. Bortezomib and zoledronic acid on angiogenic and vasculogenic activities of bone marrow macrophages in patients with multiple myeloma. Eur J Cancer. 2010;46:420–429. doi: 10.1016/j.ejca.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–392. [PubMed] [Google Scholar]
- 14.Schmidmaier R, Simsek M, Baumann P, Emmerich B, Meinhardt G. Synergistic antimyeloma effects of zoledronate and simvastatin. Anticancer Drugs. 2006;17:621–629. doi: 10.1097/01.cad.0000215058.85813.02. [DOI] [PubMed] [Google Scholar]
- 15.Aviles A, Nambo MJ, Neri N, Castaneda C, Cleto S, Huerta-Guzman J. Antitumor effect of zoledronic acid in previously untreated patients with multiple myeloma. Med Oncol. 2007;24:227–230. doi: 10.1007/BF02698044. [DOI] [PubMed] [Google Scholar]
- 16.Berenson J, Dimopoulos M, Chen Y-M. Improved survival in patients with multiple myeloma and high BALP levels treated with zoledronic acid compared with pamidronate: univariate and multivariate models of hazard ratios. 48th ASH Annual Meeting and Exposition; 2006. abstract 3589. [Google Scholar]
- 17.McCloskey EV, Dunn JA, Kanis JA, MacLennan IC, Drayson MT. Long-term follow-up of a prospective, double-blind, placebo-controlled randomized trial of clodronate in multiple myeloma. Br J Haematol. 2001;113:1035–1043. doi: 10.1046/j.1365-2141.2001.02851.x. [DOI] [PubMed] [Google Scholar]
- 18.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, Lipton A, Keller A, Ballester O, Kovacs M, Blacklock H, Bell R, Simeone JF, Reitsma DJ, Heffernan M, Seaman J, Knight RD. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J. Clin. Oncol. 1998;16:593–602. doi: 10.1200/JCO.1998.16.2.593. [DOI] [PubMed] [Google Scholar]
- 19.Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Owen RG, Feyler S, Ashcroft AJ, Ross F, Byrne J, Roddie H, Rudin C, Cook G, Jackson GH, Child JA National Cancer Research Institute Haematological Oncology Clinical Study Group. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O, Siegel D, Lokhorst H, Kumar S, Rajkumar SV, Niesvizky R, Moulopoulos LA, Durie BG IMWG. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia. 2009;23:1545–1556. doi: 10.1038/leu.2009.89. [DOI] [PubMed] [Google Scholar]
- 21.Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV International Myeloma Working Group. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 22.Zometa (zoledronic acid) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011. Available at: http://www.pharma.us.novartis.com/product/pi/pdf/Zometa.pdf. [Google Scholar]
- 23.Terpos E, Christoulas D, Kokkoris P, Anargyrou K, Gavriatopoulou M, Migkou M, Tsionos K, Dimopoulos MA. Increased bone mineral density in a subset of patients with relapsed multiple myeloma who received the combination of bortezomib, dexamethasone and zoledronic acid. Ann Oncol. 2010;21:1561–1562. doi: 10.1093/annonc/mdq259. [DOI] [PubMed] [Google Scholar]
- 24.Pennisi A, Li X, Ling W, Khan S, Zangari M, Yaccoby S. The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am J Hematol. 2009;84:6–14. doi: 10.1002/ajh.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A, Vadhan-Raj S, von Moos R, Willenbacher W, Woll PJ, Wang J, Jiang Q, Jun S, Dansey R, Yeh H. Randomized, double-blind study of denosumab versus zoledronic Acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 26.Dimopoulos MA, Kastritis E, Anagnostopoulos A, Melakopoulos I, Gika D, Moulopoulos LA, Bamia C, Terpos E, Tsionos K, Bamias A. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica. 2006;91:968–971. [PubMed] [Google Scholar]
- 27.Weitzman R, Sauter N, Eriksen EF, Tarassoff PG, Lacerna LV, Dias R, Altmeyer A, Csermak-Renner K, McGrath L, Lantwicki L, Hohneker JA. Critical review: updated recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in cancer patients--May 2006. Crit Rev Oncol Hematol. 2007;62:148–152. doi: 10.1016/j.critrevonc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Dimopoulos MA, Kastritis E, Anagnostopoulos A, Melakopoulos I, Gika D, Moulopoulos LA, Bamia C, Terpos E, Tsionos K, Bamias A. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20:117–120. doi: 10.1093/annonc/mdn554. [DOI] [PubMed] [Google Scholar]
- 29.Anderson KC, Alsina M, Bensinger W, Biermann JS, Chanan-Khan A, Cohen AD, Devine S, Djulbegovic B, Gasparetto C, Huff CA, Jagasia M, Medeiros BC, Meredith R, Raje N, Schriber J, Singhal S, Somlo G, Stockerl-Goldstein K, Tricot G, Vose JM, Weber D, Yahalom J, Yunus F National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Multiple Myeloma, v. 1.2012. National Comprehensive Cancer Network. 2011:1–59. doi: 10.6004/jnccn.2009.0061. [DOI] [PubMed] [Google Scholar]
- 30.Bird JM, Owen RG, D'Sa S, Snowden JA, Pratt G, Ashcroft J, Yong K, Cook G, Feyler S, Davies F, Morgan G, Cavenagh J, Low E, Behrens J Haemato-oncology Task Force of British Committee for Standards in Haematology (BCSH) and UK Myeloma Forum. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol. 2011;154:32–75. doi: 10.1111/j.1365-2141.2011.08573.x. [DOI] [PubMed] [Google Scholar]
- 31.Reece D, Sebag M, White D, Song K. A Canadian perspective on the use of bisphosphonates in the clinical management of multiple myeloma. New Evidence in Oncology. Canadian Vision for Oncology. 2011 Mar;:1–7. [Google Scholar]


