Abstract
Background: Heart transplantation (HTX) has become an established therapy for patients with end-stage heart failure. Endomyocardial biopsy (EMB) still represents the gold standard for routine surveillance of heart transplant rejection. The objective of this article is to report our experience regarding the use of EMB in monitoring heart transplant recipients. Methods: We evaluated retrospectively all patients who underwent orthotopic HTX between 2000 and 2011 at our hospital. From all patients, we created a follow-up, determined the number of EMB events and described the complications associated with this procedure. Results: HTX was performed in 142 cases at our center in the last 11 years (1.3% of the total of 10693 cardiac surgical operations in that period). Further 9 patients visited our department for monitoring after HTX performed at an external center (total: 151). For all patients, a total of 1896 EMB events have been recorded. The majority of biopsies were performed through the right internal jugular vein. The overall complication rate was 1% (n=19). Conclusions: The histological examination of right ventricular EMB still represents the gold standard of care for cardiac allograft rejection monitoring. EMB is an invasive, but safe and dedicated diagnostic procedure. However, the usefulness of recent non-invasive diagnostic approaches as an adjunct tool in monitoring for rejection remains to be further analyzed.
Keywords: Endomyocardial biopsy, cardiac transplantation, allograft rejection, histopathological evaluation, immunosuppression
Introduction
Heart transplantation (HTX) has become an established therapy in the treatment of terminal heart failure [1,2]. While the advances in immunosuppressive therapy has led to a concomitant decrease in cardiac allograft rejection, the risk of acute transplant failure as a consequence of allograft rejection still represents one of the major complications of HTX [3,4]. Thus close monitoring of heart transplant recipients to timely diagnose significant transplant rejection in order to modify immunosuppressive therapy is a major challenge for cardiologists and heart surgeons involved in the management of affected patients.
The history of EMB dates back to the last century, when Kent and Sutton first performed a transthoracic needle biopsy of the human ventricular myocardium in 1956 [5] and Caves and colleagues [6] and Richardson [7] first successfully used a flexible bioptome for percutaneous access through the right internal jugular vein. Later, in 1984, Anderson and colleagues described the access through the right femoral vein and femoral artery for access to the right and left ventricle as an alternative vascular approach [8]. In the meantime, EMB became a widely used routine procedure for the diagnosis of unexplained ventricular dysfunction [9]. Currently, right ventricular endomyocardial biopsy (EMB) still represents the clinical gold standard for screening for graft rejection after HTX and is actually the only tool of diagnosis and classification of allograft rejection [10].
Diagnosis of cellular allograft rejection relies mainly on the presence of interstitial mononuclear (lymphoid) aggregates within myocardial tissue. The number and extent of cellular aggregates and the presence or absence of myocardial damage (cell necrosis) defines the different degrees of cellular rejection on histopathological evaluation of EMB [11]. A standardized grading system for histological assessment of cardiac allograft rejection on EMB has been published by the International Society for Heart and Lung Transplantation (ISHLT) in 1990 [12], and revised in 2005 [13].
In the present study we report our experience with EMB in patients who underwent cardiac transplantation between 2000 and 2011 at our heart center and describe the complications and limitations of this invasive procedure, analyze the clinic-pathological spectrum, and discuss future directions for detecting transplant rejection.
Patients and methods
All patients who underwent an HTX from 2000 to 2011 at the Center for Cardiac Surgery, University Hospital of Erlangen, Germany have been included in this retrospective analysis. These were 142 cases (1.3%) of all 10693 consecutive open heart procedures performed at our department in the same period (Figure 1). Additionally, we included 9 patients who underwent HTX in other hospitals, but did their routine follow-up examinations at our department because of changing residence.
Figure 1.
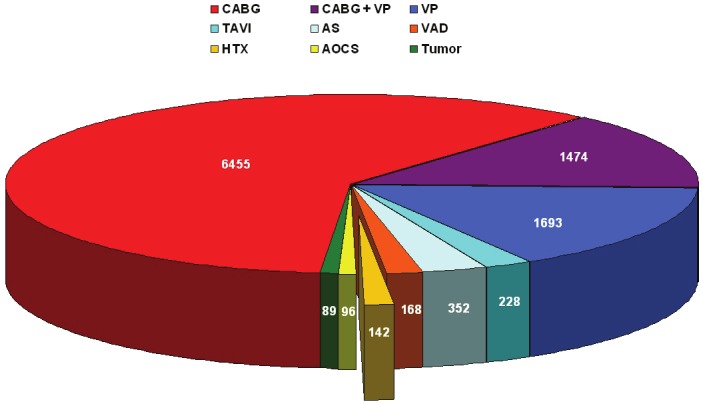
Cardiac Surgery at the University of Erlangen between 2000 and 2011. CABG = Coronary Artery Bypass Grafting; VP = Valve Procedures; TAVI = Transcatheter Aortic Valve Implantation; AS = Aortic Surgery; VAD = Ventricular Assist Device; HTX = Heart Transplantation; AOCS = Any Other Cardiac Surgery.
From all these 151 patients, 1896 EMBs were obtained either as routine surveillance protocol biopsies or as a diagnostic tool for cases with allograft dysfunction and for clinically suspected rejection. The normal biopsy schedule was: weekly for the first month, every 2 weeks for the next month, once for the next 4 weeks, once for the next 6 weeks, then every 3 months for the next two years, and afterwards every 6 months for the next years.
Biopsies were performed without further premedication. Usually, we used the right internal jugular vein approach. The vein puncture was performed under local anaesthesia (2% lidocaine) followed by insertion of a 7-French sheath in Seldinger technique. Under x-ray control, we used a special biopsy-forceps to harvest 2-6 (usually 4) fragments of myocardial tissue from the apical segment of the right side of the interventricular septum (IVS). Harvested myocardial tissue pieces were then sent immediately for further histopathological evaluation in normal saline.
All patients were continuously monitored via electrocardiogram during the EMB and got an intravenous antibiosis (1500 mg cefuroxim) after the procedure. On the Ward, a transthoracic echocardiogram (TTE), a 12-lead electrocardiogram (ECG) and a complete blood screening were performed after all EMB procedures. Additionally, all cardiac transplant recipients underwent right and left heart catheterization once every year after HTX.
EMB specimens (usually 4 biopsy fragments) were processed at the same day using a fast embedding program to enable an initial result within 4-6 hours. For histological assessment, a total of six tissue sections from different cutting levels were prepared from the paraffin block, mounted on glass slides and stained routinely with hematoxylin and eosin. Histological evaluation was then performed by two pathologists, of them at least one with sufficient experience in cardiac allograft rejection assessment. The presence or absence of perivascular or interstitial cellular aggregates and evidence of myocyte damage were used for assigning a rejection grade according to both the old and the revised ISLHT classification. Cases with equivocal findings were then subjected to deeper cutting. No special stains were used routinely for detection of humoral rejection. However, biopsies were judged for the presence of histological findings suggestive of humoral rejection. C4d immunostaining was performed in patients with histological suspicion of humoral rejection and those with clinically suspected or previously proven humoral rejection.
Standard initial post-HTX regimen of immunosuppression included cyclosporine A (CsA), azathioprine and steroids. In 2002 this regimen was changed to CsA, mycophenolate mofetil (MMF) and steroids. If repeatedly severe allograft rejections appeared, CsA was substituted with tacrolimus. Mammalian target of rapamycin (mTOR) inhibitors (everolimus) were used since 2004, either as calcineurin inhibitor (CNI)-free immunosuppression or combined with CNI after progressive renal insufficiency or repeated allograft rejection. Concomitant oral prednisone was given 10 mg for the first three months, 7.5 mg for the next three months and 5 mg lifelong.
Results
From the151 patients, there were altogether 1896 EMB events. Routinely, the right internal jugular vein was the vascular access. Only in 7 from these 151 patients (4.6%) biopsies were performed through the left or right femoral vein due to vascular stenosis of the jugular vein.
The majority of our recovered patients were men (n=125, 82.8%). The mean age of the 125 men at the time of transplantation was 53.4 ± 9.9 years (range 16.4 – 68.2 years). The mean age of the 26 females was 47.8 ± 17.3 years (range 3.7 – 69.1 years).
A total follow-up of 607.1 patient years was created (mean 4.0 years). The follow-up varied from one day to approximately 11 years. The 30 day mortality was 10.6% (16/151). The one year mortality was 26.5% (40/151).
Among the 1896 biopsies, 765 (40.3%) showed no evidence of cellular rejection (scored grade 0R). Among the remainder, mild (grade 1R), moderate (grade 2R) and severe (grade 3R) rejection was seen in 856 (45.1%), 137 (7.2%) and 25 (1.3%) EMB according to the ISHLT guidelines [12] and [13]. Only in 53 cases (2.8%) EMB was anatomically or technically not possible, and in 60 cases (3.2%) the harvested EMB fragments contained no myocardial tissue (Figure 2). Thus taken together, moderate to severe cellular allograft rejection (grade 2R or 3R) necessitating a cortisone treatment over 3 days was seen in 8.5% (n=162) biopsies. Repeated moderate or severe allograft rejection required modification of immunosuppressive therapy in 44 of the 151 cardiac transplant patients (29.1%).
Figure 2.
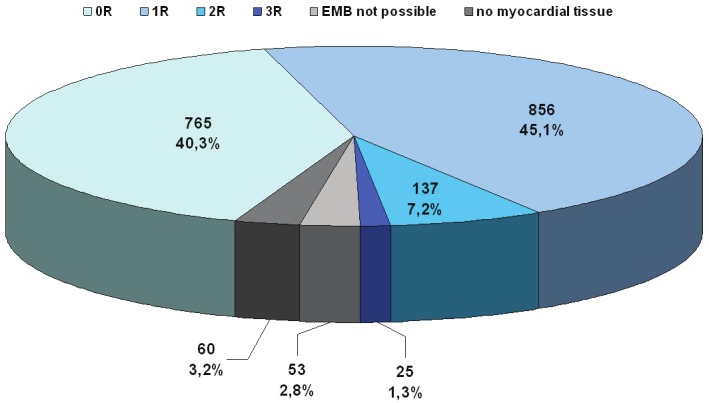
Distribution of 1896 biopsies scored by the International Society for heart and Lung Transplantation (ISHLT) guidelines [Billingham 1990], and revised in 2005 [Stewart 2005]. Grade 0R = no rejection; grade 1R = mild; grade 2R = moderate; grade 3R = severe rejection.
Accordant to our biopsy time schedule (see Patients and Methods) the majority of EMB were performed during the first month (Figure 3). Interestingly, most grade 2R or 3R rejections occurred during the first 36 months (n=157, 96.9%). There were only 5 incidences of 2R/3R rejection after 36 months. We could not identify any clinical characteristics which could identify similar patients at risk for this.
Figure 3.
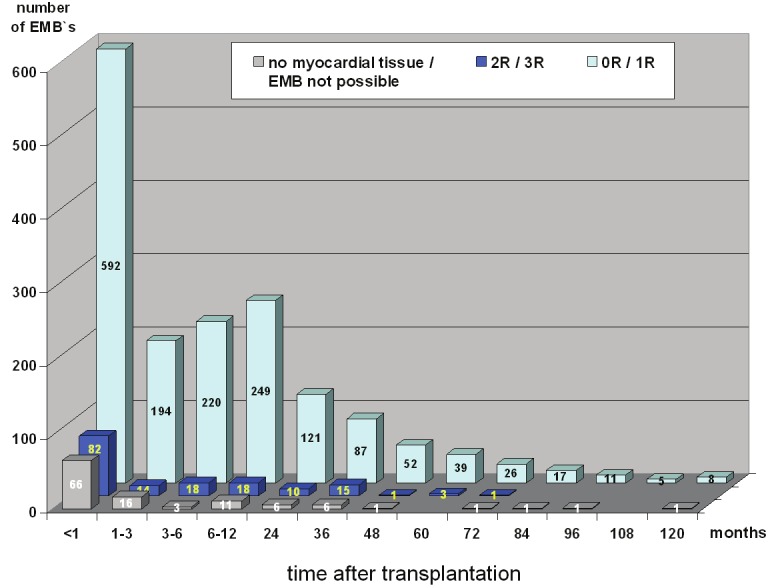
Time schedule of 1896 performed biopsies scored by the ISHLT guidelines. Grade 0R = no rejection; grade 1R = mild; grade 2R = moderate; grade 3R = severe rejection.
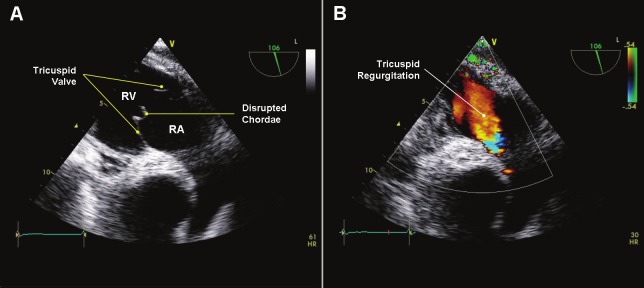
The rate of serious acute complications and long-term sequelae was 1.0% (19 of 1896 EMB) in our study. Twelve patients developed moderate or severe tricuspid regurgitation with increased pulmonary artery systolic pressure (Figure 4). Subsequently, six of these patients received mechanical or biological tricuspid valve prosthesis. In six other patients the routine transthoracic echocardiography showed pericardial effusions around the right and left ventricle with signs of tamponade. Afterwards, these patients underwent an inferior pericardiocentesis on the same day. Another patient developed supraventricular tachycardia during the EMB, but remained asymptomatic with stable vital signs. We did not detect any case of death, hemothorax, permanent arrhythmias, embolic stroke or pulmonary embolism related to the EMB procedure.
Figure 4.
Transesophageal echocardiography: A: Transgastric view showed the right ventricle inflow tract with a disrupted chordae on the tricuspid valve. B: The Colour-Doppler sonography demonstrated a severe tricuspid regurgitation.
Histopathological features on EMB
The histopathological features seen on EMB were not different from previous studies and representative examples of the different grades of cellular rejection are depicted in Figures 5 and 6. Finding of Quilty effect was relatively common (28%) and most cases did not receive a subclassification into type A or type B Quilty effect (Figure 7). Some of these cases showed multiple Quilty lesions with focal evidence of isolated myocyte damage within the most superficial myocardium and have not been considered as evidence of rejection (Figure 7). However, the precise classification and clinical relevance of these findings remains controversial.
Figure 5.
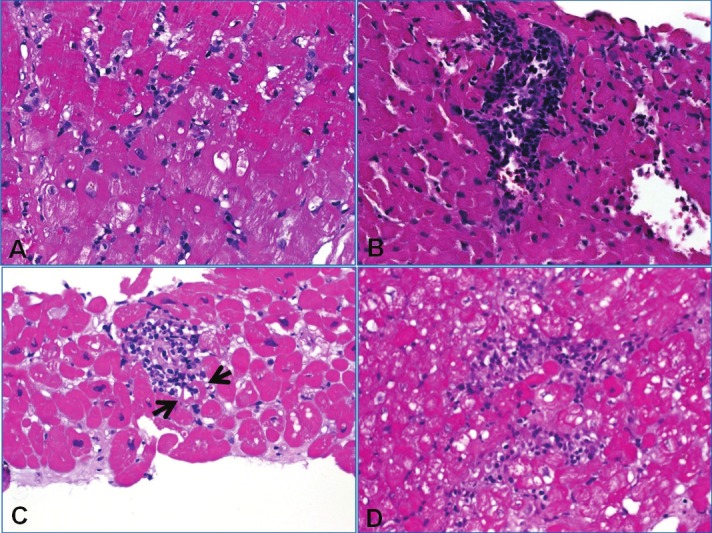
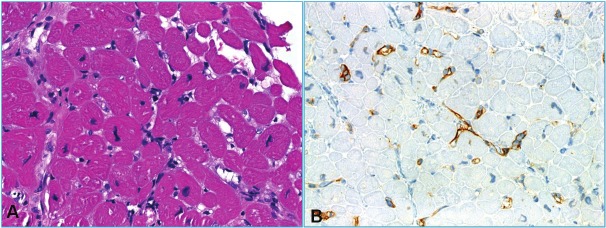
A: EMB negative for cellular and humoral rejection (grade 0R) showing mild interstitial edema suggesting mild ischemic effect (note isolated inflammatory cells without forming aggregates). B: Grade 1R revealed compact interstitial mononuclear cell aggregate without evident myocyte damage. C: This case of 2R showed a focus of myocyte damage (muscle fiber between arrows was infiltrated and damaged by mononuclear cells. D: This less compact inflammatory aggregate encased multiple degenerating cardiomyocytes (2R).
Figure 6.
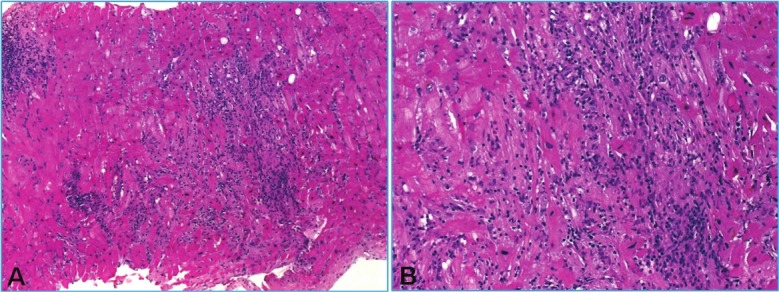
A: Severe rejection (3R) with one compact (left) and one diffuse infiltrate with extensive myocyte damage that superficially may mimic ischemic damage. B: higher magnification of A.
Figure 7.
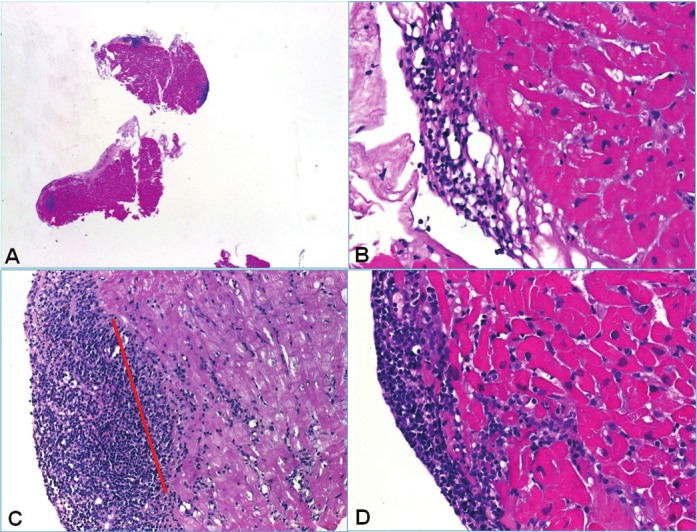
Spectrum of Quilty and Quilty-like findings. A: Overview of this case showed multiple endocardial and subendocardial aggregates. B: Type A Quilty with the aggregate completely confined to the endocardial connective tissue and respecting the adjacent myocardium. C: This larger Quilty replaced the most superficial subendocardial myocardium but no evidence of unequivocal myocyte damage was seen (red line points to the probable original endocardium-myocardium-border). D: Another type B Quilty with clear-cut evidence of subendocardial myocyte damage. Such cases are difficult to classify properly.
Inconclusive biopsies showed usually fragments of fibrous and fatty tissue or fresh thrombotic material in patients with a most recent history of HTX (Figure 8). A few cases showed isolated but more diffuse aggregates of lymphoid cells limited to a biopsy fragment and not reaching the periphery of the biopsy tissue associated with evidence of myocyte damage. There seems to be an inconsistency and difficulty in judging such cases and their classification as either grade 2R if associated with additional foci of rejection or grade 3R if, though isolated, are judged as evidence of diffuse rejection (Figure 8). Biopsies with findings suggestive of antibody-mediated rejection according to the ISHLT guidelines (endothelial swelling, intravascular accumulation of macrophage, interstitial edema, hemorrhage and intravascular thrombi combined with variable degrees of myocardial injury and myocyte necrosis) have been subjected to c4d immunostaining (Figure 9). These cases commonly showed mild interstitial edema.
Figure 8.
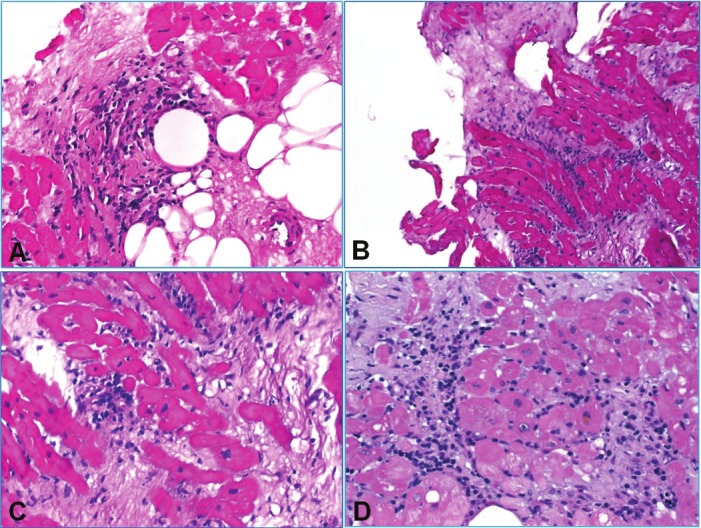
Examples of difficult-to-classify cases. A: This biopsy showed a small old scar associated with a mononuclear aggregate without fiber damage (probably perioperative scar or ischemic damage). Presence of fibrofatty tissue suggests deep localization and an increased risk of perforation. B: Ischemic change from another case showing interstitial scarring with scattered mononuclear inflammatory cells. C: Another case with scarring with minute mononuclear aggregates and evidence of active myocyte damage. Although it looks ischemic, such findings are highly suspicious of cellular rejection and should be classified as such. D: Another biopsy with loose mononuclear aggregates encasing some myocytes and associated with prominent fibrosis. Although this finding might be suggestive of post-ischemic change or site of previous biopsy, it cannot be definitely distinguished from cellular rejection and should therefore be classified as evidence of cellular rejection.
Figure 9.
A: On HE-stained section, humoral rejection showed edematous change and signs of endothelial damage in small capillaries highlighted by nuclear enlargement and hyperchromasia, note absence of cellular rejection. B: Strong C4d immunostaining in endothelial cells.
Discussion
EMB is still the gold standard for routine surveillance of cardiac allograft rejection. This article represents a large retrospective study over 11 years, in which 1896 right ventricle EMB were analyzed and their acute and long-term complications were evaluated in heart transplant patients. In contrast to other institutions, we preferably used the right internal jugular vein approach.
In the present study, we observed a serious acute complication rate and long-term sequelae of 1%. Therefore, our series is consistent with previously published series that the overall complication rate varies between 0.2% and 5.5% [14-17].
One major complication among our patients was moderate or severe tricuspid regurgitation. The incidence of this complication is consistent with previously published series which showed a prevalence ranging from 3.2% to 25% [18,19]. In our study, 12 patients developed moderate or severe tricuspid regurgitation and six of them received mechanical or biological tricuspid valve prosthesis afterwards. Notably, none of these patients died as a consequence of this tricuspid valve failure. Tricuspid regurgitation is a common problem after heart transplantation and its etiology is multifactorial, but a biopsy-induced flail leaflet and a disrupted chordae are two of the most important mechanisms [18,20]. However, no previous study could find a significant correlation between accidental chordal tissue biopsy and the development of serious tricuspid regurgitation [14,19].
Another serious complication associated with the EMB was the pericardial effusions due to iatrogenic cardiac perforation. In our series, 6 patients developed signs of cardiac tamponade after EMB. Other study groups reported similar perforation rates (0.3%) during this invasive procedure [21,22]. Although the incidence of pericardial tamponade is so low, we feel it is necessary to perform echocardiography after each biopsy. Because we did not perform echocardiography immediately before EMB, it was impossible to evaluate the formation of small or large pericardial effusions as a direct consequence of the biopsy procedure. After inferior pericardiocentesis on the same day as EMB, all of these 6 patients experienced total recovery. The rare demonstration of fibrofatty tissue should alert to the possibility of perforation risk following EBM and indicates necessity of close monitoring of such patients. Thus this rare finding should be immediately reported to the clinician after initial biopsy evaluation. However, fibrofatty tissue may on occasion be seen in the right ventricle and does not necessarily indicate epicardial origin. Based on our experience with allograft rejection after HTX we developed the above mentioned biopsy schedule. Concerning the frequency and duration of routine EMB’s in clinically stable patients, the individual adjustment of medication as well as controlling via biopsies is particular notably.
In our protocol, we preferably used the right jugular vein for vascular access. However, the pros and contras for either jugular or femoral approach remain controversial. The jugular vein access could be associated with a higher rate of complications, such as pneumothorax, hemothorax and carotid artery injury, whereas the femoral vein access is coherent with greater patient discomfort particularly during walking and requires the patient to remain in bed for a couple of hours post-procedure [9,14]. In our survey, we had no case of pneumothorax, hemothorax, permanent arrhythmias, embolic stroke, pulmonary embolism or death related to the EMB. Furthermore, we saw no biopsy induced peri-procedural infection; nevertheless we recommend the use of antimicrobial prophylaxis.
Another critical point is the pathological interpretation of the EMB; it could be subjective in some borderline and challenging cases and can results in interobserver variability in grading. Although much effort have been made to improve the consistency, reliability and reproducibility of histopathological evaluation of EMB, in our experience in routine practice, there still exist several issues that make EMB assessment in the practice not that easy and reproducible as the currently applied standardized evaluation systems might imply. One confusing issue is the so-called type B Quilty effects that is in our experience not uncommonly associated with evidence of superficial myocyte damage and would thus suggest the possibility of cellular rejection [23-25]. From a histological view point, classification of this finding as 0R seems contradictory as the same finding within the deeper myocardium would qualify as 1R or even 2R rejection based on the number and extent of infiltrates and myocyte damage. It would be of interest and potential clinical significant to compare cases with type B Quilty with myocardial damage associated with an additional focus of myocyte damage within deeper myocardium to cases with two or more classical foci of myocardial damage. Thus, more large studies are needed to evaluate the clinical meaning of type B Quilty when associated with additional foci of rejection so that an alternative grading would result into a grade 2R or even higher grade. Another critical issue concerns the definition of diffuse rejection in practical terms. In our experience, this represents one of the most confusing findings in EMB, not only for those pathologists with a limited experience in EMB evaluation but also for those with experience and interest in this topic. Thus, a small area with less compact cellular infiltrate that intermingles between myocyte might be defined by many pathologists as diffuse, but as a single non-diffuse focus by others based on the argument that this area represents only a small part of one of four EMB fragments. Furthermore, the presence of interstitial infiltrates with accompanying fibrosis or old myocyte damage might be difficult or even impossible to reliably classify as prior biopsy site, previous ischemic damage with reactive inflammatory infiltrates or even a partially healing focus of cellular rejection with associated organization.
Diagnosis of acute anti-body mediated rejection still represents a further controversial issue. The cause of difficulty in diagnosing humoral rejection relies in the fact that histological features of this serious complication are usually subtle or even almost absent. The presence of interstitial edema and evidence of endothelial cell damage or activation represent hint to this diagnosis and warrant immunohistochemical staining for C4d and other related markers for detection of humoral rejection. Additionally, suggestions are made for the histological diagnosis of acute antibody-mediated rejection using immunohistochemical staining against C4d and macrophages [26-29].
Nevertheless, despite its growing applicability and wide acceptance, EMB is an invasive and error-prone procedure and may be associated with both a risk of procedural complications and long-term sequelae, respectively [11,14]. According to these limitations, there has been extensive effort to develop non-invasive monitoring techniques that might reduce the need for further EMB, with focus placed on monitoring the recipient’s immune respond to detect the onset of rejection or nowadays, to develop an assay that directly interrogates the health of the donated heart [30-33]. Furthermore, various imaging modalities, such as echocardiography, computer tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) offer great potential for non-invasive detection and evaluation of both acute and chronic allograft rejection after heart transplantation [34]. Intragraft gene expression profiling may be a future option to complement histopathological evaluation [35].
In conclusion, in the presented study we have reviewed the usefulness of EMB in monitoring heart transplant patients. EMB is an invasive, but safe procedure with rare serious complications. At this time, despite the search of new biomarkers and new non-invasive monitoring techniques, the histological examination of the EMB remains the gold standard for routine surveillance of heart transplantation rejection.
Acknowledgement
We thank all colleagues at our heart failure and transplantation ambulance, University Hospital Erlangen, for their untiring assistance and supervision of all heart transplant recipients. Furthermore, we thank Dr. Friedrich Einhaus, anaesthesiologist at the Department of Anesthesiology, University Erlangen-Nuremberg, for his assistance in performing and interpreting the echocardiography.
Declaration
The authors have no funding, financial relationships or conflicts of interest to disclose.
References
- 1.Frazier OH. Current status of cardiac transplantation and left ventricular assist devices. Tex Heart Inst J. 2010;37:319–21. [PMC free article] [PubMed] [Google Scholar]
- 2.Vega JD, Moore J, Murray S, Chen JM, Johnson MR, Dyke DB. Heart transplantation in the United States, 1998-2007. Am J Transplant. 2009;9:932–41. doi: 10.1111/j.1600-6143.2009.02568.x. [DOI] [PubMed] [Google Scholar]
- 3.Patel JK, Kittleson M, Kobashigawa JA. Cardiac allograft rejection. Surgeon. 2011;9:160–7. doi: 10.1016/j.surge.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Boyle A. Current status of cardiac transplantation and mechanical circulatory support. Curr Heart Fail Rep. 2009;6:28–33. doi: 10.1007/s11897-009-0006-8. [DOI] [PubMed] [Google Scholar]
- 5.Kent G, Sutton DC, Sutton GC. Needle biopsy of the human ventricular myocardium. Q Bull Northwest Univ Med Sch. 1956;30:213–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Caves PK, Stinson EB, Graham AF, Billingham ME, Grehl TM, Shumway NE. Percutaneous transvenous endomyocardial biopsy. JAMA. 1973;225:288–91. [PubMed] [Google Scholar]
- 7.Richardson PJ. King’s endomyocardial bioptome. Lancet. 1974;1:660–1. doi: 10.1016/s0140-6736(74)93204-8. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JL, Marshall HW. The femoral venous approach to endomyocardial biopsy: comparison with internal jugular and transarterial approaches. Am J Cardiol. 1984;53:833–7. doi: 10.1016/0002-9149(84)90414-4. [DOI] [PubMed] [Google Scholar]
- 9.From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc. 2011;86:1095–102. doi: 10.4065/mcp.2011.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy J, Frantz R, Cooper L. Endomyocardial biopsy. In: Murphy J, Lloyd M, editors. Mayo Clinic Cardiology Concise Textbook. Minnesota: Rochester; 2007. p. 1481. [Google Scholar]
- 11.Mengel M, Sis B, Kim D, Chang J, Famulski KS, Hidalgo LG, Einecke G, de Freitas DG, Tymchak W, Burton J, Halloran PF. The molecular phenotype of heart transplant biopsies: relationship to histopathological and clinical variables. Am J Transplant. 2010;10:2105–15. doi: 10.1111/j.1600-6143.2010.03182.x. [DOI] [PubMed] [Google Scholar]
- 12.Billingham ME, Cary NR, Hammond ME, Kemnitz J, Marboe C, McCallister HA, Snovar DC, Winters GL, Zerbe A. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9:587–93. [PubMed] [Google Scholar]
- 13.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Saraiva F, Matos V, Gonçalves L, Antunes M, Providência LA. Complications of endomyocardial biopsy in heart transplant patients: a retrospective study of 2117 consecutive procedures. Transplant Proc. 2011;43:1908–12. doi: 10.1016/j.transproceed.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz A, Kindermann I, Kindermann M, Mahfoud F, Ukena C, Athanasiadis A, Hill S, Mahrholdt H, Voehringer M, Schieber M, Klingel K, Kandolf R, Böhm M, Sechtem U. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation. 2010;122:900–9. doi: 10.1161/CIRCULATIONAHA.109.924167. [DOI] [PubMed] [Google Scholar]
- 16.Holzmann M, Nicko A, Kühl U, Noutsias M, Poller W, Hoffmann W, Morguet A, Witzenbichler B, Tschöpe C, Schultheiss HP, Pauschinger M. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation. 2008;118:1722–8. doi: 10.1161/CIRCULATIONAHA.107.743427. [DOI] [PubMed] [Google Scholar]
- 17.Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: a seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol. 1992;19:43–7. doi: 10.1016/0735-1097(92)90049-s. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen V, Cantarovich M, Cecere R, Giannetti N. Tricuspid regurgitation after cardiac transplantation: how many biopsies are too many? J Heart Lung Transplant. 2005;24:S227–31. doi: 10.1016/j.healun.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Fiorelli AI, Coelho GH, Oliveira JL Jr, Aiello VD, Benvenuti LA, Santos A, Chi A, Tallans A, Igushi ML, Bacal F, Bocchi EA, Stolf NA. Endomyocardial biopsy as risk factor in the development of tricuspid insufficiency after heart transplantation. Transplant Proc. 2009;41:935–7. doi: 10.1016/j.transproceed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Mielniczuk L, Haddad H, Davies RA, Veinot JP. Tricuspid valve chordal tissue in endomyocardial biopsy specimens of patients with significant tricuspid regurgitation. J Heart Lung Transplant. 2005;24:1586–90. doi: 10.1016/j.healun.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Frustaci A, Pieroni M, Chimenti C. The role of endomyocardial biopsy in the diagnosis of cardiomyopathies. Ital Heart J. 2002;3:348–53. [PubMed] [Google Scholar]
- 22.Felker GM, Hu W, Hare JM, Hruban RH, Baughman KL, Kasper EK. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine (Baltimore) 1999;78:270–83. doi: 10.1097/00005792-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Zakliczynski M, Nozynski J, Konecka-Mrowka D, Pyka L, Trybunia D, Swierad M, Maruszewski M, Zembala M. Quilty effect correlates with biopsy-proven acute cellular rejection but does not predict transplanted heart coronary artery vasculopathy. J Heart Lung Transplant. 2009;28:255–9. doi: 10.1016/j.healun.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Pardo-Mindán FJ, Lozano MD. “Quilty effect” in heart transplantation: is it related to acute rejection? J Heart Lung Transplant. 1991;10:937–41. [PubMed] [Google Scholar]
- 25.Forbes RD, Rowan RA, Billingham ME. Endocardial infiltrates in human heart transplants: a serial biopsy analysis comparing four immunosuppression protocols. Hum Pathol. 1990;21:850–5. doi: 10.1016/0046-8177(90)90055-a. [DOI] [PubMed] [Google Scholar]
- 26.Baba HA, Wohlschläger J, Stypmann J, Hiemann NE. Heart transplantation. Pathology, clinical work-up and therapy. Pathologe. 2011;32:95–103. doi: 10.1007/s00292-010-1409-8. [DOI] [PubMed] [Google Scholar]
- 27.Arias LF, Fernández R, Fernández D, Jaramillo JC, Gil M, López García-Asenjo JA. C4D immunostaining in surveillance endomyocardial biopsies from well-functioning heart allografts. Transplant Proc. 2010;42:1793–6. doi: 10.1016/j.transproceed.2009.12.060. [DOI] [PubMed] [Google Scholar]
- 28.Miller DV, Roden AC, Gamez JD, Tazelaar HD. Detection of C4d deposition in cardiac allografts: a comparative study of immunofluorescence and immunoperoxidase methods. Arch Pathol Lab Med. 2010;134:1679–84. doi: 10.5858/2009-0511-OAR1.1. [DOI] [PubMed] [Google Scholar]
- 29.Berry GJ, Angelini A, Burke MM, Bruneval P, Fishbein MC, Hammond E, Miller D, Neil D, Revelo MP, Rodriguez ER, Stewart S, Tan CD, Winters GL, Kobashigawa J, Mehra MR. The ISHLT working formulation for pathologic diagnosis of antibody-mediated rejection in heart transplantation: evolution and current status (2005-2011) J Heart Lung Transplant. 2011;30:601–11. doi: 10.1016/j.healun.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Snyder TM, Khush KK, Valantine HA, Quake SR. Universal noninvasive detection of solid organ transplant rejection. Proc Natl Acad Sci U S A. 2011;108:6229–34. doi: 10.1073/pnas.1013924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin BA, Taylor DO. Surrogate markers of rejection. Curr Opin Organ Transplant. 2010;15:645–9. doi: 10.1097/MOT.0b013e32833d7e31. [DOI] [PubMed] [Google Scholar]
- 32.Koestenbauer S, Stiegler P, Stadlbauer V, Mayrhauser U, Leber B, Schweiger M, Wasler A, Prenner G, Sereinigg M, Zelzer S, Stojakovic T, Scarpatetti M, Griesbacher A, Greilberger J, Tscheliessnigg K. Myeloperoxidase and carbonyl proteins: promising markers for non-invasive monitoring of graft rejection after heart transplantation. J Heart Lung Transplant. 2010;29:1352–7. doi: 10.1016/j.healun.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Morrow T. Blood test can replace invasive heart biopsy. Manag Care. 2010;19:46–7. [PubMed] [Google Scholar]
- 34.Wu YL, Ye Q, Ho C. Cellular and Functional Imaging of Cardiac Transplant Rejection. Curr Cardiovasc Imaging Rep. 2011;4:50–62. doi: 10.1007/s12410-010-9055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holweg CT, Potena L, Luikart H, Yu T, Berry GJ, Cooke JP, Valantine HA, Mocarski ES. Identification and classification of acute cardiac rejection by intragraft transcriptional profiling. Circulation. 2011;123:2236–43. doi: 10.1161/CIRCULATIONAHA.109.913921. [DOI] [PMC free article] [PubMed] [Google Scholar]