Abstract
Aims: Darbepoetin-α (DPO), a long-acting erythropoietin analog, has been shown to protect the liver against cholestatic injury, to exert an antifibrotic effect, and to increase the survival time in a model of common bile duct ligation. Here we evaluate whether these tissue-protective effects are caused by DPO induced regulation of hepatobiliary transporters. Main methods: C57BL/6J mice underwent common bile duct ligation and were treated with either DPO or physiological saline. Time dependent (2, 5, 14, 28 days after bile duct ligation) protein expression of different hepatobiliary transporters which have been established to play an important role in hepatocellular (i) bile acid uptake, (ii) bile acid excretion, and (iii) retrograde bile acid efflux were assessed. mRNA and protein expression of Lhx2, an important negative regulator of hepatic stellate cell activation, was determined. Key findings: Saline treated cholestatic mice impress with increased mRNA expression of Lhx2 as a defense mechanism, while there is less need for such an upregulation in mice treated with DPO. Whereas Ntcp (slc10a1) protein expression is suppressed as early as 2 days after bile duct ligation to 40% in untreated animals, DPO treated mice exhibit decreased protein level not before day 5. Similarly, the steady decline of Mrp4 (abcc4) protein level during extrahepatic cholestasis in control treated animals does not occur upon DPO application. Significance: The collected data show that DPO affects expression of hepatobilliary transporters during obstructive cholestasis but do not provide sufficient evidence to demonstrate a direct correlation between this regulation and hepatoprotection by DPO.
Keywords: Bile duct ligation, cholestasis, erythropoietin, slc10a1, abcc4
Introduction
Intrahepatic accumulation of bile acids causes proinflammation, oxidative stress, mitochondrial damage and severe hepatocellular injury, ultimately leading to liver fibrosis and cirrhosis [1,2]. Functional characterization, cloning, and localization of hepatobiliary transporter proteins provided a molecular understanding of the mechanisms underlying bile flow and the retention of toxic bile acids in cholestatic disorders [3]. Hepatic uptake and efflux transporters, located on either sinusoidal or canalicular membrane, contribute to the vectorial transport of biliary constituents, such as bile acids, bilirubin glucuronides, and a variety of xenobiotics from sinusoidal blood into bile [4].
Regulation of hepatobiliary transporter systems in cholestasis may be regarded as a protective feedback mechanism of the hepatocytes to minimize the hepatic uptake or to enhance the hepatic efflux of bile acids [5]. On the other hand, regulation of hepatobiliary transporters is possibly only a secondary event which is insufficient to prevent the hepatocellular accumulation of toxic compounds.
Pharmacological therapy for cholestatic disorders is limited. Although used in an empirical manner, ursodeoxycholic acid is the only disease-modifying drug therapy with evidence of efficacy. However, a majority of patients are incomplete responders to ursodeoxycholic acid [6]. Increasing information on the molecular mechanisms of bile acid transport has added new information to the usage of empiric treatment strategies and extended their application [7].
We have recently shown that Darbepoetin-α (DPO), a long-acting erythropoietin analog, protects the liver against cholestatic injury, exerts an antifibrotic effect, and increases the survival time in a model of common bile duct ligation (BDL) [8]. In the present study, we determined the time dependent expression profiles of different hepatobiliary transporters (see Figure 1) during obstructive cholestasis and examined whether changes in expression are associated with hepatoprotection shown by DPO.
Figure 1.
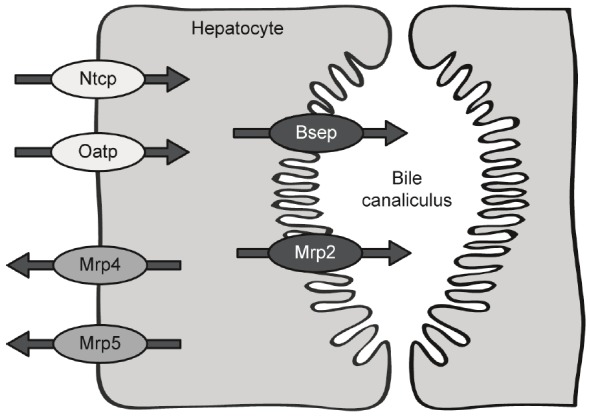
Localization of hepatocellular transporters on basolateral and canalicular hepatocyte membrane.
Materials and methods
Animal model
Male C57BL/6J mice were purchased from Charles River Laboratories (Sulzfeld, Germany) and were used at 8 - 10 weeks of age with a body weight of 23 - 26 g. Animals were kept on water and standard laboratory chow ad libitum. The experiments were conducted in accordance with EU Directive 2010/63/EU for animal experiments and the German legislation on protection of animals. Mice were anesthetized with isoflurane and BDL was performed after midline laparotomy. The common bile duct was ligated 3 times with 5-0 silk and transected between the 2 distal ligations. Sham operation was performed similarly except for ligation and transection of the bile duct (n = 6). A total of eighty BDL mice were randomized into two groups. Darbepoetin-α (10 μg/kg body weight, Aranesp; Amgen Europe, Breda, The Netherlands) or the same volume of isotonic saline was injected intraperitoneally in a blinded fashion every third day beginning at the first day after BDL. To obtain blood and liver samples, mice (10 animals per group at each time point) were sacrificed at days 2, 5, 14, and 28 after BDL.
Hematological measurements and histopathology
Animals were anesthetized and exsanguinated by puncture of the vena cava inferior. Plasma concentration of total bilirubin served as parameter of cholestasis. Liver tissue samples were fixed in formalin for 2 - 3 days and embedded in paraffin. Five-micrometer sections were stained with Hematoxylin and Eosin (H&E) for routine examination and quantification of bile infarcts. All samples from a series of experiments were stained simultaneously and evaluated in a blinded fashion. For histomorphometric analysis images of twenty random low-power fields (10× magnification, Olympus BX 51, Hamburg, Germany) were acquired with a Color View II FW camera (Color View, Munich, Germany) and evaluated by means of an image analysis system (Adobe Photoshop, Adobe Systems, Uxbridge, UK). Bile infarcts were quantified in H&E stained sections in a similar fashion and the percentage of the focal necrosis surface to the total liver section area was assessed.
Real-time PCR
Total RNA from liver tissue was isolated using a RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. Total RNA were eluted in 30 μl of nuclease-free water, quantified spectrophotometrically at 260 nm, and kept at -80°C until use. First strand cDNA was synthesized by reverse transcription of 2 μg of total RNA using oligo(dT)18 primer (Biolabs, Frankfurt am Main, Germany) and Superscript II RNaseH-Reverse Transcriptase (Invitrogen, Karlsruhe, Germany) in the presence of dNTP’s, 5 x first strand buffer and dithiothreitol at 72°C for 10 min and 42°C for 60 min. The reverse transcriptase was inactivated by 95°C for 5 min. Expression levels of murine collagen-(I)-α1 and Lhx2 mRNA were quantified relative to control samples (LightCycler System 1.5 Roche, Mannheim, Germany) and normalized to the expression of the housekeeping gene GAPDH. For our approach we used SYBR Green I (Roche, Manheim, Germany) for the detection of dsDNA amplified in the PCR. Primers (Lhx2 forward: 5’-GTCCAGGACCCGGGGCAGAT-3’, reverse: 5’-CGAGGCGTTGGACAGCTCCG-3’; collagen-(I)-α1 forward: 5’-GAAACCCGAGGTATGCTTGA-3’, reverse: 5-GACCAGGAGGACCAGGAAGT-3’) were designed spanning introns to eliminate false positive signals from contaminating genomic DNA as well as to anneal at about 55°C to combine both reactions of GAPDH and target gene in the same run. The real-time PCR program included a 10 min denaturation step followed by 40 - 50 amplification cycles. After each LightCycler run, a melting curve analysis was performed to analyze the PCR products. LightCycler PCR products were separated by 1% agarose (peqGOLD Universal Agarose; peQLab Biotechnologie, Erlangen, Germany) gel electrophoresis containing 0.5 μg/ml ethidium bromide to confirm the right amplification products. For the quantification of gene expression, duplicates were run in the LightCycler for both genes. The reaction without cDNA template was also performed as a negative control. Specificity of the amplification was verified by melt-curve analysis and evaluation of efficiency of PCR amplification.
Immunoblot analysis
For Western blot analysis of protein levels of Lhx2, Ntcp (scl10a1), Oatp2b1 (slco2b1), Mrp2 (abcc2), Bsep (abcb11), Mrp4 (abcc4), Mrp5 (abcc5), and β-actin liver tissue was homogenized in lysis buffer (10 mM Tris pH 7.5, 10 mM NaCl, 0.1 mM EDTA, 0.5% Triton-X 100, 0.02% NaN3, and 0.2 mM PMSF, protease inhibitor cocktail), incubated for 30 min on ice and centrifuged for 15 min at 10,000 x g. Protein content were assayed by bicinchoninic acid (BCA) method (Pierce, Biotechnology) with bovine serum albumin (BSA) (Pierce, Biotechnology) as standard. Per lane 20 μg was separated on a 8% (abcc2, abcb11, abcc4, and abcc5), 10% (slco2b1) or 12% (Lhx2, slc10a1) SDS gel and transferred to a polyvinyldifluoride membrane (Immobilon-P; Millipore, Eschborn, Germany). After blockade with 2% BSA (Santa Cruz Biotechnology, Santa Cruz, CA, USA), membranes were incubated over night at 4°C with a goat polyclonal anti-Lhx2-antibody (1:1,000; Santa Cruz Biotechnology), a rabbit polyclonal anti-scl10a1-antibody (1:1,000; abcam, Cambridge, UK), a rabbit polyclonal anti-slco2b1-antibody (1:1,000; abcam), a goat polyclonal anti-abcc2-antibody (1:400; Santa Cruz Biotechnology), a rabbit polyclonal anti-abcb11-antibody (1:200; abcam), a goat polyclonal anti-abcc4-antibody (1:500; abcam), and a goat polyclonal anti-abcc5-antibody (1:250; Santa Cruz Biotechnology) followed by a secondary peroxidase-linked goat anti-rabbit antibody (slc10a1, slco2b1, abcb11; 1:10,000; Cell Signaling Technology) or by a secondary peroxidase-linked donkey anti-goat antibody (Lhx2, abcc4; 1:10,000; abcc2; 1:25,000; abcc5; 1:15,000, Santa Cruz Biotechnology).
Protein expression was visualized by means of luminol-enhanced chemiluminescence (ECL plus; Amersham Pharmacia Biotech, Freiburg, Germany) and digitalized with ChemiDoc™ XRS System (Bio-Rad Laboratories, Munich, Germany). Signals were densitometrically assessed (Quantity One; Bio-Rad Laboratories) and normalized to the β-actin signals (mouse monoclonal anti-β-actin antibody; 1:20,000; Sigma).
Statistics
Data are presented as mean ± s.e. To compare BDL groups with sham operated animals for significant differences One Way Analysis of Variance for normally distributed populations or Kruskal-Wallis Analysis of Variance on Ranks for non-normal populations was performed. To determine significant differences between means of saline and DPO treated animals at the same time point unpaired t-test for normally distributed populations was performed. Shapiro-Wilk test was used to test for a normally distributed population. Data were considered significant when p < 0.05. Statistical analysis was performed using the Sigma Plot software package (Version 12.0, Systat Software Inc, San Jose, CA, USA).
Results
Time profiles of bilirubin concentration and bile infarcts after BDL
Impaired bile excretion resulted in elevated total bilirubin concentration in plasma of BDL mice reaching a maximum at 14 days. Administration of DPO significantly reduced bilirubin levels at day 2, when compared to saline treated controls (Table 1). The retention of bile salts elicited a toxic response and led to cellular damage of hepatocytes as revealed by histomorphometric analysis of biliary infarcts in liver tissue. These clusters of injured hepatocytes could be significantly diminished by DPO treatment at both day 2 and 14 post BDL (Table 1).
Table 1.
Bilirubin concentration, bile infarcts, and mRNA expression of collagen-(I)-α1 after BDL
| sham | 2 days after BDL | 5 days after BDL | 14 days after BDL | 28 days after BDL | |||||
|---|---|---|---|---|---|---|---|---|---|
| con | DPO | con | DPO | con | DPO | con | DPO | ||
| bilirubin (mmol/l) | 11 ± 0 | 247 ± 33 | 161 ± 17# | 343 ± 26 | 331 ± 30 | 651 ± 42 | 554 ± 58 | 371 ± 44 | 302 ± 41 |
| bile infarcts (%) | 0.1 ± 0.1 | 5.7 ± 1.3 | 1.8 ± 0.7# | 3.9 ± 1.2 | 2.3 ± 0.8 | 6.4 ± 0.8 | 2.8 ± 1.0# | 0.7 ± 0.2 | 0.5 ± 0.1 |
| mRNA collagen-(I)-α1/GAPDH (rel. density) | 0.15 ± 0.03 | 2.25 ± 0.57 | 0.33 ± 0.08# | 2.65 ± 0.68 | 1.34 ± 0.36 | 5.77 ± 1.54 | 2.02 ± 0.41# | 6.09 ± 2.0 | 3.88 ± 0.66 |
Plasma bilirubin concentration, histologically assessed bile infarcts and hepatic mRNA expression of collagen-(I)-α1 in sham operated mice (n = 3 – 4) and in animals at 2, 5, 14, and 28 days after BDL with administration of either 10 μg/kg body weight darbepoetin-α (DPO) or physiological saline (con) every third day, beginning 24 h after surgery (n = 6 - 10 per group and time point). Data are given as mean ± s.e.
p < 0.05 versus control at the respective time point.
Data are adopted from (Sigal et al. 2010).
Time profiles of hepatic stellate cell activation and collagen deposition after BDL
The major source of extracellular matrix proteins in liver fibrosis are activated hepatic stellate cells (HSCs) [9,10]. The LIM homeobox gene Lhx2 is an important negative regulator of HSC activation and fibrogenesis. Lhx2-/- embryos contain numerous activated HSCs and display a progressively increased deposition of extracellular matrix proteins associated with liver fibrosis. Overexpression of Lhx2 in cultured stellate cells leads to decreased cell activation [9,10]. In the present study, Lhx2 mRNA was highly up-regulated in cholestatic mice without DPO therapy at day 2 (Figure 2A). Lhx2 protein level was slightly increased in DPO treated animals at day 2, whereas sham treated mice showed significantly elevated level of Lhx2 at day 5 after BDL (Figure 2B). Activation of stellate cells led to marked increase of collagen-(I)-1α mRNA expression, which was significantly reduced by DPO application at both day 2 and 14 after BDL (Table 1).
Figure 2.
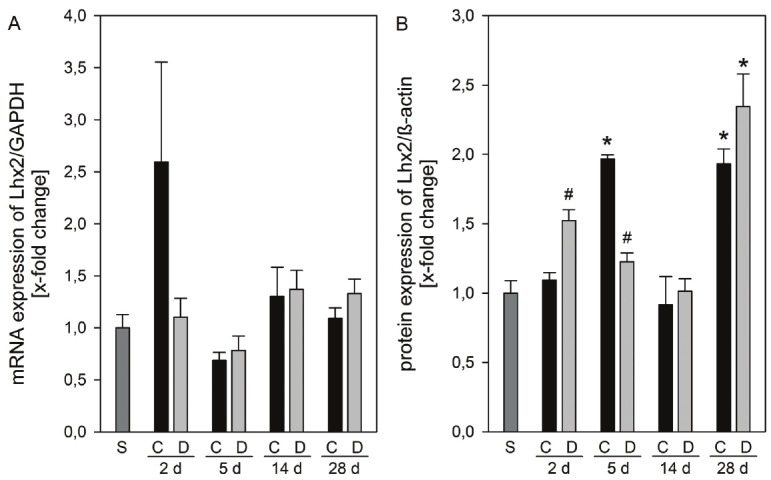
mRNA and protein expression of Lhx2. mRNA expression of Lhx2 (A) and protein expression of Lhx2 (B) in liver tissue of sham operated animals (n = 3 - 4) and of animals at 2, 5, 14, and 24 days after BDL with administration of either 10 μg/kg body weight darbepoetin-α (D) or physiological saline (C) every third day, beginning 24 h after surgery (n = 3 - 5 per group and time point). The expression levels were normalized to GAPDH or β-actin, respectively. Data are given as mean ± s.e. *p < 0.05 versus sham (S); #p < 0.05 versus control (C) at the respective time point.
Expression time profiles of hepatic transporters after BDL
Expression of various hepatic transport proteins during extrahepatic cholestasis (see Figure 1) was examined by Western Blot analysis. The vectorial transport of bile acids in hepatocytes includes carrier-mediated uptake at the basolateral membrane, translocation through the cell, and active transport across the canalicular plasma membrane into bile or efflux via the basolateral membrane back into sinusoidal blood [3,5].
Bile acid uptake across the basolateral membrane
Quantitatively relevant uptake transporters in the basolateral membrane include the sodium taurocholate contransporting polypeptide (NTCP/Ntcp) and the organic anion transporting polypeptides (OATPs/Oatps) [3]. Compared with sham-operated animals, the relative protein expression of Ntcp declined by 60% at 2 days after BDL in untreated animals, while the expression was slightly increased after treatment with DPO. Ntcp level further decreased to 30% at 28 days after ligation in both saline and DPO treated mice (Figure 3A). Extrahepatic cholestasis downregulated liver Oatp2b1 expression at 2 days to about 50% compared to sham operated mice. This downregulation remained unaffected by DPO treatment (Figure 3B).
Figure 3.
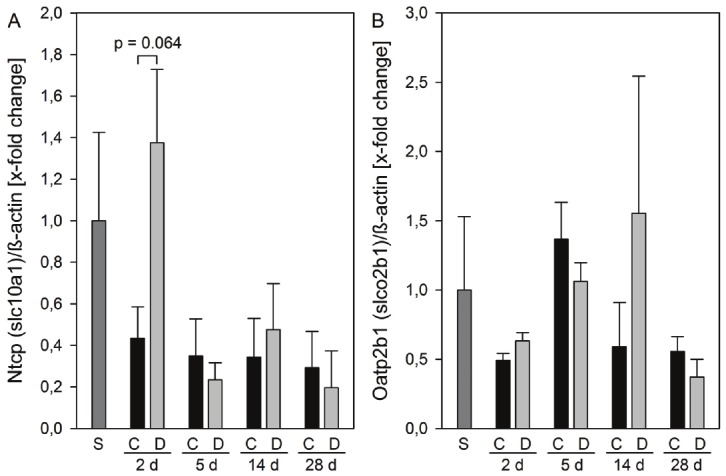
Protein expression of basolateral membrane transporters. Protein expressions of Ntcp (A) and Oatp2b1 (B) in liver tissue of sham operated animals (n = 3) and of animals at 2, 5, 14, and 24 days after BDL with administration of either 10 μg/kg body weight darbepoetin-α (D) or physiological saline (C) every third day, beginning 24 h after surgery (n = 6 per group and time point). The expression levels were normalized to β-actin. Data are given as mean ± s.e.
Bile acid excretion across the canalicular membrane
Both, multidrug resistance protein 2 (MRP2/Mrp2) and bile salt exporting pump (BSEP/Bsep) are major contributors to bile acid independent and bile acid dependent bile flow via the canalicular membrane into bile [3]. In the liver, Mrp2 protein expression time-dependently decreased in all cholestatic groups to finally 50% at 28 days after BDL. This decline remained unaffected by DPO treatment (Figure 4A). Expression of Bsep showed unique changes; it decreased by 60% until 14 days after BDL in saline treated animals, and thereafter increased 2.3-fold at 28 days of extrahepatic cholestasis (Figure 4B). Quite similar results were observed in DPO treated animals, however, the Bsep protein level already started to increase at 14 days after BDL (Figure 4B).
Figure 4.
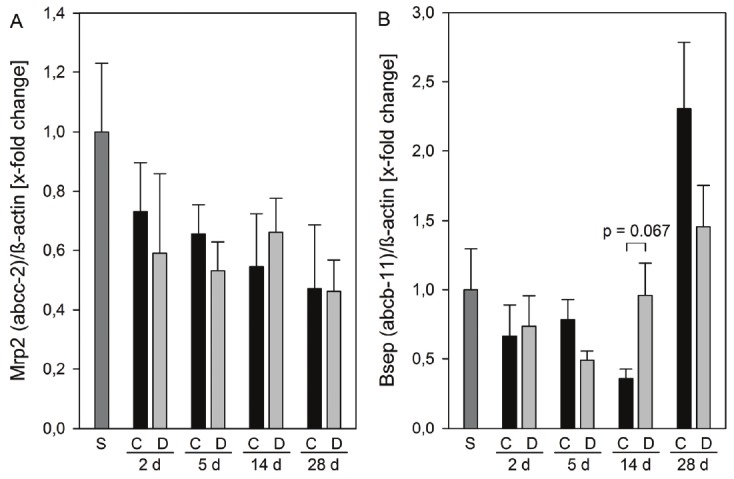
Protein expression of canalicular membrane transporters. Protein expressions of Mrp2 (A) and Bsep (B) in liver tissue of sham operated animals (n = 3) and of animals at 2, 5, 14, and 24 days after BDL with administration of either 10 μg/kg body weight darbepoetin-α (D) or physiological saline (C) every third day, beginning 24 h after surgery (n = 6 per group and time point). The expression levels were normalized to β-actin. Data are given as mean ± s.e.
Retrograde bile acid efflux across the basolateral membrane
MRP4/Mrp4 and Mrp5/Mrp5 are unidirectional pumps releasing organic anions into the sinusoidal blood, particularly when the activity of canalicular efflux pumps is impaired [3,7,11]. Recent research suggests that Mrp4 rather than Mrp3 represents the major hepatic basolateral bile acid export pump in obstructive cholestasis in mice [12,13]. However, liver Mrp4 protein expression was unchanged at 2 and 5 days after BDL in saline treated animals, while a decrease to 35% at 14 days and thereafter an increase to 120% at 28 days were observed. In contrast DPO treated mice revealed nearly unchanged Mrp4 protein expression until day 14 after BDL (Figure 5A). The role of Mrp5 in bile acid transport is currently under investigation and it is speculated that Mrp5 could possibly contribute to the alternative bile acid elimination in cholestasis [11,14]. Obstructive cholestasis decreased Mrp5 protein expression in mouse liver to about 40% at all time-points studied. There were no differences between treatment groups (Figure 5B).
Figure 5.
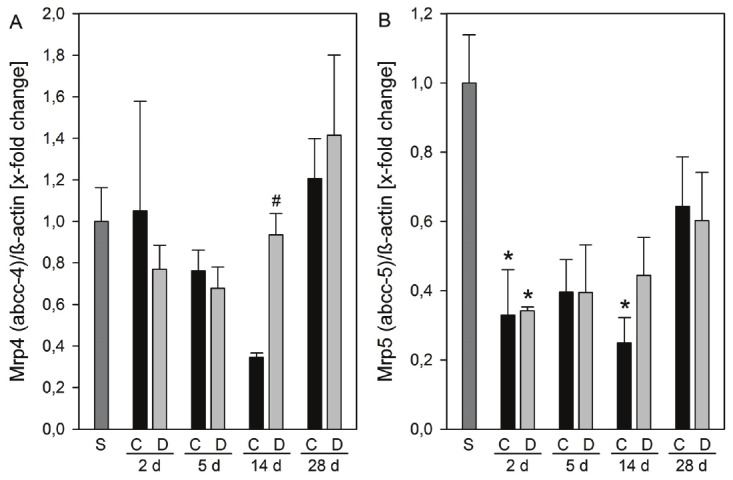
Protein expression of efflux basolateral membrane transporters. Protein expressions of Mrp4 (A) and Mrp5 (B) in liver tissue of sham operated animals (n = 3) and of animals at 2, 5, 14, and 24 days after BDL with administration of either 10 μg/kg body weight darbepoetin-α (D) or physiological saline (C) every third day, beginning 24 h after surgery (n = 6 per group and time point). The expression levels were normalized to β-actin. Data are given as mean ± s.e. *p < 0.05 versus sham (S); #p < 0.05 versus control (C) at the respective time points.
Discussion
Bile acid induced liver injury as a consequence of extrahepatic cholestasis may progress to fibrosis and cirrhosis, ultimately leading to death unless the obstructive lesion is removed or the bile duct is bypassed. There is accumulating evidence that several hepatic transporters undergo adaptive regulation in response to cholestatic liver injury [3,7]. These adaptions are presumed to minimize the hepatic retention of bile acids and other potentially toxic substrates. However, it is quite possible that this response only reflects a non-effective consequence to the accumulation of toxic bile constitutes in hepatocytes. In the present study, we characterized the time-dependent effect of extrahepatic cholestasis on the expression of different hepatobiliary transport proteins which have been established to play an important role in (i) bile acid uptake across the basolateral membrane, (ii) bile acid excretion across the canalicular membrane, and (iii) retrograde bile acid efflux across the basolateral membrane (see Figure 1).
The protein level of the predominant basolateral uptake transporter Ntcp has been shown to be downregulated in obstructive cholestasis model of common BDL up to 7 days [5,13,15]. The present study confirms the downregulation of Ntcp protein as early as 2 days after BDL and extends our knowledge indicating that the expression remains decreased up to 28 days. The sodium independent transport is mediated by the OATPs/Oatps which belong to the solute carrier organic transporter family. The OATPs were thought to be secondary active transporters and the main human OATPs (1A2, 1B1, 1B3, and 2B1) are expressed at the basolateral membrane of the hepatocyte and primarily transport unconjugated bile salts [16]. It has been shown that protein expression of members of the Oatp1 family (1a4, 1b2) is unchanged [17] or slightly reduced [18] during obstructive cholestasis in rats. First characterized with rather limited substrate specificity compared to other OATPs [19], in more recent studies the substrate specificity of OATP2B1 has been broadened, indicating that OATP2B1 might be involved in elimination of numerous endo- and xenobiotics [20]. Here we demonstrate a slight reduction of Oatp2b1 protein expression as early as 2 days after BDL.
In liver, MRP2/Mrp2 serves as the canalicular efflux pump for many organic anions, particularly for bilirubin glucuronosides. The absence of functional Mrp2 from the canalicular membrane causes conjugated hyperbilirubinemia [21]. However, loss of Mrp2 function is usually well tolerated by compensatory upregulation of other membrane transporters [21]. Mrp2 protein expression was considerably decreased in obstructive cholestasis in rats during different time periods [18,22-25]. For mice, results are conflicting. The group of Mennone reported both no change in protein expression at 7 days after BDL in mice maintained in an equivalent 129/Sv and C57BL6 mixed background [13] as well as downregulation of Mrp2 protein expression by 50% at the same time point in FVB/129 mice [26]. Wagner et al. showed that Mrp2 protein level remained preserved at 1, 3, and 7 days after common BDL in C57BL6 mice [27]. Herein, we observed a time-dependent decline from 70% at 2 days to 50% at 28 days after BDL in C57BL6 mice. The second main canalicular export pump Bsep is primarily expressed in the liver and mice deficient for Bsep develop intrahepatic cholestasis [16]. Mutations in human BSEP lead to the inherited cholestatic disorder progressive familial intrahepatic cholestasis type 2 [4]. BSEP has been most recently suggested as a potential therapeutic target in cholestatic disorders [28]. However, in contrast to humans, mice lacking the Bsep gene exhibit only mild, non-progressive, but persistent intrahepatic cholestasis [4]. Our findings are consistent with previous studies in mice in which Bsep protein level remained unchanged up to 7 days after BDL [26,27,29]. Moreover, we show that Bsep protein expression reduces to about 40% at 2 weeks and doubles thereafter at 4 weeks post BDL.
It is speculated that Mrp4 represents the major hepatic basolateral bile acid transporter in animal models of obstructive cholestasis [12,13]. Induction of hepatic Mrp4 expression is considered to be an adaptive response counteracting intracellular bile acid toxicity by increased efflux of bile acids [12]. Herein, we observed a transient decline of Mrp4 protein level and only minor induction at 4 weeks after BDL. The role of Mrp5 in bile acid transport remains elusive but possible because it is localized in basolateral membranes and expressed in the liver [14]. An increase of MRP5 protein was detected in livers of patients with primary biliary cirrhosis [11]. Surprisingly, we observed a decline of Mrp5 protein level after obstructive cholestasis.
Delayed necro-inflammation and macrophage infiltration upon DPO application suppressed the activation of HSCs, resulting in attenuation of fibrogenesis [8]. Whereas mRNA expression of Lhx2, a negative regulator of HSC activation, is rapidly upregulated as a defense mechanism in cholestatic mice, there is less need for this upregulation in mice treated with DPO. The limited increase of Lhx2 mRNA in DPO treated cholestatic mice seems to be a consequence of the anti-inflammatory effects mediated by DPO.
The collected data do not provide sufficient evidence to demonstrate a direct correlation between hepatoprotection by DPO and changes in hepatobiliary transporter expression during obstructive cholestasis. However, DPO treatment of bile duct ligated mice results in a preservation of basic protein levels of two transporter systems. Whereas Ntcp protein expression is suppressed as early as 2 days after BDL to 40% in untreated animals, DPO treated mice exhibit decreased protein level not before day 5. Similarly, the steady decline of Mrp4 protein level during extrahepatic cholestasis in control treated animals does not occur upon DPO application. Bile acid accumulation during obstructive cholestasis results in a systemic release of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), and interleukin-6 (IL-6), which is accompanied by activation of NF-kappaB [30,31]. Pro-inflammatory cytokines are potent inhibitors of transporter expression [7]. In livers of patients with inflammation-induced cholestasis, expressions of NTCP, OATP2, and BSEP were reduced [32]. IL-1β treatment of hepatocytes markedly reduced Ntcp RNA level and Ntcp promoter activity [33], whereas reduced IL-1β and TNF-α upon Kupffer cell depletion blocks cytokinemediated Ntcp suppression in sepsis-associated cholestasis [34]. Erythropoietin (EPO) acts as a potent anti-inflammatory immune modulator by specifically targeting NF-kB p65-driven inflammatory effector pathways [35] and thus impairs formation of pro-inflammatory cytokines [36-38]. We have already shown that DPO treatment of bile duct ligated mice markedly reduced the infiltration of macrophages into the liver and inhibited the perpetuation of the necro-inflammatory reaction [8]. Concluding, the herein observed delayed suppression of Ntcp and Mrp4 protein levels in DPO treated animals, particularly at the early stage of cholestasis, is rather a result of this reduced necroinflammation than a direct action of DPO.
Several signal transduction events have been implicated in the tissue protective effects of EPO and its analogues including induction of JAK2 (Janus kinase-2) and STAT-5 (signal transducer and activator of transcription-5) dependent signaling cascades as well as activation of JNK (c-Jun N-terminal kinase) and MAPK (mitogen-activated protein kinase) pathways [37]. We and others have previously demonstrated that EPO analogues attenuate liver injury in a model of fulminant hepatic failure. The protection is mediated by decreased activation of the JNK pathway and thus reduced activities of TNF-α and IL-1β [36,38]. Reduced Ntcp gene expression in cholestatic liver injury has been attributed to JNK-dependent suppression of key Ntcp promoter activators, primarily HNF1 (hepatocyte nuclear factor 1) and RXR:RAR (nuclear receptor heterodimer retinoid X receptor:retinoic acid receptor) [33]. Thus, the inhibited suppression of Ntcp expression in bile duct ligated animals treated with DPO might conceivably be caused via DPO dependent reduction of JNK activity. However, suppressed JNK activity could as well be mediated through anti-inflammatory effects of DPO, because JNK pathway has been shown to be activated by pro-inflammatory cytokines [39].
In conclusion, on the basis of the data presented a cause-and-effect relationship between changes in hepatobiliary transporter expression and hepatoprotection by DPO could not be established. It is quite possible that the observed changes are a secondary effect of anti-inflammatory action of DPO. Increasing information on the cause-and-effect relationship of possible treatment strategies will represent a major step towards the development of novel, more effective therapeutic strategies.
Acknowledgement
This work was supported in part by the Deutsche Forschungsgemeinschaft, Bonn-Bad Godesberg, Germany (Ei 768/1-2), and by the Bundesministerium für Bildung und Forschung, Germany (01GN0986). The authors kindly thank Berit Blendow, Dorothea Frenz, and Maren Nerowski (Institute for Experimental Surgery, University of Rostock) for excellent technical assistance.
Declaration of conflict of interest
All authors declare that there are no conflicts of interest.
References
- 1.Sokol RJ, Devereaux M, Dahl R, Gumpricht E. “Let there be bile”--understanding hepatic injury in cholestasis. J Pediatr Gastroenterol Nutr. 2006;43(Suppl 1):S4–S9. doi: 10.1097/01.mpg.0000226384.71859.16. [DOI] [PubMed] [Google Scholar]
- 2.Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keppler D. Cholestasis and the role of basolateral efflux pumps. Z Gastroenterol. 2011;49:1553–1557. doi: 10.1055/s-0031-1281755. [DOI] [PubMed] [Google Scholar]
- 4.Kosters A, Karpen SJ. Bile acid transporters in health and disease. Xenobiotica. 2008;38:1043–1071. doi: 10.1080/00498250802040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gartung C, Ananthanarayanan M, Rahman MA, Schuele S, Nundy S, Soroka CJ, Stolz A, Suchy FJ, Boyer JL. Down-regulation of expression and function of the rat liver Na+/bile acid cotransporter in extrahepatic cholestasis. Gastroenterology. 1996;110:199–209. doi: 10.1053/gast.1996.v110.pm8536857. [DOI] [PubMed] [Google Scholar]
- 6.Jonker JW, Stedman CA, Liddle C, Downes M. Hepatobiliary ABC transporters: physiology, regulation and implications for disease. Front Biosci. 2009;14:4904–4920. doi: 10.2741/3576. [DOI] [PubMed] [Google Scholar]
- 7.Roma MG, Crocenzi FA, Sanchez Pozzi EA. Hepatocellular transport in acquired cholestasis: new insights into functional, regulatory and therapeutic aspects. Clin Sci (Lond) 2008;114:567–588. doi: 10.1042/CS20070227. [DOI] [PubMed] [Google Scholar]
- 8.Sigal M, Siebert N, Zechner D, Menschikow E, Abshagen K, Vollmar B, Eipel C. Darbepoetin-alpha inhibits the perpetuation of necro-inflammation and delays the progression of cholestatic fibrosis in mice. Lab Invest. 2010;90:1447–1456. doi: 10.1038/labinvest.2010.115. [DOI] [PubMed] [Google Scholar]
- 9.Wandzioch E, Kolterud A, Jacobsson M, Friedman SL, Carlsson L. Lhx2-/- mice develop liver fibrosis. Proc Natl Acad Sci U S A. 2004;101:16549–16554. doi: 10.1073/pnas.0404678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes SN, Aleksunes LM, Augustine L, Scheffer GL, Goedken MJ, Jakowski AB, Pruimboom-Brees IM, Cherrington NJ, Manautou JE. Induction of hepatobiliary efflux transporters in acetaminophen-induced acute liver failure cases. Drug Metab Dispos. 2007;35:1963–1969. doi: 10.1124/dmd.107.016170. [DOI] [PubMed] [Google Scholar]
- 12.Chai J, Luo D, Wu X, Wang H, He Y, Li Q, Zhang Y, Chen L, Peng ZH, Xiao T, Wang R, Chen W. Changes of organic anion transporter MRP4 and related nuclear receptors in human obstructive cholestasis. J Gastrointest Surg. 2011;15:996–1004. doi: 10.1007/s11605-011-1473-2. [DOI] [PubMed] [Google Scholar]
- 13.Mennone A, Soroka CJ, Cai SY, Harry K, Adachi M, Hagey L, Schuetz JD, Boyer JL. Mrp4-/- mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology. 2006;43:1013–1021. doi: 10.1002/hep.21158. [DOI] [PubMed] [Google Scholar]
- 14.Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G923–G932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]
- 15.Zollner G, Fickert P, Silbert D, Fuchsbichler A, Stumptner C, Zatloukal K, Denk H, Trauner M. Induction of short heterodimer partner 1 precedes downregulation of Ntcp in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G184–G191. doi: 10.1152/ajpgi.00215.2001. [DOI] [PubMed] [Google Scholar]
- 16.Nicolaou M, Andress EJ, Zolnerciks JK, Dixon PH, Williamson C, Linton KJ. Canalicular ABC transporters and liver disease. J Pathol. 2012;226:300–315. doi: 10.1002/path.3019. [DOI] [PubMed] [Google Scholar]
- 17.Geier A, Dietrich CG, Trauner M, Gartung C. Extrahepatic cholestasis downregulates Oatp1 by TNF-alpha signalling without affecting Oatp2 and Oatp4 expression and sodium-independent bile salt uptake in rat liver. Liver Int. 2007;27:1056–1065. doi: 10.1111/j.1478-3231.2007.01523.x. [DOI] [PubMed] [Google Scholar]
- 18.Brcakova E, Fuksa L, Cermanova J, Kolouchova G, Hroch M, Hirsova P, Martinkova J, Staud F, Micuda S. Alteration of methotrexate biliary and renal elimination during extrahepatic and intrahepatic cholestasis in rats. Biol Pharm Bull. 2009;32:1978–1985. doi: 10.1248/bpb.32.1978. [DOI] [PubMed] [Google Scholar]
- 19.Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120:525–533. doi: 10.1053/gast.2001.21176. [DOI] [PubMed] [Google Scholar]
- 20.Hagenbuch B, Gui C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica. 2008;38:778–801. doi: 10.1080/00498250801986951. [DOI] [PubMed] [Google Scholar]
- 21.Nies AT, Keppler D. The apical conjugate efflux pump ABCC2 (MRP2) Pflugers Arch. 2007;453:643–659. doi: 10.1007/s00424-006-0109-y. [DOI] [PubMed] [Google Scholar]
- 22.Hyogo H, Tazuma S, Nishioka T, Ochi H, Yamaguchi A, Numata Y, Kanno K, Sakomoto M, Asamoto Y, Tsuboi K, Nakai K, Yasumiba S, Sunami Y, Kajiyama G. Phospholipid alterations in hepatocyte membranes and transporter protein changes in cholestatic rat model. Dig Dis Sci. 2001;46:2089–2097. doi: 10.1023/a:1011934108920. [DOI] [PubMed] [Google Scholar]
- 23.Kanno K, Tazuma S, Niida S, Chayama K. Unique reciprocal changes of hepatocellular membrane transporter expression and fluidity in rats with selective biliary obstruction. Hepatol Res. 2003;26:157–163. doi: 10.1016/s1386-6346(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 24.Paulusma CC, Kothe MJ, Bakker CT, Bosma PJ, van Bokhoven I, van Marle J, Bolder U, Tytgat GN, Oude Elferink RP. Zonal down-regulation and redistribution of the multidrug resistance protein 2 during bile duct ligation in rat liver. Hepatology. 2000;31:684–693. doi: 10.1002/hep.510310319. [DOI] [PubMed] [Google Scholar]
- 25.Trauner M, Arrese M, Soroka CJ, Ananthanarayanan M, Koeppel TA, Schlosser SF, Suchy FJ, Keppler D, Boyer JL. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology. 1997;113:255–264. doi: 10.1016/s0016-5085(97)70103-3. [DOI] [PubMed] [Google Scholar]
- 26.Mennone A, Soroka CJ, Harry KM, Boyer JL. Role of breast cancer resistance protein in the adaptive response to cholestasis. Drug Metab Dispos. 2010;38:1673–1678. doi: 10.1124/dmd.110.034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, Zatloukal K, Guo GL, Schuetz JD, Gonzalez FJ, Marschall HU, Denk H, Trauner M. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–838. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- 28.Stieger B, Beuers U. The canalicular bile salt export pump BSEP (ABCB11) as a potential therapeutic target. Curr Drug Targets. 2011;12:661–670. doi: 10.2174/138945011795378496. [DOI] [PubMed] [Google Scholar]
- 29.Donner MG, Schumacher S, Warskulat U, Heinemann J, Haussinger D. Obstructive cholestasis induces TNF-alpha- and IL-1 -mediated periportal downregulation of Bsep and zonal regulation of Ntcp, Oatp1a4, and Oatp1b2. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1134–G1146. doi: 10.1152/ajpgi.00079.2007. [DOI] [PubMed] [Google Scholar]
- 30.Liu TZ, Lee KT, Chern CL, Cheng JT, Stern A, Tsai LY. Free radical-triggered hepatic injury of experimental obstructive jaundice of rats involves overproduction of proinflammatory cytokines and enhanced activation of nuclear factor kappaB. Ann Clin Lab Sci. 2001;31:383–390. [PubMed] [Google Scholar]
- 31.Plebani M, Panozzo MP, Basso D, De Paoli M, Biasin R, Infantolino D. Cytokines and the progression of liver damage in experimental bile duct ligation. Clin Exp Pharmacol Physiol. 1999;26:358–363. doi: 10.1046/j.1440-1681.1999.03042.x. [DOI] [PubMed] [Google Scholar]
- 32.Zollner G, Fickert P, Zenz R, Fuchsbichler A, Stumptner C, Kenner L, Ferenci P, Stauber RE, Krejs GJ, Denk H, Zatloukal K, Trauner M. Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology. 2001;33:633–646. doi: 10.1053/jhep.2001.22646. [DOI] [PubMed] [Google Scholar]
- 33.Li D, Zimmerman TL, Thevananther S, Lee HY, Kurie JM, Karpen SJ. Interleukin-1 beta-mediated suppression of RXR:RAR transactivation of the Ntcp promoter is JNK-dependent. J Biol Chem. 2002;277:31416–31422. doi: 10.1074/jbc.M204818200. [DOI] [PubMed] [Google Scholar]
- 34.Sturm E, Havinga R, Baller JF, Wolters H, van Rooijen N, Kamps JA, Verkade HJ, Karpen SJ, Kuipers F. Kupffer cell depletion with liposomal clodronate prevents suppression of Ntcp expression in endotoxin-treated rats. J Hepatol. 2005;42:102–109. doi: 10.1016/j.jhep.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Nairz M, Schroll A, Moschen AR, Sonnweber T, Theurl M, Theurl I, Taub N, Jamnig C, Neurauter D, Huber LA, Tilg H, Moser PL, Weiss G. Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-kappaB-inducible immune pathways. Immunity. 2011;34:61–74. doi: 10.1016/j.immuni.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Minh K, Klemm K, Abshagen K, Eipel C, Menger MD, Vollmar B. Attenuation of inflammation and apoptosis by pre- and posttreatment of darbepoetin-alpha in acute liver failure of mice. Am J Pathol. 2007;170:1954–1963. doi: 10.2353/ajpath.2007.061056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nairz M, Sonnweber T, Schroll A, Theurl I, Weiss G. The pleiotropic effects of erythropoietin in infection and inflammation. Microbes Infect. 2012;14:238–246. doi: 10.1016/j.micinf.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben-Ari Z, Zilbermints V, Pappo O, Avlas O, Sharon E, Greif F, Cheporko Y, Ravid A, Shapiro R, Hochhauser E. Erythropoietin increases survival and attenuates fulminant hepatic failure injury induced by D-galactosamine/lipopolysaccharide in mice. Transplantation. 2011;92:18–24. doi: 10.1097/TP.0b013e31821cdea5. [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, Stravitz RT, Dent P, Hylemon PB. Down-regulation of cholesterol 7alpha-hydroxylase (CYP7A1) gene expression by bile acids in primary rat hepatocytes is mediated by the c-Jun N-terminal kinase pathway. J Biol Chem. 2001;276:15816–15822. doi: 10.1074/jbc.M010878200. [DOI] [PubMed] [Google Scholar]


