Abstract
This study evaluated the properties of endogenous nitric oxide synthases (NOS) and annexin-A1 (ANXA1) and determined how they can be exploited in the N-methyl-N-nitro-N-nitrosoguanidine (MNNG)-induced gastric carcinogenesis and myenteric denervation model. Male Wistar rats were treated with MNNG and/or aminoguanidine (AG) for 20 weeks. In another set of experiments, rats with nondenervated and denervated stomachs were treated with MNNG or water for 28 weeks. Fragments of the pyloric region were processed for histopathology, NOS activity, and immunohistochemistry to explore the activity and expression of constitutive (cNOS) and inducible (iNOS) NO synthase and their relationship with annexin-A1 (ANXA1) expression. NO inhibition by AG increased the percentage of animals with adenocarcinomas (~29%) compared with the untreated MNNG group (~4%). Myenteric denervation did not alter NOS activity. cNOS activity was significantly greater in nondernervated and denervated stomachs with or without lesions (P<0.001) than iNOS activity (P<0.01), as confirmed by immunohistochemistry. Further, cNOS activity in normal stomachs and outside the lesion area was considerably higher than inside it (P<0.01). By densitometric analysis of nondenervated and denervated stomachs, ANXA1 expression was modulated in epithelial and inflammatory cells (mast cells and neutrophils), wherein significant alterations were induced by lesion development and myenteric denervation. In conclusion, NO protects against the development of gastric adenocarcinomas. The pattern of ANXA1 expression was not associated with NOS activity or expression, suggesting that NO and ANXA1 act in gastric tumors in disparate pathways.
Keywords: Benzalkonium chloride, immunohistochemistry, lipocortin-1, N-methyl-N-nitro-N-nitrosoguanidine, nitric oxide synthase, inflammation
Introduction
Inflammation is a critical component in the progression of cancer. Several cancers arise from sites of infection, chronic irritation, and inflammation [1-4]. Nitric oxide (NO) and annexin A1 (ANXA1) are believed to regulate many of the pathophysiological processes that link inflammation to cancer development and progression. NO and ANXA1 have protumor and antitumor effects [5,6].
Nitric oxide (NO) is a small, short-lived biomolecule that functions as a signaling molecule in development, immunity, and carcinogenesis. NO is a product of the conversion of L-arginine into L-citrulline by nitric oxide synthase (NOS). NOS has three isoforms: type 1 (neuronal or brain NOS; nNOS) and type 3 (endothelial NOS; eNOS) are Ca2+-dependent and constitutively expressed (cNOS), generated primarily by neurons and endothelial cells, respectively; type 2 NOS (macrophage or inducible NOS; iNOS) is Ca2+-independent and is induced in various cell types by inflammatory cytokines, lipopolysaccharides, and other stimuli. The NOS isoforms are tissue-specific, and their expression pattern can be altered under pathological conditions [7-9]. eNOS and nNOS exist in the stomach, and although iNOS is not detected in normal gastric tissues, it is significantly induced on exposure to endotoxin.
In certain tumor tissues, NO enhances tumor angiogenesis and induces vasodilatation, thus accelerating tumor growth. Other tumors, including gastric and colon cancers, express less NOS than normal tissue by immunohistochemistry, and there is a link between the loss of NO and carcinogenesis [10]. Further, NO mediates tumoricidal activity in vivo, as demonstrated using sodium nitroprusside, a generator of NO that inhibits MNNG-induced gastric carcinogenesis [11].
ANXA1, a 37-kDa protein that binds calcium and phospholipids binding, is an anti-inflammatory mediator, regulating leukocyte migration, cell proliferation and death signaling, apoptosis, and carcinogenesis [12]. ANXA1 expression is reduced in certain cancers but increased in other cancer types. Many clinical tumor cases report that the loss of ANXA1 contributes to cancer cell resistance to apoptosis and implicate it as a negative biomarker in cancer development and progression [12].
The enteric nervous system can be exploited in carcinogenesis, especially in gastric tumors. Available human and animal data have shown that intrinsic denervation is protective against the development of tumors, but the reasons for this protection remain unknown [13-16]. As the NO and ANXA1 regulate many of the pathophysiological processes that link inflammation to cancer development and progression with protumor and antitumor effects, we examined the effects of inhibiting NO production on the incidence of gastric tumors that are induced by N-methyl-N-nitro-N-nitrosoguanidine (MNNG), the activity and expression of cNOS and iNOS in nondenervated and denervated rat stomachs with and without tumors, and the relationship between NOS activity and ANXA1 expression.
Materials and methods
Animals
Male Wistar rats, weighing 100–150 g at the beginning of the experiment, were provided by the Central Animal House, Ribeirão Preto School of Medicine and maintained in the Animal Facilities of the Department of Pathology. The animals were housed in plastic cages (4 animals/cage) under a 12-hour light/12-hour dark cycle in rooms with natural ventilation at 24 ± 2°C and received a standard maintenance diet (Nuvilab-CR-Nuvital Nutrients Prodvet Ltda., Brazil) and tap water ad libitum. The experiments were performed per the Committee on Care and Uses of Laboratory Animals of the National Research Council, NIH (USA) and the Ethics Committee for Animal Experimentation (CEEA) of FAMERP (Protocol 6193/2008), São José do Rio Preto, SP.
Experimental design
To determine the effects of inhibiting NO production on the incidence of MNNG-induced gastric tumors, four experimental groups were evaluated: in MNNG Group (n = 38), animals ingested a solution of MNNG (Aldrich Chemical Co., Inc., Milwaukee, WI) in distilled and deionized water at 100 mg/L [13]; MNNG+AG Group (n = 38) animals ingested MNNG and aminoguanidine (AG – 50 mg/Kg/day) [17]; AG Group (n = 20) ingested distilled and deionized water and AG at the same concentration as in MNNG+AG Group; and Control Group (n = 12) animals ingested distilled and deionized water. All animals were weighed weekly, and fluid intake (MNNG or MNNG plus AG; water plus AG or water) and survival rate was measured daily. After 20 weeks, the animals were sacrificed; the first adenocarcinoma was detected at this time point. The macroscopic analysis of stomachs was performed to detected tumor incidence and morphology.
To investigate the relationship between intrinsic denervation, NOS activity and ANXA1 expression another set of experiments was performed. Four experimental groups were evaluated: Nondenervated (ND) and Denervated (D) Groups (n = 5/ group) animals ingested distilled and deionized water; ND+MNNG and D+MNNG (n = 10/group), with MNNG-induced gastric lesions.
For myenteric denervation, male Wistar rats, weighing 100–150 g, were anesthetized i.m. with ketamine hydrochloride and thiazine chloride (0.15 ml/0.05 ml/100 g of weight). Each stomach was exteriorized by midline upper abdominal laparotomy and isolated from the peritoneal cavity through a small fenestration in a plastic sheet for topical application of 0.6% benzalkonium chloride (BAC; v/v, Aldrich Chemical Co.; diluted in saline). The isolated stomachs were wrapped with gauze that was soaked in BAC or saline and kept moist for 30 min. The serosal surface of the stomachs was washed thoroughly with saline, the organs were returned to the abdominal cavity, and the abdominal wall was sutured. Treatment with BAC was effective, leading to a decrease of approximately 45% of the population of myenteric neurons [13].
Sixteen weeks after surgery, the animals in ND+MNNG and D+MNNG Groups ingested a solution of MNNG in distilled and deionized water (100 mg/L) for 28 weeks. The animals were sacrificed 2 months after the final intake of MNNG, and the stomachs were harvested for histopathological analysis, NOS activity assay, and immunohistochemistry.
Macroscopic and histopathological analysis
After sacrifice, the stomachs were removed, opened along the greater curvature, rinsed with saline solution, and examined for the presence of tumors. Fragments of the pyloric region of the stomach (antrum) were fixed in 4% buffered formalin for 12 h, washed in tap water, dehydrated in an ethanol series, and embedded in paraffin. The gastric antrum was analyzed in 4-μm sections that were stained with hematoxylin-eosin and toluidine blue 0.5%; the lesions were classified as benign or malignant, based on the general classification of the epithelial lesions.
Assay for NOS activity
NOS (cNOS and iNOS) activity was measured inside and outside of tumors of nondenervated and denervated stomachs (n = 10 fragments of the pyloric region per group). The full thickness of pyloric gastric wall was collected and frozen at -70°C until they were processed for NOS activity, as described [18,19].
Briefly, tissue was homogenized and centrifuged at 100,000 g for 10 min at 4°C. Supernatants were assayed for NOS activity by measuring the conversion of L-[14C]-arginine to [14C]-citrulline, as described, except that the incubation was performed at room temperature for 60 min. The protein content of the supernatant was measured using Coomassie blue per the manufacturer’s recommendations (Coomassie Blue Reagent; Pierce Chemical, Rockford, IL). NOS activity was expressed in picomoles of citrulline per milligram of protein per hour (pmol/mg protein/hour).
Immunohistochemistry
Immunohistochemistry was performed using streptavidin-peroxidase (SP) and polyclonal antibodies against iNOS, eNOS, nNOS (Santa Cruz Biotechnology, USA), and ANXA1 (Zymed Laboratories, Cambridge, UK). Deparaffinized sections were incubated in citrate buffer, pH 6.0 at 96°C for 20 min in a pressure cooker to retrieve antigens, washed 3 times with distilled water, and incubated with 3% hydrogen peroxide for 30 min in methanol to block endogenous peroxidase activity.
After being washed 3 times with phosphatebuffered saline (PBS, pH 7.4), the sections were blocked with 10% goat serum for 15 min to reduce nonspecific binding and incubated with rabbit polyclonal anti-iNOS, eNOS, nNOS (1:100), or anti-ANXA1 (1:1000) in 2% bovine serum albumin (BSA) at 4°C overnight. The sections were washed 3 times with PBS, incubated with the universal LSABTM2 Kit/HRP secondary antibody (DAKO, USA) per the manufacturer’s protocol, washed 3 times with PBS, and developed with 3,3 0-diaminobenzidine (DAKO). At the end of the reaction, the sections were washed thoroughly in distilled water, counterstained with hematoxylin, and mounted on glass slides. As a control, some sections were incubated with 2% BSA, without primary antibody.
ANXA1 immunostaining was quantified by densitometric analysis on an arbitrary scale from 0 to 255 with AxioVision software on a Zeiss-Axioskop II light microscope, and the data were expressed as mean ± standard error (SE).
Data analysis
For survival rate and tumor incidence, data are reported as mean ± SEM and were submitted to the test of proportions, normal approximation for the difference in the percentage of animals with tumors, to Student´s t-test and oneway analysis of variance (ANOVA), and then to the Tukey–Kramer multiple comparisons test. The proportions were considered distinct when the observed z was greater than the critical z. Differences with values of P<0.05 were considered significant. For densitometric analysis, statistical differences between groups were determined by Student´s t test and one-way analysis of variance (ANOVA) and, if significant, followed by Bonferroni test. In all cases, a probability value < 0.05 was considered significant.
Results
Effects of inhibition of NO production on MNNG-induced gastric tumors
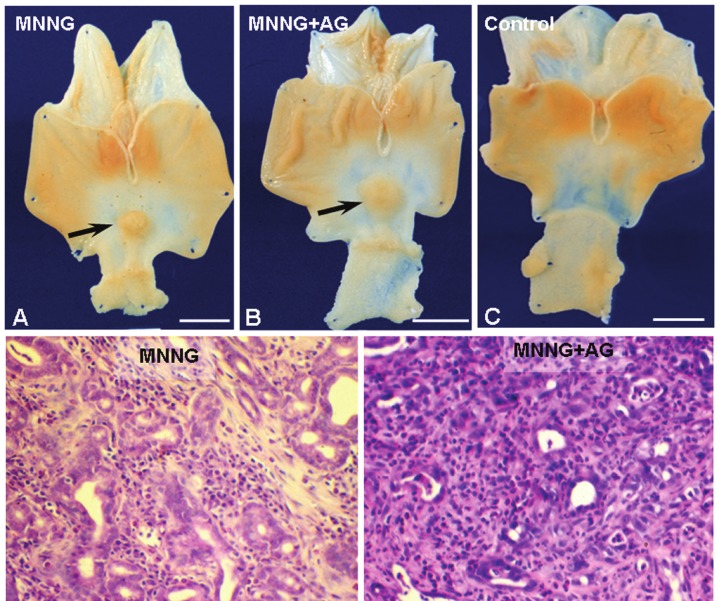
Sixty-three percent of the animals in MNNG Group (treated with MNNG) and 55% of MNNG+AG Group (treated with MNNG and aminoguanidine-AG) survived the experimental period (20 weeks), versus 100% of animals in AG and Control Groups that remained in good overall health. On initial consumption of the carcinogen, there were no differences in body weight or fluid ingestion between groups. After several weeks, body weight and liquid diet intake declined significantly in the groups that received MNNG compared with animals that received only water or water plus AG. Only animals that were treated with MNNG developed adenocarcinomas in the antrum. The tumors were raised, with a wide base and sessile or ulcerated lesions (Figure 1A-C). Morphologically, there were no differences between lesions in the groups that were treated with MNNG. Histologically, all tumors were adenocarcinomas (Figure 1D and 1E). The first adenocarcinoma was detected 20 weeks after MNNG was first ingested. The MNNG + AG Group had more animals with tumors (28.57%) compared with MNNG Group (4.16%) according z test.
Figure 1.
Effect of aminoguanidine on MNNG-induced gastric carcinogenesis. Macroscopic analysis of stomachs after treatment with MNNG (A), MNNG plus aminoguanidine (MNNG+AG; B), and water (Control; C). Raised lesions (arrows). Bars: 10 mm. Histopathological analysis of adenocarcinomas in animals treated with MNNG (D) and MNNG+AG (E). Stain: hematoxylin & eosin. Bars: 50 μm.
NOS activity in gastric tissue
Myenteric denervation did not alter NOS activity in gastric tissues without lesions (ND and D Groups) (Figure 2A), which was mediated primarily by cNOS. The generation of L-citrulline from cNOS in normal gastric tissue (ND and D Groups) and outside the tumoral area (ND+MNNG and D+MNNG Groups) was considerably higher than inside the tumor (Figure 2B and 2C). As in ND and D Groups, iNOS levels were low in gastric tissues with lesions (inside or outside the tumor) of ND+MNNG and D+MNNG Groups (Figure 2B and 2C).
Figure 2.
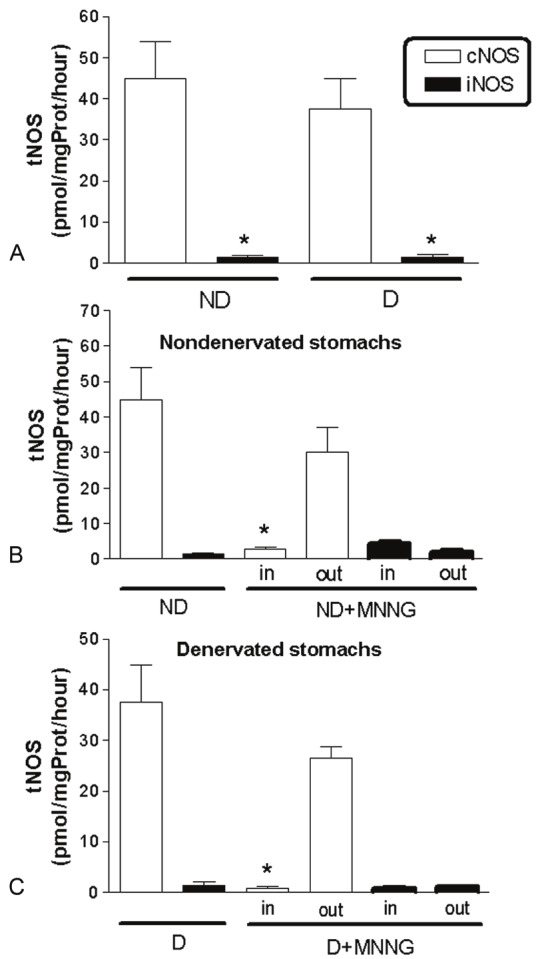
Measurement of total NOS (tNOS) activity in fragments of nondenervated (ND) and denervated (D) stomachs without lesions and with lesions (ND+MNNG and D+MNNG Groups, respectively). Data are mean ± SE of [14C]-citrulline generation and expressed as pmol/mg protein/hour (n = 10 fragments per group). iNOS activity was lower than cNOS in all experimental groups (A-C). No differences in NOS activity were detected after myenteric denervation (A, ND and D Groups, * P<0.001). The cNOS activity inside tumors (in; ND+MMG and D+MNNG Groups) was significantly lower than in ND and D stomachs (* P < 0.001) and outside tumors (out; ND+MNNG Group, * P<0.01; D+MNNG Group, * P < 0.001) (B and C).
NOS expression in gastric tissue
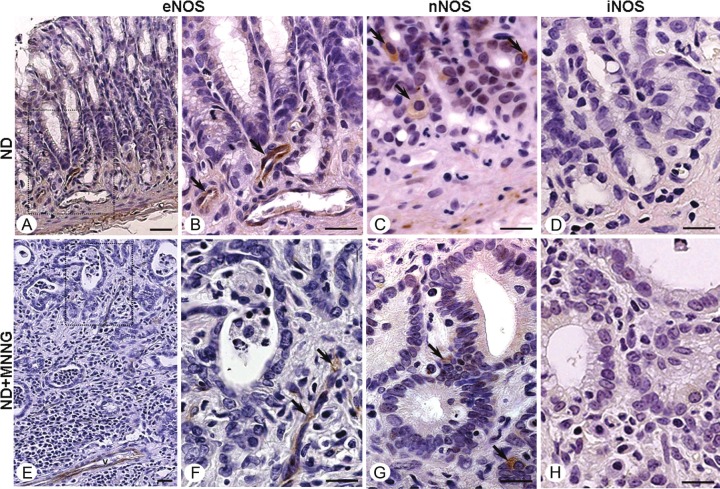
By immunohistochemical analysis of the gastric antrum, myenteric denervation did not alter NOS expression in nondenervated or denervated stomachs confirming the NOS assay data. All normal gastric mucosa samples (ND and D Groups) and tumor tissues (ND+MNNG and D+MNNG Groups) were immunoreactive for nNOS and eNOS (Figure 3A-C and 3E-G). nNOS was highly expressed in epithelial cells (Figure 3C and 3G), muscle, and the myenteric plexus, and eNOS was detected in endothelial cells (Figure 3B and 3F). iNOS was undetected by immunohistochemistry in normal and gastric tumor tissues (Figure 3D and 3H).
Figure 3.
NOS immunostaining in nondenervated (ND) stomachs. Normal gastric mucosa (ND Group; A) and adenocarcinoma (ND+MNNG Group; E) showing eNOS expression in endothelial cells (arrows; B and F – squared area of figures A and E, respectively) and nNOS expression in epithelial cells (arrows; C and G). No immunoreactivity for iNOS was observed in normal and tumor tissues (D and H). Counterstain: hematoxylin. Bars: 10 μm.
ANXA1 expression in gastric tissue
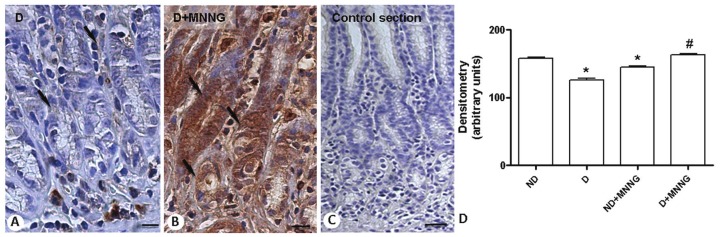
ANXA1 immunoreactivity was analyzed in all experimental groups (ND; D; ND+MNNG; D+MNNG) and was detected in epithelial and inflammatory cells, primarily mast cells and neutrophils. Epithelial cells in the stomachs of D Group expressed low levels of ANXA1 compared with ND Group. Development of adenocarcinomas in the stomachs of ND+MNNG Group was associated with lower ANXA1 immunoreactivity in the epithelium compared with ND Group, whereas adenocarcinomas from the stomachs of D+MNNG Group stained robustly for ANXA1 versus D Group (Figure 4A and 4B). No signal was detected in the control sections (Figure 4C). Densitometric analysis of the epithelial cells confirmed the morphological findings, demonstrating significant modulation of ANXA1 under various experimental conditions (Figure 4D).
Figure 4.
ANXA1 expression in epithelial cells of denervated stomachs without or with lesions (D and D+MNNG Groups, respectively). Strong ANXA1 immunoreactivity in epithelial cells from gastric lesions (arrows; B) compared with cells of D Group (arrows; A). Control of reaction (C). Counterstain: hematoxylin. Bars: 10 μm. (D) Densitometric analysis of epithelial cells immunostained for ANXA1 in nondenervated (ND), denervated (D), ND+MNNG and D+MNNG Groups. Values (arbitrary units) are expressed as mean ± SE of sections analyzed from 5 rats/ group. * P <0.001 versus ND Group; # P <0.001 versus D and ND+MNNG Groups.
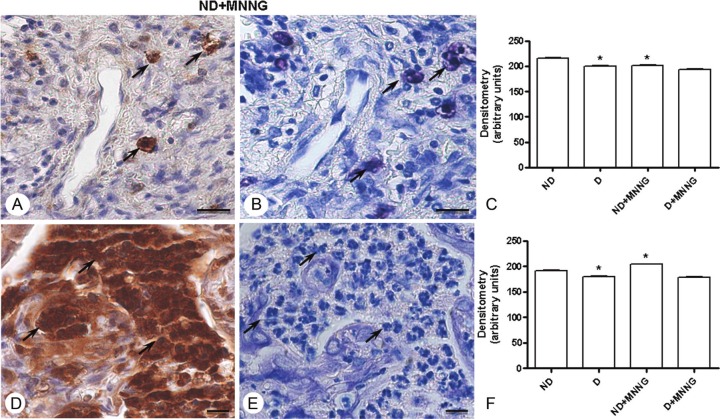
As in epithelial cells, inflammatory cells (mast cells and neutrophils) from denervated stomachs expressed low levels of ANXA1 in their cytoplasm compared with those from nondenervated stomachs. The development of lesions in nondenervated stomachs (ND+MNNG Group) was associated with lower ANXA1 immunoreactivity in mast cells (Figure 5A), whereas neutrophils presented an increase in ANXA1 levels compared with cells from ND Group (Figure 5D).
Figure 5.
ANXA1 expression in inflammatory cells. Adenocarcinoma of nondenervated stomachs (ND+MNNG Group) with ANXA1-positive mast cells (arrows; A) and neutrophils (arrows; B). Counterstain: hematoxylin. Adjacent histological section after staining with 0.5% toluidine blue confirms that positive cells are mast cells (arrows; C) and neutrophils (D). Bars: 10 μm. Densitometric analysis of mast cells (E) and neutrophils (F) immunostained for ANXA1. Values (arbitrary units) are expressed as mean ± SE of sections analyzed from 5 rats/ group. ANXA1 levels significantly reduced in inflammatory cells of denervated stomachs (D Group) compared with nondenervated (ND Group). In D+MNNG Group, diminished ANXA1 expression was detected in mast cells, whereas neutrophils exhibited a significant increase in ANXA1 expression compared with cells of ND Group. * P < 0.001 versus ND Group.
In denervated stomachs (D+MNNG Group), no differences in ANXA1 expression was noted in these inflammatory cells.
The densitometric analysis confirmed the modulation of ANXA1 expression in inflammatory cells, as shown in Figure 5C (mast cells) and 5F (neutrophils). Toluidine blue-stained histological sections confirmed that the immunostained cells were mast cells (Figure 5B) and neutrophils (Figure 5E).
Discussion
In MNNG-induced gastric carcinogenesis, we demonstrate that NOS activity inhibits the development of gastric lesions through treatment with AG. We find that the anti-inflammatory mediator NO and ANXA1 have disparate functions in the progression of gastric cancer. By immunohistochemistry of NOS and ANXA1 in the stomachs on chemical denervation and treatment with MNNG, we confirmed the presence of constitutive NOS (cNOS) and the modulation of ANXA1 in various cell types that regulate tumor development.
Initially, to confirm the protective effect of endogenous NO on tumor development, animals were treated with MNNG and AG, a NOS inhibitor, for 20 weeks. AG increased the number of animals with adenocarcinomas, indicating that inhibition of NO synthesis accelerates MNNG-induced gastric carcinogenesis in rats. That we did not observe iNOS by citrulline assay or immunohistochemistry suggests that AG blocks the cNOS isoform. Despite being a selective inhibitor of iNOS with a 50- to 500-fold higher affinity [20], AG also suppresses cNOS activity by the alteration of its prosthetic heme moiety in a time- and metabolism-dependent manner that could be attenuated by the presence of the natural substrate, L-arginine [21].
NO has been demonstrated to mediate tumoricidal activity using sodium nitroprusside, and it has been suggested that this effect is related to the suppression of antral epithelial proliferation [11]. Thus, the reduced expression of NOS in stomach tumors and the absence of iNOS, consequently decreasing NO production, might accelerated the pathogenesis and maintenance of cancer [7].
We examined the effects of myenteric denervation on NOS activity in gastric tissues. Compared with iNOS, cNOS had higher activity in nondenervated and denervated stomachs, but no differences in the generation of L-citruline were observed between groups. Similarly, nondenervated and denervated stomachs that were treated with MNNG experienced increased cNOS activity in extratumoral tissues versus those inside tumors, suggesting that decreased NOS activity was linked to tumor development.
We complemented these functional data by measuring cNOS and iNOS expression in normal tissues and stomach lesions. Both isoforms of cNOS, eNOS and nNOS, were detected in these tissues, whereas iNOS was not expressed, confirming the NOS assay. These findings are consistent with data on normal and cancerous human gastric tissues [22], wherein the expression of cNOS isoforms in stomach tumors was significantly lower than in normal stomach mucosa, whereas iNOS expression in tumor tissues was significantly lower versus cNOS expression.
Our study suggests that lesion development is associated with a reduction in cNOS activity that is not influenced by myenteric denervation. The mechanisms by which NOS expression declines in tumor tissues are unknown; identifying them could increase our understanding of carcinogenesis. Nishio and colleagues [23] suggested that endogenous NO that is derived from both cNOS and iNOS regulates mucosal defense in the inflamed stomach, in part by decreasing acid secretion, and maintains mucosal integrity under such conditions.
The glucocorticoid-modulated ANXA1 downregulates leukocyte extravasation and the release of inflammatory mediators by directly inhibiting cytosolic phospholipase A2 and the expression of inducible cyclooxygenase and NOS [24]. Alterations in ANXA1 expression occur in many tumors, rising in pancreatic, hepatic, and esophageal and esophagogastric junction adenocarcinomas, but declining in esophageal, prostate, thyroid, and nasopharyngeal carcinomas [25,26]. Further, ANXA1 is a critical mediator of apoptosis and a substrate receptor for epidermal growth factor [27,28]. Thus, we examined ANXA1 expression in gastric tissues and its relationship with NOS activity and myenteric denervation in the MNNG-induced carcinogenesis model.
Under all experimental conditions, non-denervated and denervated stomachs with or without adenocarcinomas were positive for ANXA1 in the epithelium and inflammatory cells, particularly mast cells and neutrophils, confirming previous data [29-31]. Myenteric denervation significantly decreased ANXA1 levels in the epithelium compared with nondenervated stomachs. ANXA1 has antiproliferative effects in epithelial cells [32], and its expression increased in epithelial cells of human nasal polyps on treatment with glucocorticoids [30]. Further, increased activity of the ANXA1 promoter in lung epithelial cells is linked to cellular activation in the control of the local and systemic responses that are induced by lipopolysaccharide in mice [33]. Thus, reduced expression of ANXA1 in the epithelium of denervated stomachs suggests its impaired activation compared with the gastric epithelium of non-denervated stomachs.
The development of epithelial lesions in non-denervated stomachs caused a significant decrease in ANXA1 in the epithelium compared with nondenervated stomachs without lesions, whereas it increased in denervated stomachs with adenocarcinomas compared with denervated stomachs without lesions.
Studies involving 1072 patients with gastric cancer reported that loss of ANXA1 expression was significantly associated with lymph node metastasis, advanced disease stage, and poor histological differentiation [26], indicating an effect of reduced ANXA1 levels in disease progression. The function of ANXA1 in tumor progression was determined in prostate cancer cells in vitro, in which ANXA1 levels increased, reducing cell viability through the induction of caspase-mediated apoptosis, colony formation, and the proliferative effects of epidermal growth factor [34]. Likely, increases in ANXA1 levels in denervated stomachs suppress the development of gastric tumors, reducing the number of lesions [13].
As in epithelial cells, myenteric denervation was associated with lower ANXA1 expression in mast cells and neutrophils. Despite the altered ANXA1 expression in denervated stomachs, no differences in protein levels were observed in inflammatory cells after tumor development. However, adenocarcinomas in nondenervated stomachs harbored mast cells with lower levels of ANXA1 versus cells in nondenervated stomachs without lesions.
ANXA1 expression is altered in mast cells in inflammatory diseases and cancer. In the trachea and mesentery of rats, mast cells express more ANXA1 after treatment with dexamethasone, suggesting that glucocorticoids inhibit degranulating mast cells [29,30,35]. Similarly, pretreatment of mice with Ac2-26 (the N-terminal region of ANXA1) increases the number of intact mast cells in the pleural cavity and decreases the release of histamine in a model of ovalbumin-induced allergic inflammation, confirming the protective effect of ANXA1 in their activation [36]. In head and neck tumors, mast cells in the stromal tumor and peritumoral area express high levels of ANXA1 in their cytoplasmic granules, although the number of degranulated cells in these regions rises compared with the control [31]. In gastric MNNG-induced adenocarcinomas, the number of degranulated and intact mast cells increases, especially in denervated stomachs that harbor phenotypically altered mast cells [37]. This finding, in conjunction with decreased levels of ANXA1, suggests that mast cell activation is affected through the release of mediators that function in tumorigenesis. However, this effect of ANXA1 was not associated with iNOS activity or expression in this gastric carcinogenesis model.
Neutrophils in nondenervated stomachs with adenocarcinomas (ND+MNNG Group) upregulated ANXA1 compared with those in non-denervated stomachs without lesions (ND Group). ANXA1, on the plasma membrane of adherent neutrophils, is inhibitory, reducing the extent of transmigration across endothelial cells [29]. ANXA1 mediates the apoptosis of neutrophils, an important mechanism of limiting the inflammatory response [38]. Thus, myenteric denervation modulates ANXA1 levels in neutrophils in gastric adenocarcinomas, exacerbating the inflammatory response that is induced by the carcinogenesis.
In this context and under our experimental conditions, NO protects against the development of gastric adenocarcinomas. Further, we examined the properties of endogenous ANXA1 and NOS and determined how they can be exploited in the MNNG-induced carcinogenesis and myenteric denervation model, concluding that they act in disparate pathways in gastric tumors. The molecular basis of NOS and ANXA1 expressions and their functions in the progression of gastric cancer should be investigated in follow-up studies. Agents, such as activators or modulators of NO and ANXA1, are potentially novel therapeutic targets, particularly in gastric cancer.
Acknowledgments
This work was supported by a grant from Fundação de Amparo à Pesquisa do Estado de São Paulo (2008/05722-6 to CFE). The authors would like to thank Mrs. Laura Midori Kawasse, Mrs. Marcia Aparecida Oliva Destido, Mrs. Fabíola Leslie Antunes Mestriner, and Mr. Domingos Zanchetta Netto for excellent technical assistance.
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence T. Inflammation and cancer: a failure of resolution? Trends Pharmacol Sci. 2007;28:162–165. doi: 10.1016/j.tips.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 5.Tse GM, Wong FC, Tsang AK, Lee CS, Lui PC, Lo AW, Law BK, Scolyer RA, Karim RZ, Putti TC. Stromal nitric oxide synthase (NOS) expression correlates with the grade of mammary phyllodes tumour. J Clin Pathol. 2005;58:600–604. doi: 10.1136/jcp.2004.023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastian BC. Annexins in cancer and autoimmune diseases. Cell Mol Life Sci. 1997;53:554–556. doi: 10.1007/s000180050071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang YZ, Cao YQ, Wu JN, Chen M, Cha XY. Expression of nitric oxide synthase in human gastric carcinoma and its relation to p53, PCNA. World J Gastroenterol. 2005;11:46–50. doi: 10.3748/wjg.v11.i1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keklikoglu N, Koray M, Kocaelli H, Akinci S. iNOS expression in oral and gastrointestinal tract mucosa. Dig Dis Sci. 2008;53:1437–1442. doi: 10.1007/s10620-007-0061-5. [DOI] [PubMed] [Google Scholar]
- 9.Pautz A, Art J, Hahn S, Nowag S, Voss C, Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide. 2010;23:75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Doi C, Noguchi Y, Marat D, Saito A, Fukuzawa K, Yoshikawa T, Tsuburaya A, Ito T. Expression of nitric oxide synthase in gastric cancer. Cancer Lett. 1999;144:161–167. doi: 10.1016/s0304-3835(99)00222-0. [DOI] [PubMed] [Google Scholar]
- 11.Iishi H, Tatsuta M, Baba M, Yamamoto R, Uehara H, Nakaizumi A. Inhibition of experimental gastric carcinogenesis, induced by N-methyl-N’-nitro-N-nitrosoguanidine in rats, by sodium nitroprusside, a nitric oxide generator. Eur J Cancer. 1998;34:554–557. doi: 10.1016/s0959-8049(97)10074-0. [DOI] [PubMed] [Google Scholar]
- 12.Lim LH, Pervaiz S. Annexin 1: the new face of an old molecule. FASEB J. 2007;21:968–975. doi: 10.1096/fj.06-7464rev. [DOI] [PubMed] [Google Scholar]
- 13.Polli-Lopes AC, Zucoloto S, de Queirós Cunha F, da Silva Figueiredo LA, Garcia SB. Myenteric denervation reduces the incidence of gastric tumors in rats. Cancer Lett. 2003;190:45–50. doi: 10.1016/s0304-3835(02)00584-0. [DOI] [PubMed] [Google Scholar]
- 14.Meneses AC, Lopes MA, Rocha A, Fatureto MC, Lopes GP, Lopes ER, Chapadeiro E. Megas and cancer. Cancer of the large intestine in chagasic patients with megacolon. Arq Gastroenterol. 1989;26:13–16. [PubMed] [Google Scholar]
- 15.Garcia SB, Oliveira JS, Pinto LZ, Muccillo G, Zucoloto S. The relationship between megacolon and carcinoma of the colon: an experimental approach. Carcinogenesis. 1996;17:1777–1779. doi: 10.1093/carcin/17.8.1777. [DOI] [PubMed] [Google Scholar]
- 16.Garcia SB, Aranha AL, Garcia FR, Basile FV, Pinto AP, de Oliveira EC, Zucoloto S. A retrospective study of histopathological findings in 894 cases of megacolon: what is the relationship between megacolon and colonic cancer? Rev Inst Med Trop Sao Paulo. 2003;45:91–93. doi: 10.1590/s0036-46652003000200007. [DOI] [PubMed] [Google Scholar]
- 17.Castañeda AA, Denning JW, Chang L, Mercer DW. Does upregulation of inducible nitric oxide synthase (iNOS) render the stomach more susceptible to damage? J Surg Res. 1999;84:174–179. doi: 10.1006/jsre.1999.5637. [DOI] [PubMed] [Google Scholar]
- 18.Salter M, Knowles RG, Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 1991;291:145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- 19.Cunha FQ, Assreuy J, Moss DW, Rees D, Leal LM, Moncada S, Carrier M, O’Donnell CA, Liew FY. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: role of TNF-alpha and IL-1-beta. Immunology. 1994;81:211–215. [PMC free article] [PubMed] [Google Scholar]
- 20.Wolff DJ, Lubeskie A. Aminoguanidine is an isoform-selective, mechanism-based inactivator of nitric oxide synthase. Arch Biochem Biophys. 1995;316:290–301. doi: 10.1006/abbi.1995.1040. [DOI] [PubMed] [Google Scholar]
- 21.Jianmongkol S, Vuletich JL, Bender AT, Demady DR, Osawa Y. Aminoguanidine-mediated inactivation and alteration of neuronal nitricoxide synthase. J Biol Chem. 2000;275:13370–13376. doi: 10.1074/jbc.275.18.13370. [DOI] [PubMed] [Google Scholar]
- 22.Rajnakova A, Goh PM, Chan ST, Ngoi SS, Alponat A, Moochhala S. Expression of differential nitric oxide synthase isoforms in human normal gastric mucosa and gastric cancer tissue. Carcinogenesis. 1997;18:1841–1845. doi: 10.1093/carcin/18.9.1841. [DOI] [PubMed] [Google Scholar]
- 23.Nishio H, Hayashi Y, Terashima S, Takeuchi K. Role of endogenous nitric oxide in mucosal defense of inflamed rat stomach following iodoacetamide treatment. Life Sci. 2006;79:1523–1530. doi: 10.1016/j.lfs.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Parente L, Solito E. Annexin 1: more than an anti-phospholipase protein. Inflamm Res. 2004;53:125–132. doi: 10.1007/s00011-003-1235-z. [DOI] [PubMed] [Google Scholar]
- 25.Wang KL, Wu TT, Resetkova E, Wang H, Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Hamilton SR, Albarracin CT. Expression of annexin A1 in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Clin Cancer Res. 2006;12:4598–4604. doi: 10.1158/1078-0432.CCR-06-0483. [DOI] [PubMed] [Google Scholar]
- 26.Yu G, Wang J, Chen Y, Wang X, Pan J, Li Q, Xie K. Tissue microarray analysis reveals strong clinical evidence for a close association between loss of annexin A1 expression and nodal metastasis in gastric cancer. Clin Exp Metastasis. 2008;25:695–702. doi: 10.1007/s10585-008-9178-y. [DOI] [PubMed] [Google Scholar]
- 27.Mussunoor S, Murray GI. The role of annexins in tumour development and progression. J Pathol. 2008;216:131–140. doi: 10.1002/path.2400. [DOI] [PubMed] [Google Scholar]
- 28.Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 29.Oliani SM, Paul-Clark MJ, Christian HC, Flower RJ, Perretti M. Neutrophil interaction with inflamed postcapillary venule endothelium alters annexin 1 expression. Am J Pathol. 2001;158:603–615. doi: 10.1016/S0002-9440(10)64002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sena AA, Provazzi PJ, Fernandes AM, Cury PM, Rahal P, Oliani SM. Spatial expression of two anti-inflammatory mediators, annexin 1 and galectin-1, in nasal polyposis. Clin Exp Allergy. 2006;36:1260–1267. doi: 10.1111/j.1365-2222.2006.02570.x. [DOI] [PubMed] [Google Scholar]
- 31.Silistino-Souza R, Rodrigues-Lisoni FC, Cury PM, Maniglia JV, Raposo LS, Tajara EH, Christian HC, Oliani SM. Annexin 1: differential expression in tumor and mast cells in human larynx cancer. Int J Cancer. 2007;120:2582–2589. doi: 10.1002/ijc.22639. [DOI] [PubMed] [Google Scholar]
- 32.Kamal AM, Flower RJ, Perretti M. An overview of the effects of annexin 1 on cells involved in the inflammatory process. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):39–47. doi: 10.1590/s0074-02762005000900008. [DOI] [PubMed] [Google Scholar]
- 33.Damazo AS, Yona S, D’Acquisto F, Flower RJ, Oliani SM, Perretti M. Critical protective role for annexin 1 gene expression in the endotoxemic murine microcirculation. Am J Pathol. 2005;166:1607–1617. doi: 10.1016/S0002-9440(10)62471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsiang CH, Tunoda T, Whang YE, Tyson DR, Ornstein DK. The impact of altered annexin I protein levels on apoptosis and signal transduction pathways in prostate cancer cells. Prostate. 2006;66:1413–1424. doi: 10.1002/pros.20457. [DOI] [PubMed] [Google Scholar]
- 35.Damazo AS, Paul-Clark M, Straus A, Takahashi H, Perretti M, Oliani SM. Analysis of the annexin-1 expression in the rat trachea. Study of mast cell heterogeneity. Annexins. 2004;1:12–18. [Google Scholar]
- 36.Bandeira-Melo C, Bonavita AG, Diaz BL, E Silva PM, Carvalho VF, Jose PJ, Flower RJ, Perretti M, Martins MA. A novel effect for annexin 1-derived peptide ac2-26: reduction of allergic inflammation in the rat. J Pharmacol Exp Ther. 2005;313:1416–1422. doi: 10.1124/jpet.104.080473. [DOI] [PubMed] [Google Scholar]
- 37.Estofolete CF, Botelho-Machado C, Taboga SR, Zucoloto S, Polli-Lopes AC, Gil CD. Effects of myenteric denervation on extracellular matrix fibers and mast cell distribution in normal stomach and gastric lesions. Cancer Cell Int. 2010;10:18. doi: 10.1186/1475-2867-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Acquisto F, Perretti M, Flower RJ. Annexin-A1: a pivotal regulator of the innate and adaptive immune systems. Br J Pharmacol. 2008;155:152–169. doi: 10.1038/bjp.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]