Abstract
Objective: To investigate the mechanism underlying the effect of hyperbaric oxygen (HBO) on hypoxic/ischemic brain damage (HIBD) in a neonatal rat model. Methods: A total of 30 neonatal SD rats aged 7 days were randomly assigned into control group, HIBD group and HBO group (n=10 per group). Following HIBD modeling in neonatal rats, HBO treatment was performed for consecutive 7 days. Immunohistochemistry was done to measure the expression of bone morphogenetic protein-4 (BMP-4) and nestin in the hippocampus. In situ hybridization was employed to detect the mRNA expression of BMP-4 and nestin in the hippocampus. TUNEL staining was done to detect the apoptosis of nerve cells. Results: HIBD was successfully established in the present study. Among three groups, the protein expression of BMP-4 in the hippocampus was the highest in the HBO group, and the smallest in the HIBD group. The BMP-4 expression in the HIBD group was significantly lower than that in the control group. The protein expression of nestin in the hippocampus was the highest in the HBO group, and the smallest in the HIBD group. The nestin protein expression in the hippocampus of HIBD group was significantly lower than that in the control group. The mRNA expression of BMP-4 in the hippocampus was the highest in the HBO group, and the smallest in the HIBD group. The mRNA expression of nestin in the hippocampus was the highest in the HBO group, and the smallest in the HIBD group. The number of apoptotic cells was the largest in the HIBD group, and the number of apoptotic cells in the HBO group was still larger than that in the control group (P<0.01). Conclusion: HBO may promote the neurological recovery in neonatal rats with HIBD, which may be attributed to the increased protein and mRNA expression of BMP-4 and nestin in the hippocampus and the inhibition of neural apoptosis.
Keywords: Neonatal rat, ischemic/hypoxic brain damage, hyperbaric oxygen, bone morphogenetic protein – 4, nestin
Introduction
With the development of perinatology, the survival rate of critically ill newborns including premature infant and low birth weight infants continues to increase. Thus, the incidence and mortality of neonatal acquired brain damage, especially the hypoxic-ischemic brain damage (HIBD), has an increasing tendency. It is estimated that the incidence of HIBD is about 6/1000 live births, and 25-30% of survived neonates with HIBD often present with long-term sequelas. Thus, HIBD has become a major cause influencing the quality of life of children worldwide [1-3]. Neonatal HIBD refers to the hypoxia/ischemia induced brain injury secondary to perinatal suffocation and is characterized by a series of abnormalities in the central nervous system [4]. HIBD is the most common cause of brain damage in perinatal full-term infants [1,5]. HIBD can result in severe sequelas including cerebral palsy, epilepsy and mental retardation. However, no effective strategy has been developed for the treatment of HIBD so far [6,7]. In respect of significantly detrimental influence of neonatal hypoxic-ischemic encephalopathy (HIE) on the quality of life of infants, it is imperative to investigate the pathogenesis of HIBD, which may provide evidence for the effective treatment of HIBD. The permanent brain damage following HIBD is attributed to the sensitivity of neural stem cells (NSCs) in the subventricular zone (SVZ) to ischemia/hypoxia, which leads to the apoptosis and necrosis [8]. Hyperbaric oxygen (HBO) has been used in the treatment of neonatal HIBD. Animal experiments and clinical trials have shown that HBO performed within 6 h after HIBD may achieve favorable outcome and promote the long term neurological recovery [9-12]. In addition, a lot of Chinese studies also demonstrate that HBO can reduce the disability and mortality of HIBD [13]. However, there are still controversies on the therapeutic efficacy and the exact mechanism of HBO on HIBD. For this, HIBD animal model was established in the present study and HBO was employed to treat HIBD. Immunohistochemistry, in situ hybridization and TUNEL staining were performed to investigate the mechanism of therapeutic effect of HBO on HIBD. Our findings may provide theoretical evidence for the clinical treatment of HIBD with HBO.
Materials and methods
Animals, grouping and treatment
Neonatal rats aged 7 days (n=30) and weighing 10-12 g (specific pathogen free) were purchased from the Experimental Animal Center of Academy of Military Medical Sciences. These animals were randomly assigned into 3 groups: 1) normal control group (n=10): rats received intramuscular injection of 0.9% NaCl at 10μl/g/d for consecutive 7 days; 2) HIBD group: neonatal rats were introduced with HIBD but received no treatment; (3) HBO group: at 6 h after HIBD, rats were treated with HBO. Compression was done within 10 min (0.002 MPa/min), the pressure at 0.2 MPa was maintained for 20 min, and decompression completed within 10 min (0.002 MPa/min). HBO treatment was performed for 7 days. At 7 days after treatment, animals were sacrificed for further detection.
Main reagent
Goat anti-rat BMP-4 polyclonal antibody, mouse anti-rat nestin monoclonal antibody, in situ hybridization kits for nestin and BMP-4, cell apoptosis detection kit, DAB kit, NBF/BCIP kit, SABC kit, tetrazolium red (Zhongshan Biotech Co., Ltd) and other reagents (analytically pure) were used in the present study.
HIBD modeling and sample collection
The 7-day old SD rats were anesthetized with anhydrous ether for 0.5-1 min. The animal was placed in left palm under a microscope. The forefinger and middle finger were used to fix the head of the animal, and the thumb to fix the bilateral forelimbs. A midline incision was made at the neck, and the subcutaneous fat was separated. The left common carotid artery was separated through the inner side of sternocleidomastoid, and ligated with 5-0 suture twice followed by disconnecting the common carotid artery. Hemostasis was done with gelatin sponge. At 2-3 h after surgery, animals were placed in a closed chamber (10 L) and received hypoxic treatment (8% oxygen) at 37°C at a flow rate of 0.5 L/min for 2.5 h. In the normal control group, animals did not receive any treatment. In the HIBD group, animals receive both ligation of common carotid artery and hypoxia for 2.5 h. In the HBO group, animals received HBO treatment at 6 h after HIBD. At the predesigned time points, animals were anesthetized with ether, and thoracotomy was done. The heart was exposed and a catheter was inserted via the aorta. Perfusion was done with cold normal saline at 20 ml/kg to remove blood and the brain was collected. The macroscopic features of the brain were recorded and then the brain was placed in a freezing microtome at -20°C for balance for 2 h. Coronal sections (8 μm) were collected along the suprachiasmatic midpoint into the polylysine-coated slides followed by fixation in acetone for 20 min. The slides were stored at -20°C or immediately used in the following experiments [14,15].
Tetrazolium red staining
One rat was sacrificed in each group and the brain was collected and stained with 2% tetrazolium red. The white region was defined as the ischemic region.
HE staining
The frozen sections were treated sequentially with xylene, ethanol at different concentration, distilled water (2 min), Harris hematoxylin (1 min), 10% ethanol in acid (several seconds), flowing water (1 min), ethanol at different concentrations (75%, 85% and 95%; 2 min for each), 1% eosin (1 min), 95% ethanol ()several seconds), 100% ethanol (twice; 10 min for each) and xylene (twice; 10 min for each). Then, these sections were mounted and observed under a light microscope and representative photographs were captured.
NISSL staining
The dried sections were immersed in distilled water for 5 min, and then sequentially treated with ethanol at increasing concentrations (75%, 85%, 95% and 100%; 5 min for each) and then with ethanol at decreasing concentrations (100%, 95%, 85% and 75%). After incubation in 1% toluidine blue at 500C, staining was performed in an incubator at 560C for 20 min. Following treatment in distilled water for 10 min, sections were incubated with 70% ethanol (min), 95% ethanol, absolute ethanol I (1 min) and absolute ethanol II (1 min) and then with xylene I (10 min) and xylene II (10 min). After mounting, sections were observed under a light microscope and representative photographs were captured.
Detection of protein expression of BMP-4 and nestin in hippocampus
Immunohistochemistry was performed with routine SABC technique. Sections were fixed in acetone and allowed to stay at room temperature for 20 min. After treatment in xylene and ethanol at different concentrations, sections were incubated with 0.6% methanol in H2O2 for 20 min. Following washing in PBS thrice (5 min for each), sections were blocked in goat serum at room temperature for 20 min. Then, these sections were incubated with mouse anti-rat nestin monoclonal antibody (1:200) at 37°C for 90 min. Following washing in PBS thrice (10 min for each), sections were incubated with biotin conjugated goat anti-mouse IgG at 37°C fpr 30 min. Following washing in PBS thrice (10 min for each), sections were treated with SABC at 37°C for 30 min. After washing in PBS four times (5 min for each), visualization was done with DAB for 5 min. Following complete washing in water, dehydration, and transparentization, mounting was done with neutral gum. In the negative control, the primary antibody was replaced with 0.01mol/L PBS and secondary antibody was replaced with normal goat serum. Sections were observed under a light microscope. Cells with brown granules in the cytoplasm were considered to be positive for target proteins.
Detection of mRNA expression of BMP-4 and nestin in hippocampus
In situ hybridization was done according to the manufacturer’s instructions with modification. In brief, sections were fixed in 4% paraformaldehyde for 30 min. After washing in 0.01 M PBS twice (10 min for each), sections were treated with 0.3% Triton-X 100 for 30-60 s. After washing in 0.01 M PBS thrice (10 min for each), sections were treated with 2×SSC twice (10 min for each) and then with probe at 42°C overnight. After incubation in 4×SSC, 2×SSC, 1×SSC and 0.5×SSC (10 min for each), sections were washing in 0.01 M PBS thrice (10 min for each), and treated with alkaline phosphatase conjugated digoxin antibody (1:500) for 4 h at 37°C. After washing in 0.01 M PBS thrice (10 min for each), sections were treated with TSM1 for 20 s and TSM2 for 20 s. Visualization was done with NBF/BCIP for 20 s. After dehydration, transparentization, and mounting, sections were observed under a microscope and representative photographs were captured. In the negative control group, probe or antibody was not used. mRNA of BMP-4 and nestin is found in the cytoplasm. Cells with blue granules in the cytoplasm were considered to be positive for target genes.
Detection of apoptotic nerve cells
Detection of apoptosis was done according to the manufacturer’s instructions with slight modification. In brief, sections were treated with 4% paraformaldehyde at room temperature for 30 min. After washing in PBS thrice (10 min for each), sections were incubated with 0.3% H2O2 in methanol at room temperature for 30 min. After washing in PBS thrice (10 min for each), sections were incubated with 0.1%TritonX-100 at 37°C for 30 min. Following washing in PBS thrice (10 min for each), sections were incubated with TUNEL reaction solution at 37°C for 60 min. Following washing in PBS thrice (10 min for each), sections were treated with POD at 37°C for 60 min. Following washing in PBS thrice (10 min for each), sections were treated with DAB at room temperature for 5-20 min. After washing in PBS thrice (10 min for each), sections were observed under a light microscope. In the negative control, the TUNEL reaction solution was replaced with 50 μl of 0.1 M PBS. Cells with yellow-brown granules in the nucleus were considered to be apoptotic cells.
Statistical analysis
Data are expressed as mean ± standard deviation ( x̅ ±s) and statistical analysis was done with SPSS version 12.0. Analysis of variance was used to comparisons of means among three groups.
Results
Behaviors of rats with HIBD
After ligation of left common carotid artery, hypoxia for 10 min induced dysphoria, hypoxia for 15-20 min caused cyanosis and deep and rapid breathing, hypoxia for 20-30 min led to unstable standing and dragging step of right hind limb during creeping; hypoxia for 35-60 significantly reduced the activity; hypoxia for more than 1 h resulted in lethargy and irritability in 90% of rats with HIBD. At 1 h after posthypoxic re-oxygenation, rats circled towards left side. For rats receiving hypoxia alone, abnormal behaviors were not observed.
Macroscopic observation
For normal rats and those receiving hypoxia alone, the bilateral hemispheres were symmetrical and brain edema was not observed at any time point. At 3 h after ischemia/hypoxia, there were no changes in bilateral hemispheres; at 6 h after ischemia/hypoxia, mild edema was noted in the left brain which was pale; at 24 h after ischemia/hypoxia, obvious edema was found in the left brain which was pale (especially in the frontoparietal lobes), intraventricular hematocele was also noted; at 3 d after ischemia/hypoxia, the brain edema was slightly attenuated, but pale region was still found in the frontoparietal lobes; at 7 days after ischemia/hypoxia, the bilateral hemispheres were basically symmetrical; at 14 d after ischemia/hypoxia, the left hemisphere was shrunken; at 21 d after ischemia/hypoxia, the left hemisphere was atrophy, the cortex was thickened, the hippocampus deformed, the cerebral ventricle was enlarged and several vacuoles were observed.
Tetrazolium red staining
In the HIBD group and HBO group, the ischemic hemisphere was white and the intact hemisphere was red. The normal brain was stained red.
Pathological examination of brain of rats with HIBD
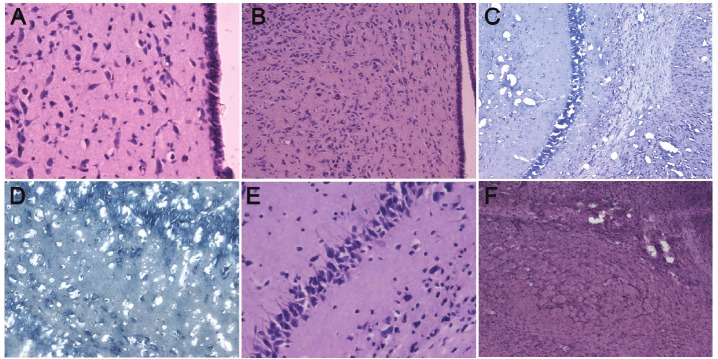
In the control group, the brain had clear structural layers and cells presented with clear borderline. The nucleus located at the center of the cells, the nucleolus was clear and Nissl body was evenly distributed around the nucleus. At 3 h after ischemia/hypoxia, focal karyopyknosis of neurons was found in the left cortex and striatum; at 6 h after hypoxia/ischemia, the lesions in the striatum was enlarged and more lesions were observed, and small lesions were also noted in the hippocampus and thalamus; at 24 h after hypoxia/ischemia, massive necrosis was found in the cortex, striatum, hippocampus and thalamus, and degradation or absence of cells was noted; at 3 days after hypoxia/ischemia, proliferation of glial cells was noted around the lesions, and a lot of pyknotic nuclei and nuclear debris were found at the center of lesions; at 7 days after hypoxia/ischemia, only a few pyknotic nuclei, and the proliferative glial cells increased as compared to those on day 3; at 14 and 21 days after hypoxia/ischemia, massive loss of neurons was found in the lesions, and glial scars were observed in the lesions of cortext, striatum, hippocampus and thalamus (Figure 1A-F).
Figure 1.
Pathological examination of brain of HIBD neonatal rats. A: 3 h after hypoxia/ischemia (HIBD group), pathological examination of thalamus showed focal karyopyknosis of neurons (HE staining, 400×); B: 6 h after hypoxia/ ischemia (HIBD group), pathological examination of thalamus showed neurons with karyopyknosis increased and the number of small lesions was elevated (HE staining, 100×); C: 1 d after hypoxia/ischemia (HIBD group), pathological examination of the brain: massive necrosis in the hippocampus and striatum, and degradation or absence of cells (Nissl staining, 100×); D: 1 d after hypoxia/ischemia (HIBD group), pathological examination of the brain: massive necrosis in the hippocampus, and degradation or absence of cells (Nissl staining, 400×); E: 3 d after hypoxia/ischemia (HIBD group), pathological examination of the hippocampus: a lot of neurons with karyopyknosis at the center of lesions, surrounding glial cell proliferation (HE staining, 400×); F: 21 h after hypoxia/ischemia (HIBD group), pathological examination of thalamus: proliferation of astrocytes surrounding the lesions and glial scars were found, and cavity formed due to liquefaction necrosis of the lesions (HE staining, 100×).
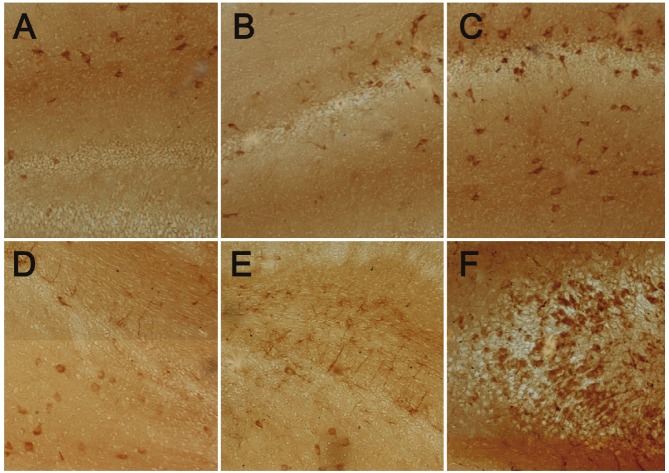
Protein expression of BMP-4 and nestin in hippocampus in immunohistochemistry
BMP-4 and nestin are expressed in the cytoplasm and yellow-brown in immunohistochemistry. The BMP-4 expression in the hippocampus was the highest in the HBO group, and the lowest in the HIBD group. The BMP-4 expression in the HIBD group was significantly lower than that in the control group. The nestin expression in the hippocampus was the highest in the HBO group, and the lowest in the HIBD group. The nestin expression in the HIBD group was markedly reduced as compared to the control group (Figure 2A-F).
Figure 2.
Protein expression of BMP-4 and nestin in hippocampus. A: Protein expression of BMP-4 in hippocampus of the control group. Positive cells were yellow (DAB; 100×); B: Protein expression of BMP-4 in hippocampus of affected hemisphere of the HIBD group. Positive cells were yellow (DAB; 100×); C: Protein expression of BMP-4 in hippocampus of affected hemisphere of the HBO group. Positive cells were yellow (DAB; 100×); D: Protein expression of nestin in hippocampus of the control group. Positive cells were yellow (DAB; 100×); E: Protein expression of nestin in hippocampus of affected hemisphere of the HIBD group. Positive cells were yellow (DAB; 100×); F: Protein expression of nestin in hippocampus of affected hemisphere of the HBO group. Positive cells were yellow (DAB; 100×).
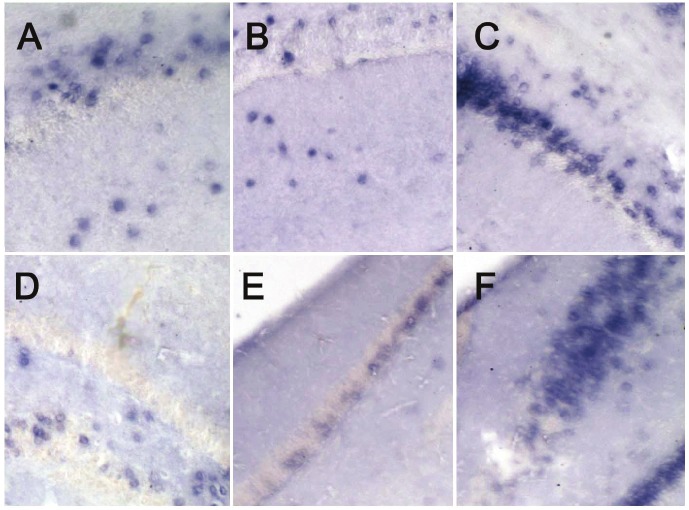
mRNA expression of BMP-4 and nestin in hippocampus in in situ hybridization
The mRNA of BMP-4 and nestin locates in the cytoplasm, and cells with blue granules in the cytoplasm were regarded as positive cells. The mRNA expression of BMP-4 in the hippocampus was the highest in the HBO group, and the lowest in the HIBD group. The mRNA expression of BMP-4 in the hippocampus of HIBD group was significantly lower than that in the control group. In addition, the mRNA expression of nestin in the hippocampus was the highest in the HBO group, and the lowest in the HIBD group. The mRNA expression of nestin in the hippocampus of HIBD group was significantly lower than that in the control group. (Figure 3A-F).
Figure 3.
mRNA expression of BMP-4 and nestin in hippocampus. A: mRNA expression of BMP-4 in hippocampus of the control group. Positive cells were blue (NBF/BCIP; 100×); B: mRNA expression of BMP-4 in hippocampus of affected hemisphere of the HIBD group. Positive cells were blue (NBF/BCIP; 100×); C: mRNA expression of BMP-4 in hippocampus of affected hemisphere of the HBO group. Positive cells were blue (NBF/BCIP; 100×); D: mRNA expression of nestin in hippocampus of the control group. Positive cells were blue (NBF/BCIP; 100×); E: mRNA expression of nestin in hippocampus of affected hemisphere of the HIBD group. Positive cells were blue (NBF/BCIP; 100×); F: mRNA expression of nestin in hippocampus of affected hemisphere of the HBO group. Positive cells were blue (NBF/BCIP; 100×).
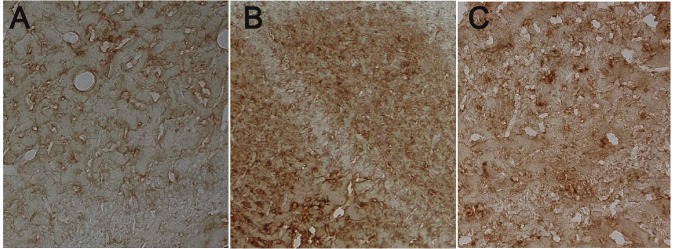
Apoptosis of nerve cells in brain
Among three groups, the number of apoptotic cells was the largest in the HIBD group. In the HBO group, the number of apoptotic cells was significantly higher than that in the control group, but markedly lower than that in the HIBD group (Figure 4A-C) (P<0.01, Table 1).
Figure 4.
Apoptotic cells in the brain of different groups. A: Apoptotic neurons in the brain of control group. Positive cells were yellow (DAB, 100×); B: Apoptotic neurons in the affected hemisphere of HIBD group. Positive cells were yellow (DAB, 100×); C: Apoptotic neurons in the affected hemisphere of HBO group. Positive cells were yellow (DAB, 100×).
Table 1.
Number of apoptotic cells in the brain of three groups (n=10, x̅ ±s)
P<0.01 vs control group;
P<0.01 vs HIBD group.
Discussion
The brain development of 7-day-old rats is similar to that of humans in neonatal stage, and the hypoxia/ischemia induced brain injury in these rats is also comparable to neonatal HIBD in humans. Thus, the 7-day-old rats have been widely applied in the neonatal HIBD animal model to investigate the pathogenesis and treatment of HIBD [13]. Anesthesia is a key factor assuring the survival of animals in the surgery and hypoxia. Ether is a favorable anesthetic in the preparation of HIBD animal model in which rats recover rapidly and are tolerant to hypoxia. Accurate ligation of common carotid artery is a key step for successful preparation of HIBD animal model. Before ligation, the blood flow should be confirmed and favorable separation of surrounding tissues is also crucial to reduce bleeding because excessive bleeding may influence the tolerance to hypoxia. In the present study, hypoxia/ischemia induced brain injury was introduced to neonatal SD rats, and tetrazolium red staining and pathological examination showed the HIBD animal model was successfully established. The circling towards left side was attributed to the imbalance as a result of hypoxia/ischemia induced injury of subcortical motor center. The paralysis of ipsilateral limbs is the behavioral characteristic of rats with HIBD. In addition, the neonatal rats are usually resistance to hypoxia, and thus hypoxia alone often fails to induce pathological changes in the brain. At 3 h after hypoxia/ischemia, pathological changes began to occur; 24 h after hypoxia/ischemia, the pathological changes were the most obvious; at 3 days after hypoxia/ischemia, surrounding glial cells began to proliferate; 14 and 21 days after hypoxia/ischemia, a lot of neurons were lost and glial scars were found. These pathological changes were similar to those in neonates with HIBD. The above findings demonstrated that the HIBD animal model was successfully established. When compared with methods described in previous studies, this method is time-saving and requires less oxygen, the instruments are simple and site requirement is low [15]. Thus, this method is a simple tool to rapidly prepare neonatal HIE model with low cost and high success rate.
BMPs are a group of secretory glycoproteins and more 20 BMPs have been identified to date. Except for BMP1, all BMPs belong to the superfamily of transforming growth factor β (TGF-β) and BMPs have numerous biological functions. Studies have shown that BMPs involve in the osteogenesis and bone development in the embryonic stage, in the repair of bone defect in adults and the pathogenesis of several bone diseases. In addition, they also play important roles in the genesis of adipose tissues, kidney, liver, bone and nervous system. There is evidence showing that BMPs play crucial roles in the development of nervous system at different stages and different sites. Moreover, BMPs have complex effect on the neural stem cells and exert distinct effects on neural stem cells at different sites and different developmental stages. BMPs can induce the differentiation of neural stem cells into neurons and also promote their differentiation into astrocytes. Even at the same site or at the same developmental stage, there are still controversies, even conflicting results, on the role of BMPs currently [16].
BMP-4 is a member of BMP family and an important factor affecting the development of nervous system. Studies have confirmed that BMP-4 plays important roles in the development of nervous system at different stages and different sites. BMP-4 can induced the differentiation of neural stem cells (NSCs) into neurons and promote the differentiation of these cells into astrocytes. In addition, BMP-4 is able to induce the NSCs to differentiate into cholinergic cells and support the differentiation into catecholamine neurons, which is dependent on specific signaling pathway. Moreover, BMP-4 can also promote the differentiation of NSCs into dorsal nervous system and neural crest cells, and facilitate the differentiation into glial cells [16]. NSCs have multilineage differentiation potentials. After brain injury, NSCs are activated to differentiate into different neural cells which then migrate into the injured sites for construction of neurofunction. Thus, NSCs play important roles in the repair of brain injury. Nestin is mainly expressed in the neurula, and its expression reduces with the differentiation. The mature cells have no nestin expression. Thus, nestin has been used as a marker of NSCs [17,18]. In respect of the source of nestin following brain injury, there are two opinions currently. Some investigators propose that the astrocytes around the injured sites replay the embryonic development [19] but others suggest that the nestin positive cells are derived from the NSCs in the subependymal zone [20]. Under the physiological state, the NSCs in the brain are in a quiescent stage. In the presence of stimulation, some cytokines may activate these NSCs leading to their in situ or ectopic proliferation. In the aid of chemokines, these NSCs migrate into the injured sites and differentiate into functional cells [21]. Following the central nervous system injury, the endogenous NSCs are induced to differentiate into repair functional cells to repair the brain injury [22]. Under the hyperbaric condition, the blood oxygen pressure increases, and this further elevates the blood oxygen content and oxygen diffusion. This improves the hypoxia and the activities of mitochondrial enzymes, promotes the repair of cellular injury and recovery of normal metabolism and increases the production of free radicals, which finally induce the tolerance of nervous system to hypoxia/ischemia. In addition, HBO can also inhibit the production of some cytokines by activated microglial cells, which regulates the microglia cell mediated immune response [23].
Our results showed, the BMP-4 protein expression in the hippocampus of HIBD group was markedly lower than that in the control group at 7 days after HIBD, but that was significantly increased in the HBO group when compared with control group. In addition, at 7 days after HIBD, the nestin protein expression in the HIBD group was also significantly lower than that in control group, but that in the HBO group dramatically increased as compared to the control group. The mRNA expression of BMP-4 and nestin had consistent tendency with the protein expression of BMP-4 and nestin. These findings suggest that HBO can increase the protein and mRNA expression of BMP-4 and nestin which involve in the neurological recovery following HIBD. Furthermore, our findings revealed that the number of apoptotic cells in the HBO group was higher than that in the control group, but lower than that in the HIBD group. This indicates that HBO can inhibit the apoptosis of neural cells. Thus, we speculate that the therapeutic effect of HBO on HIBD is attributed to the induction of proliferation and differentiation of NSCs and reduction in apoptosis of neural cells, in which BMP-4 plays an important role in controlling the differentiation of NSCs into neurons and astrocytes.
Neonatal HIBD is a perinatal disease as a result of hypoxia/ischemia of any cause and reduction or discontinuation in cerebral blood flow in neonates or fetus. HIBD is a common disease in the perinatal stage and usually results in sequelae. To date, effective treatment has not developed for the HIBD. To investigate the mechanism underlying the pathogenesis of HIE may guide the clinical treatment of HIBD. HBO may exert therapeutic effect on HIBD. HBO not only inhibits the apoptosis of neural cells, but increases the protein and mRNA expression of BMP-4 and nestin in the hippocampus, a region crucial for the memory. This suggests that HBO may improve the memory of animals with HIBD, and also provide evidence for the clinical treatment of HIBD with HBO.
Acknowledgements
This work was supported by the China Postdoctoral Science Foundation (No. 20070410505).
References
- 1.Zhou CL, Chen HJ, Yu RJ. Ultrasound diagnostics of neonatal brain. Peking University Medical Press; 2007. pp. 85–88. [Google Scholar]
- 2.Vasiljević B, Maglajlić-Djukić S, Gojnić M. The prognostic value of amplitude-integrated electroencephalography in neonates with hypoxicischemic encephalopathy. Vojnosanit Pregl. 2012;69:492–499. [PubMed] [Google Scholar]
- 3.Verklan MT. The chilling details: hypoxic-ischemic encephalopathy. J Perinat Neonatal Nurs. 2009;23:59–68. doi: 10.1097/01.JPN.0000346221.48202.7e. [DOI] [PubMed] [Google Scholar]
- 4.Lynch NE, Stevenson NJ, Livingstone V, Murphy BP, Rennie JM, Boylan GB. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia. 2012;53:549–557. doi: 10.1111/j.1528-1167.2011.03401.x. [DOI] [PubMed] [Google Scholar]
- 5.Triulzi F, Parazzini C, Righini A. Patterns of damage in the mature neonatal brain. Pediatr Radiol. 2006;36:608–620. doi: 10.1007/s00247-006-0203-5. [DOI] [PubMed] [Google Scholar]
- 6.Pietrini D, Piastra M, Luca E, Mancino A, Conti G, Cavaliere F, De Luca D. Neuroprotection and hypothermia in infants and children. Curr Drug Targets. 2012;13:925–935. doi: 10.2174/138945012800675641. [DOI] [PubMed] [Google Scholar]
- 7.Wintermark P. Current controversies in newer therapies to treat birth asphyxia. Int J Pediatr. 2011;2011:848413. doi: 10.1155/2011/848413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levison SW, Rothstein RP, Romanko MJ, Snyder MJ, Meyers RL, Vannucci SJ. Hypoxia/ischemia depletes the rat perinatal subventricular zone of oligodendrocyte progenitors and neural stem cells. Dev Neurosci. 2001;23:234–247. doi: 10.1159/000046149. [DOI] [PubMed] [Google Scholar]
- 9.Badr AE, Yin W, Mychaskiw G, Zhang JH. Dual effect of HBO on cerebral infarction in MCAO rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:766–770. doi: 10.1152/ajpregu.2001.280.3.R766. [DOI] [PubMed] [Google Scholar]
- 10.Calvert JW, Zhou C, Nanda A, Zhang JH. Effect of hyperbaric oxygen on apoptosis in neonatal hypoxia-ischemia rat model. J App l Physiol. 2004;95:2072–2080. doi: 10.1152/japplphysiol.00630.2003. [DOI] [PubMed] [Google Scholar]
- 11.Veltkamp R, Siebing DA, Heiland S, Schoenffeldt-Varas P, Veltkamp C, Schwaninger M, Schwab S. Hyperbaric oxygen induces rapid protection against focal cerebral ischemia. Brain Res. 2005;1037:134–138. doi: 10.1016/j.brainres.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Nemoto EM, Betterman K. Basic physiology of hyperbaric oxygen in brain. Neurol Res. 2007;29:116–126. doi: 10.1179/016164107X174138. [DOI] [PubMed] [Google Scholar]
- 13.Gai Y, Zhang X. Approach of Therapeutic Juncture about Hyperbaric Oxygen Treatment for Critical Hypoxic-Ischemic Encephalopathy. J Appl Clin Pediatr. 2005;20:489–489. [Google Scholar]
- 14.Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 15.Yin XJ, Ju R, Feng ZC. Changes of neural stem cells in neonatal rat model of hypoxic-ischemic encephalopathy. Chin J Pediatr. 2005;43:572–575. [PubMed] [Google Scholar]
- 16.Yin XJ, Liu DY, Luo FP, Long Q, Feng ZC. Effect of basic fibroblast growth factor on expression of protein and mRNA of bone morphogenetic protein 4 in hypoxic-ischemic brain damage in newborn rats. Chin J Pediatr. 2009;47:856–861. [PubMed] [Google Scholar]
- 17.Uchida K, Okano H, Hayashi T, Mine Y, Tanioka Y, Nomura T, Kawase T. Grafted swine neuroepithelial stem cells can form myelinated axons and both efferent and afferent synapses with xenogeneic rat neurons. J Neurosci Res. 2003;72:661–669. doi: 10.1002/jnr.10628. [DOI] [PubMed] [Google Scholar]
- 18.An YH, Wan H, Zhang ZS, Wang HY, Gao ZX, Sun MZ, Wang ZC. Effect of rat Schwann cell secretion on proliferation anddifferentiation of human neural stem cells. Biomed Environ Sci. 2003;16:90–94. [PubMed] [Google Scholar]
- 19.Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization,and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmin S, Almqvist P, Lendahl U, Mathiesen T. Adult nestinex-pressing subependymal cells differentiate to astrocytes in response to brain injury. Eur J Neurosci. 1997;9:65–75. doi: 10.1111/j.1460-9568.1997.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 22.Riess P, Zhang C, Saatman KE, Laurer HL, Longhi LG, Raghupathi R, Lenzlinger PM, Lifshitz J, Boockvar J, Neugebauer E, Snyder EY, McIntosh TK. Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery. 2002;51:1043–1052. doi: 10.1097/00006123-200210000-00035. [DOI] [PubMed] [Google Scholar]
- 23.Lu PG, Feng H, Wang XR, Lu JY, Hu SL, Xia YZ, Chu WH, Gong GQ. Effects of preconditioning with hyperbaric oxygen on expression of GFAP and nestin after spinal cord injury in rats. Chin J Neurosurg Dis Res. 2008;7:149–153. [Google Scholar]