Abstract
Aims: To evaluate the frequency of somatostatin-receptor 5 (SSTR 5) in pancreatic neuroendocrine tumors by using monoclonal and polyclonal antibodies. Material and Method: we analyzed 66 proven pancreatic neuroendocrine tumors immunohistochemically with monoclonal (clone UMB-4) and polyclonal SSTR 5-antibodies. Immunoreactive score (IRS) and DAKO-score Her2/neu were evaluated. Results: Immunohistochemistry analysis demonstrated for the IRS a significant higher staining of all specimen using the monoclonal antibodies ( IRS SSTR5 poly vs IRS SSTR 5 mono; 20.0% vs 30.3% p < 0.001) by a correlation of 0.21; p = 0.04. For the HER2 score there was also a significant higher staining in the monoclonal group (Her2 SSTR 5 poly vs Her2 SSTR 5 mono; 21.5% vs 28.8% p < 0.001) by a correlation of 0.20; p = 0.08. Conclusion: Both antibodies are useful in staining of SSTR, although UMB-4 demonstrated a 10% higher SSTR 5 staining. Due to the previous underestimated expression rate of SSTR 5, current standards in diagnostics and therapy should be reconsidered. The increasing usage of long-acting pansomatostatin receptor analogues will rise the adverse effects connected to SSTR5 binding.
Keywords: Somatostatin, pancreatic tumor, neuroendocrine tumor, monoclonal antibody
Introduction
Somatostatin receptors (SSTR) are frequently expressed above-average in neuroendocrine tumors (NETs) [1]. In humans, five subtypes are differentiated: SSTR 1, 2A, 3, 4 and 5. SSTR play a decisive role in diagnostics and therapy of NETs. They are the basis for molecular in-vivo diagnostics, the antiproliferative and symptomatic biological therapy with somatostatin analogues and also for the antitumor radiation therapy, the peptide receptor radionuclide therapy (PRRT) [2-4].
The SSTR-IHC status plays another crucial role in a socio-economic point of view. On surgically removed or biopsied tissue, the SSTR density can be analysed quickly and due to the immunohistochemical analysis the indication for SSTR based diagnostics and therapy can be evaluated. It is therefore possible to avoid time-consuming additional examinations and therapies [5].
The fundamental and largest explorations about SSTR distribution in different organs were made by Prof. Reubi’s team using autoradiographic methods. The majority of the more current IHC studies which evaluate frequency and distribution of SSTR used polyclonal SSTR antibodies for the examinations. For a couple of years, a raising number of monoclonal SSTR antibodies have been developed. The already generally known high selectivity and sensibility for monoclonal antibodies was proven. In Western Blot examinations both for the monoclonal SSTR2A antibody (clone UMB-1) and monoclonal SSTR5 antibody (clone UMB-4) they demonstrated an excellent and highly selective SSTR binding without any disturbance by protein cross reactivities [6,7]. Schmid H et al. underlined the high specificity without cross reactivities of monoclonal SSTR antibodies [8]. However, despite these high specificities a comparative study concerning the use of polyclonal and monoclonal SSTR antibodies has not yet been accomplished.
Furthermore, long-acting somatostatin-analogues are recommended due to their anti-proliferative and symptomatic efficacy. Besides the well tolerated drugs there are some main adverse events as diarrhea, gallstones and hyperglycemia [9]. New developed analogues present a broader receptor spectrum which is supposed to improve treatment efficacy and lower incidence of adverse effects [9,10]. Previous studies have already shown the superiority in treatment of Cushing or Acromegaly diseases but they also report a high impact on the glucose homeostasis using new pan-somatostatin-analogues with a higher binding affinity to SSTR5 [11-13].
Is the frequency of SSTR5 distribution still underestimated in pancreatic neuroendocrine tumors? Therefore the aims of this study was to quantify the frequency of SSTR5 expression with a highly selective monoclonal antibody and moreover, to accomplish a correlation of a monoclonal with a polyclonal SSTR antibody for the first time.
Material and methods
50 patients with primary pancreatic neuroendocrine tumors underwent surgical treatment. 66 paraffin-embedded blocks were immunohistologically quantified. The paraffin-embedded blocks were generated from the Department of General and Visceral Surgery, the Laboratory of Pathology and Cytology Bad Berka und the Department of Pathology, Technical University of München.
Immunohistochemistry
The detection of SSTR-subtypes was performed using the streptavidin-biotin method and counterstaining was done with haematoxylin. The monoclonal antibody used for detection of SSTR5 (clonal UMB-4, SSTR5 mono) was produced by Epitomics, Burlingame, CA (USA) and the polyclonal one (SSTR5 mono) by Gramsch Laboratories, Schwabhausen (Germany) against the same amino acid sequence of the carboxyl terminal tail of the human SSTR5. The semi-quantitative analysis of the stained sections was done with light microscopy according to the immunoreactive score (IRS) by Remmele and Stegner and the DAKO score Her2/neu as previously described [14]. Only IRS ≥ 4 points and Her2/neu ≥ 2+ were considered positively for SSTR staining.
Statistics
Data were analysed using SigmaPlot 11.0. Spearman’s rank correlation analysis and Kendalls tau-tests were used.
Results
66 paraffin-embedded blocks of 50 patients with immunohistopathologically proven neuroendocrine pancreatic tumors have been worked on and were examined. In the polyclonal SSTR antibody group, one specimen had to be removed because of technical deficiency.
Immunohistochemistry analysis
For the IRS a significant higher staining of all specimen using the monoclonal antibodies ( IRS SSTR 5 poly vs IRS SSTR 5 mono; 20.0% vs30.3% p < 0.001) by a correlation of 0.21; p = 0.04 was seen (Table 1).
Table 1.
IRS and Her2-score of the SSTR-staining, comparison of monoclonal (UMB-4) and polyclonal antibodies in pancreatic neuroendocrine tumors
| IRS SSTR5 poly Score / Frequency (%) | Pancreas (n=65) | Her2/neu Score / Frequency (%) | Pancreas (n=65) |
| 0 | 18 (27.7%) | 0 | 29 (44.6%) |
| 1-3 | 34 (52.3%) | 1+ | 22 (33.8%) |
| 4-8 | 13 (20.0%) | 2+ | 14 (20%) |
| 9-12 | 0 (0%) | 3+ | 0 (0%) |
| SSTR5 positive total | 13/65 (20%) | 14/65 (21.5%) | |
| IRS SSTR5 mono Score / Frequency (%) | Pancreas (n=66) | Her2/neu Score / Frequency (%) | Pancreas (n=66) |
| 0 | 23 (34.8%) | 0 | 32 (48.5%) |
| 1-3 | 23 (34.8%) | 1+ | 15 (22.7%) |
| 4-8 | 15 (22.7%) | 2+ | 13 (19.7%) |
| 9-12 | 5 (7.6%) | 3+ | 6 (9.1%) |
| SSTR5 positive total | 20/66 (30.3%) | 19/66 (28.8%) |
IRS ≥ 4 points and Her2/neu ≥ 2+ were defined positively for SSTR expression.
For the HER2 score a significant higher staining in the monoclonal group (Her2 SSTR 5 poly vs Her2 SSTR 5 mono; 21.5% vs 28.8% p < 0.001) by a correlation of 0.20; p = 0.08 was observed (Table 1).
Limitations
The study is limited by its retrospective design as well as by the semiquantitative evaluation of the immunohistochemical staining.
Discussion
Somatostatin receptors are present throughout the exocrine and endocrine pancreatic tissue. The SSTR subtypes 2A, 3 and 5 are the most frequently expressed SSTR and form the basis for molecular diagnostics and pharmacological therapy with long acting SSTR analogues (octreotide, lanreotide, pasireotide) [15]. The current pharmacological therapy, but also the molecular imaging via SSTR-PET/CT, are strongly interconnected with the SSTR subtype 2A expression. However, during the last few years the important role of the SSTR5 within the pancreas has become more and more evident.
Due to the SSTR5 associated adverse events of new somatostantin-analogues which cause an inhibitory effect on insulin secretion the rate of hyperglycaemia is rising. Furthermore, immunohistochemical studies have proven a positive correlation of SSTR2A staining and the percentage decrease of growth hormone level after long-term octreotide treatment. After prolonged octreotide treatment, however, a significant reduction in SSTR2A staining was observed, which, in contrast could not be shown for the SSTR5 [16]. From these observations it can thus be postulated that even if the SSTR2A as most commonly used therapeutic target is suppressed after long-term octreotide treatment, the SSTR5 still remains as a promising therapeutic reserve target.
Thus, the aim of the present study was not only to quantify the frequency of SSTR5 expression in a large series of pancreatic neuroendocrine tumors. For the first time, the correlation of a monoclonal with a polyclonal SSTR antibody was examined too.
Frequency of SSTR subtypes
In previous studies the frequency of SSTR5 has been evaluated using polyclonal antibodies including all gastroenteropancreatic NET entities between 19 and 83%. If pancreatic NETs are looked at separately, frequencies between 56 and 78% are observed [15,17-20]. In the present study, a strict selection was made; thus, only cases of IRS ≥ 4 points und Her2/neu ≥ 2+ were observed to be strongly positive for SSTR expression. If all positive cases, including IRS ≥ 1 and Her2 ≥ 1+ are taken into account our study revealed frequencies between 52 and 72%, respectively. Therefore, the overall frequency of SSTR5 is in accordance with the results of other studies.
The correlation of IRS poly versus IRS mono resulted in a low but significant correlation (C:0.21; p=0.04). In contrast, the correlation between Her2/neu poly and mono could not reach statistical significance (C:0.20; p=0.08), despite displaying also a low positive association.
To our best knowledge, no other studies about correlation of polyclonal versus monoclonal SSTR antibodies exist. Thus, a comparison to other studies, applying SSTR is not possible yet.
Surprisingly, clearly 8-10% more positive SSTR5 stainings were noticed with the monoclonal antibody both in the Her2 and in the IRS analysis, as compared to the results obtained with the polyclonal anti-SSTR5 antibody. The reason for this discrepancy could be, that although polyclonal and monoclonal SSTR antibodies are made against the same peptide, they bind on specific but different epitopes. A polyclonal antibody consists of several antibodies with different recognition sites which assign specific subepitopes. However, there may also be an overlap in the target structures, which means that a competitive inhibition occurs, in which the first antibody on the receptor assigns the target, thus preventing the binding of the other antibodies. Compared to the polyclonal ones the selectivity of the monoclonal antibodies is clearly higher. Only a specific epitope of the carboxyl-terminal section of the peptide is detected. There is no competition at the target. Thus, the highly specific binding of UMB-4 to its target receptor without any signs of cross reactions seems to be responsible for the increased SSTR staining. In the study of Lupp et al. 2011, these excellent and highly selective qualities of UMB-4 have been proven; in particular, there were no interferences because of cross reactions to other proteins [7]. Figure 1 shows a pancreatic neuroendocrine tumor with a typical mainly cytoplasmatic SSTR5-staining demonstrated by using UMB-4. Figure 2 presents the same pancreatic specimen but with a staining caused by a polyclonal SSTR5–antibody.
Figure 1.
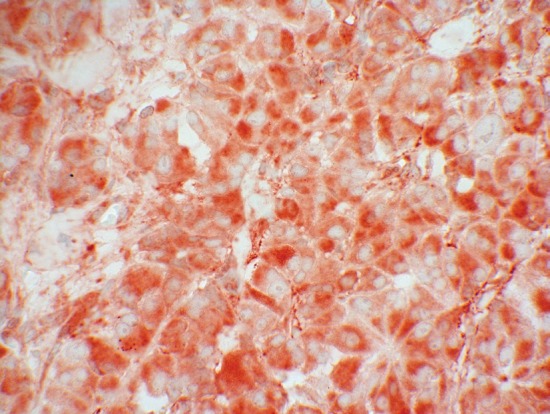
SSTR5 expression (red colour) of a pancreatic neuroendocrine tumor stained with the monoclonal antibody UMB-4.
Figure 2.
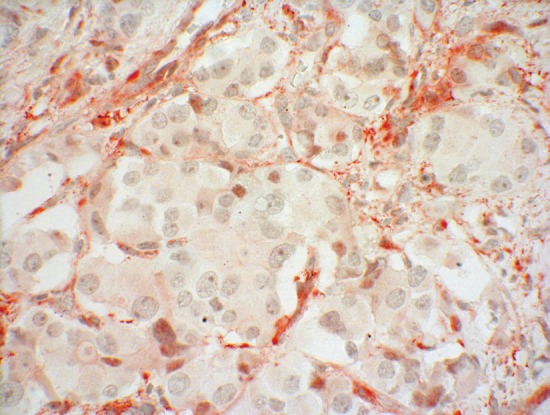
SSTR5 expression (red colour) of a pancreatic neuroendocrine tumor stained with a polyclonal anti SSTR5-antibody.
From the clinical point of view, the observation of a higher SSTR5 incidence is of special importance for application possibilities in terms of reserve receptor and with regard to the SSTR-associated adverse effects. After decreased response of SSTR2A following sustained octreotide therapy and/or in the course of cancer progression, SSTR5 can additionally become important as a new target in molecular diagnostics and therapy. However, by applying new pansomatostatin analogues (e.g. pasireotide) an increase in possible adverse effects, as e.g. hyperglycaemia, should be taken into account, since they are particularly connected to SSTR5 [10,13,21].
Conclusion
Both antibodies are useful in staining of SSTR, although UMB-4 demonstrated a 10% higher SSTR 5 staining. Due to the previous underestimated expression rate of SSTR 5, current standards in diagnostics and therapy should be reconsidered. The increasing usage of long-acting pansomatostatin receptor analogues will rise the adverse effects connected to SSTR5 binding.
Acknowledgement
We thank Sarah Osterberg for translating services.
Disclosure
The authors of the manuscript attest that we have nothing to disclose any financial or other relationships that could be construed as conflict of interest regarding to this study. Each author has contributed significantly to the submitted work and has finally proven the manuscript. Daniel Kaemmerer: had the conception, designed and performed the study and surgery, collected the tumors, drafted the manuscript and performed the immunohistochemical (IHC) assessment. Luisa Peter and Elke Fischer: performed the IHC assesssment and critically revised the manuscript. Amelie Lupp: developed the antibodies, contributed to the conception, arranged the IHC, acquired the data and critically revised the manuscript. Stefan Schulz: developed the antibodies, critically revised and approved the final manuscript. Günter Klöppel: enhanced the study with its intellectual content and critically revised the manuscript. Merten Hommann: performed the surgery, collected the tumors, and critically revised and approved the final manuscript.
References
- 1.Reubi JC. Somatostatin and other peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology. 2004;80(Suppl 1):51–56. doi: 10.1159/000080742. [DOI] [PubMed] [Google Scholar]
- 2.Kloppel G, Couvelard A, Perren A, Komminoth P, McNicol AM, Nilsson O, Scarpa A, Scoazec JY, Wiedenmann B, Papotti M, Rindi G, Plockinger U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90:162–166. doi: 10.1159/000182196. [DOI] [PubMed] [Google Scholar]
- 3.Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, Feelders RA, van Aken MO, Krenning EP. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3] octreotate: toxicity, efficacy, and survival. J. Clin. Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 4.Prasad V, Fetscher S, Baum RP. Changing role of somatostatin receptor targeted drugs in NET: Nuclear Medicine’s view. J Pharm Pharm Sci. 2007;10:321s–337s. [PubMed] [Google Scholar]
- 5.Miederer M, Seidl S, Buck A, Scheidhauer K, Wester HJ, Schwaiger M, Perren A. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:48–52. doi: 10.1007/s00259-008-0944-5. [DOI] [PubMed] [Google Scholar]
- 6.Fischer T, Doll C, Jacobs S, Kolodziej A, Stumm R, Schulz S. Reassessment of sst2 somatostatin receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-1. J Clin Endocrinol Metab. 2008;93:4519–4524. doi: 10.1210/jc.2008-1063. [DOI] [PubMed] [Google Scholar]
- 7.Lupp A, Hunder A, Petrich A, Nagel F, Doll C, Schulz S. Reassessment of sst(5) Somatostatin Receptor Expression in Normal and Neoplastic Human Tissues Using the Novel Rabbit Monoclonal Antibody UMB-4. Neuroendocrinology. 2011;94(3):255–64. doi: 10.1159/000329876. [DOI] [PubMed] [Google Scholar]
- 8.Schmid HA, Lambertini C, van Vugt HH, Barzaghi-Rinaudo P, Schafer J, Hillenbrand R, Sailer AW, Kaufmann M, Nuciforo P. Monoclonal Antibodies against the Human Somatostatin Receptor Subtypes 1-5: Development and Immunohistochemical Application in Neuroendocrine Tumors. Neuroendocrinology. 2012;95(3):232–47. doi: 10.1159/000330616. [DOI] [PubMed] [Google Scholar]
- 9.Bornschein J, Drozdov I, Malfertheiner P. Octreotide LAR: safety and tolerability issues. Expert Opin Drug Saf. 2009;8:755–768. doi: 10.1517/14740330903379525. [DOI] [PubMed] [Google Scholar]
- 10.Lamberts SW, van der Lely AJ, Hofland LJ. New somatostatin analogs: will they fulfil old promises? Eur J Endocrinol. 2002;146:701–705. doi: 10.1530/eje.0.1460701. [DOI] [PubMed] [Google Scholar]
- 11.Schmid HA. Preclinical evidences suggest new treatment options for endocrine disorders: Pasireotide (SOM230) and Everolimus (RAD001) Ann Endocrinol (Paris) 2008;69:162–163. doi: 10.1016/j.ando.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Schmid HA. Pasireotide (SOM230): development, mechanism of action and potential applications. Mol Cell Endocrinol. 2008;286:69–74. doi: 10.1016/j.mce.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Schmid HA, Brueggen J. Effects of somatostatin analogs on glucose homeostasis in rats. J Endocrinol. 2012 Jan;212:49–60. doi: 10.1530/JOE-11-0224. [DOI] [PubMed] [Google Scholar]
- 14.Kaemmerer D, Peter L, Lupp A, Schulz S, Sanger J, Prasad V, Kulkarni H, Haugvik SP, Hommann M, Baum RP. Molecular imaging with Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38:1659–1668. doi: 10.1007/s00259-011-1846-5. [DOI] [PubMed] [Google Scholar]
- 15.Zamora V, Cabanne A, Salanova R, Bestani C, Domenichini E, Marmissolle F, Giacomi N, O’Connor J, Mendez G, Roca E. Immunohistochemical expression of somatostatin receptors in digestive endocrine tumours. Dig Liver Dis. 2010;42:220–225. doi: 10.1016/j.dld.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Takei M, Suzuki M, Kajiya H, Ishii Y, Tahara S, Miyakoshi T, Egashira N, Takekoshi S, Sanno N, Teramoto A, Osamura RY. Immunohistochemical detection of somatostatin receptor (SSTR) subtypes 2A and 5 in pituitary adenoma from acromegalic patients: good correlation with preoperative response to octreotide. Endocr Pathol. 2007;18:208–216. doi: 10.1007/s12022-007-9004-0. [DOI] [PubMed] [Google Scholar]
- 17.Kulaksiz H, Eissele R, Rossler D, Schulz S, Hollt V, Cetin Y, Arnold R. Identification of somatostatin receptor subtypes 1, 2A, 3, and 5 in neuroendocrine tumours with subtype specific antibodies. Gut. 2002;50:52–60. doi: 10.1136/gut.50.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papotti M, Bongiovanni M, Volante M, Allia E, Landolfi S, Helboe L, Schindler M, Cole SL, Bussolati G. Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002;440:461–475. doi: 10.1007/s00428-002-0609-x. [DOI] [PubMed] [Google Scholar]
- 19.Papotti M, Kumar U, Volante M, Pecchioni C, Patel YC. Immunohistochemical detection of somatostatin receptor types 1-5 in medullary carcinoma of the thyroid. Clin Endocrinol (Oxf) 2001;54:641–649. doi: 10.1046/j.1365-2265.2001.01175.x. [DOI] [PubMed] [Google Scholar]
- 20.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 21.Hofland LJ, van der Hoek J, Feelders R, van der Lely AJ, de Herder W, Lamberts SW. Preclinical and clinical experiences with novel somatostatin ligands: advantages, disadvantages and new prospects. J Endocrinol Invest. 2005;28:36–42. [PubMed] [Google Scholar]


