Abstract
Immunostaining for epidermal growth factor receptor (EGFR) is important in the contemporary therapeutic strategy of colorectal carcinomas. We tried to increase detection sensitivity, and compared the high-sensitivity EGFR immunostaining with a worldwide standard, EGFR PharmDx™ (Dako). In order to pursue high-sensitivity EGFR detection, deparaffinized sections were pressure-cooked in 1 mM EDTA solution, pH 8.0. Two mouse monoclonal antibodies against EGFR, clone EGFR2.5 and DAK-H1-WT, and six kinds of secondary detection reagents, including biotin-free catalyzed signal amplification (CSA II), Simple Stain MAX-PO, PolyVue, Novolink, EnVision™ FLEX+, and MACH3, were evaluated to compare the results with those with EGFR PharmDx™, employing a combination of 2-18-C9 as the primary monoclonal antibody and EnVision™ as the secondary reagent. Furthermore, we replaced EnVision™ in the EGFR PharmDx™ kit with CSAII. EGFR detection sensitivity was higher with DAK-H1-WT than with EGFR2.5, and among the secondary reagents, the strongest signals were observed with Novolink. All 30 colorectal carcinomas showed distinct expression of EGFR with our high-sensitivity EGFR immunostaining, while only 16 (53%) gave focal positivity with EGFR PharmDx™. When EnVision™ in EGFR PharmDx™ was replaced by CSA II, strong signals were seen in all cases, and the expression pattern was comparable with our sequence. Non-neoplastic crypt epithelial cells often showed weakly signal with the standard EGFR PharmDx™, but consistently revealed strong membrane staining in the two high-sensitivity sequences. EGFR PharmDx™ frequently gave false negativity. Importantly, EGFR was consistently and sensitively detected when the secondary polymer in the EGFR PharmDx™ kit was simply replaced by CSA II.
Keywords: Colorectal cancer, epidermal growth factor receptor, immunohistochemistry, sensitivity and specificity, monoclonal antibody
Introduction
Epidermal growth factor receptor (EGFR), a 170 kD transmembrane protein, categorized in the tyrosine kinase family, regulates cell functions, including cell division and apoptosis [1,2]. Reportedly, EGFR is expressed in approximately 60% to 80% of colorectal carcinomas [3,4], and molecular targeted therapy is given to EGFR-positive cases [5].
EGFR PharmDx™, a Food and Drug Administration (FDA)-approved diagnostic kit for localizing EGFR in formalin-fixed, paraffin-embedded sections available from Dako Co., is widely utilized for determining the eligibility of anti-EGFR molecular target therapy Cetuximab against advanced colorectal carcinoma [6-9]. Cetuximab is a chimeric type anti-human EGFR monoclonal antibody with high affinity to EGFR, and it exerts anti-tumor effects by inhibiting the intracellular signal pathway. It is known that EGFR immunostaining is affected by fixation condition [10]. False negativity may result from overfixation and/or poor detection sensitivity. Criticisms have been raised by many pathologists, doubting why focal and weak membrane reactivity should be judged as positive in case of EGFR PharmDx™ immunostaining. The judging situation is in sharp contrast to human epidermal growth factor receptor type 2 (HER2) expression in breast cancer, where weak but diffuse reactivity is judged as negative [11].
In the present study, we evaluated two anti-EGFR monoclonal antibodies and various secondary detection reagents, and established high sensitivity EGFR immunostaining for colorectal cancer. Subsequently, we compared the results with those with EGFR PharmDx™ under both the standard and modified conditions. In the modified PharmDx™ method, the secondary polymer reagent (EnVision™) was replaced by the biotin-free catalyzed signal amplification system (CSAII) available also from Dako.
Materials and methods
High-sensitivity EGFR immunostaining
Samples
We analyzed a total of five advanced colorectal adenocarcinomas surgically removed in Fujita Health University Hospital, Toyoake, Japan. The tissues were routinely fixed in 10% formalin and embedded in paraffin wax. One block sampled from the normal/tumor junction was used for analysis in each case.
Immunohistochemistry
Sections were deparaffinized with xylene, and rehydrated in graded ethanol. Endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide in methanol for 30 minutes at room temperature. Hydrated heat-assisted epitope retrieval was applied using a pressure pan cooker (Delicio 6L, T-FAL, Clithy, France) for 10 minutes. Preliminary study chose 1 mM ethylenediamine tetraacetic acid (EDTA) solution, pH 8.0, for the optimal soaking solution for heating. After pressure pan cooking, the sections were left for 30 minutes at room temperature for cooling. Anti-EGFR monoclonal antibodies, clone EGFR 2.5 (diluted at 1:100, NovoCastra, Newcastle, UK) and clone DAK-H1-WT (diluted at 1:100, Dako, Glostrup, Denmark), were incubated for 30 minutes at room temperature. After rinsing in 50 mM Tris-HCl-buffered saline (TBS), pH 7.6, the sections were reacted with six different kinds of secondary detection reagents, principally according to manufacturer’s instructions. These included 1) tyramide amplification-assisted biotin-free catalyzed signal amplification (CSA II, Dako), and five different immunoperoxidase polymer reagents, such as Histofine Simple Stain MAX-PO (SSMAX, Nichirei Bioscience, Tokyo, Japan), PolyVue (Japan Tanner, Osaka, Japan), Novolink (Novocastra), EnVision FLEX+ (Dako) and MACH3 (Biocare Medical, Concord, CA, USA). Reaction products were visualized in 50 mM Tris-HCl buffer, pH 7.6, containing 20 mg/dl diaminobenzidine tetrahydrochloride and 0.006% hydrogen peroxidase. The nuclei were lightly counterstained with Mayer’s hematoxylin.
Comparison with EGFR PharmDx™
Samples
A total of 30 advanced colorectal adenocarcinomas surgically removed in Fujita Health University Hospital in the period from 2001 to 2009 were routinely fixed in 10% formalin and embedded in paraffin wax. Again, samples at the tumor/normal junction were evaluated. The patients’ age ranged from 36 years to 78, with the average of 59.9. The male to female ratio was 14:16. No preoperative treatment was given in any case.
EGFR PharmDx™ immunohistochemistry
The immunohistochemical procedure for EGFR PharmDx™ followed the manufacturer’s instruction. Briefly, sections were deparaffinized, rehydrated, and treated with proteinase K for 5 minutes at room temperature. After peroxidase inactivation, the primary antibody, clone 2-18-C9, was incubated for 30 minutes at room temperature. After rinsing in TBS, the sections were incubated with secondary polymer reagent (EnVision™) for 30 minutes at room temperature. Diaminobenzidine coloration and nuclear counterstaining followed. In order to improve the detection sensitivity of EGFR PharmDx™, the secondary reagent was simply replaced by CSA II available from the same company, Dako. We compared the findings obtained with the standard EGFR PharmDx™ with those with the modified EGFR PharmDx™ and the above-mentioned high sensitivity EGFR immunostaining.
EGFR immunoreactivity interpretation
EGFR expression on the plasma membranes was evaluated according to the intensity of staining: 0, no reactivity, 1+, weak reactivity, 2+, moderate reactivity, 3+, strong reactivity. The judgment was independently confirmed by three authors (KS, WT and YT).
Ethical issue
The present study was approved by the institutional ethical review board for clinical and epidemiological investigations at Fujita Health University, Toyoake. The approved number is 11-091. Written informed consent was obtained from each patient.
Results
High-sensitivity EGFR immunostaining
The immunostained results for EGFR using two monoclonal antibodies and six secondary detection reagents are summarized in Table 1. Representative staining patterns are illustrated in Figure 1. EGFR immunoreactivity was consistently localized on the plasma membranes.
Table 1.
EGFR immunostaining in various combinations
| Monoclonal antibody to EGFR | Intensity Score | CSA II | SSMAX | PolyVue | Novolink | EnVision™ FLEX+ | MACH3 |
|---|---|---|---|---|---|---|---|
| EGFR 2.5 | 0 | 0 (0%) | 4 (80%) | 1 (20%) | 0 (0%) | 0 (0%) | 1 (20%) |
| 1+ | 2 (40%) | 1 (20%) | 2 (40%) | 0 (0%) | 2 (40%) | 0 (0%) | |
| 2+ | 3 (60%) | 0 (0%) | 2 (40%) | 3 (60%) | 2 (40%) | 3 (60%) | |
| 3+ | 0 (0%) | 0 (0%) | 0 (0%) | 2 (40%) | 1 (20%) | 1 (20%) | |
|
| |||||||
| DAK-H1-WT | 0 | 0 (0%) | 2 (40%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 1+ | 1 (20%) | 2 (40%) | 0 (0%) | 0 (0%) | 1 (20%) | 1 (20%) | |
| 2+ | 3 (60%) | 1 (20%) | 5 (100%) | 0 (0%) | 3 (60%) | 2 (40%) | |
| 3+ | 1 (20%) | 0 (0%) | 0 (0%) | 5 (100%) | 1 (20%) | 2 (40%) | |
0: negative, 1+: weak intensity, 2+: moderate intensity, 3+: strong intensity.
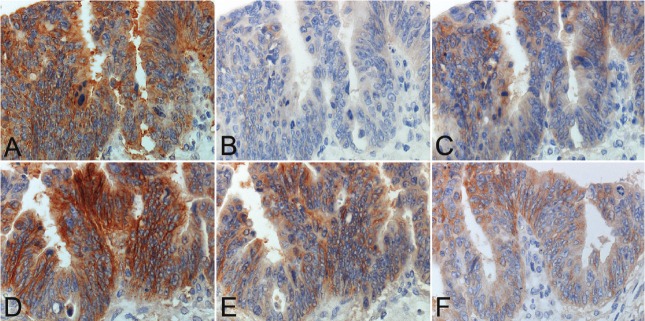
Figure 1.
Comparative study employing six different secondary reagents for detecting EGFR immunoreactivity with a mouse monoclonal antibody, clone: DAK-H1-WT in a representative case of advanced colon cancer. A. CSA II, B. SSMAX, C. PolyVue, D. Novolink, E. EnVision™ FLEX+ and F. MACH3. CSAII and Novolink give the strongest reactivity, but with cytoplasmic diffusion with CSAII. Membrane reactivity is sharply accentuated with Novolink.
The clone DAK-H1-WT consistently gave stronger immunoreactivity than the clone EGFR2.5 in any experiment employing six different kinds of the secondary reagents. The clone EGFR2.5 gave negativity in 4 of 5 (80%) lesions with SSMAX and 1 of 5 (20%) with PolyVue or MACH3. The clone DAK-H1-WT antibody showed negativity only in 2 of 5 (40%) with SSMAX. Meanwhile, the clone EGFR2.5 demonstrated 3+ immunoreactivity in 2 of 5 (40%) lesions with Novolink and 1 of 5 (20%) with EnVision™ FLEX+ or MACH3. The clone DAK-H1-WT resulted in 3+ immunoreactivity in 5 of 5 (100%) with Novolink, 2 of 5 (40%) with MACH3 and 1 of 5 (20%) with CSAII or EnVision™ FLEX+. The area of positively stained cancer tissue paralleled with the staining intensity. The strongest and consistent signals were obtained in the combination of DAK-H1-WT and Novolink. CSAII tended to show cytoplasmic diffusion of the reaction product. With our high-sensitivity method, non-neoplastic crypt epithelial cells strongly expressed EGFR on the basolateral plasma membranes.
Comparing the standard EGFR PharmDx™ with the modified EGFR PharmDx™ and the highsensitivity EGFR immunostaining
Results are summarized in Table 2 and Figure 2. Positivity with the standard EGFR PharmDx™ was observed in 16 of 30 (53%) lesions evaluated. Only in four (13%), 3+ positivity was observed. Non-neoplastic crypt epithelial cells were often weakly signaled in 23 of 30 (77%) with the standard EGFR PharmDx™. In contrast, the modified EGFR PharmDx™ employing CSA II, as well as our high-sensitivity EGFR immunostaining with DAK-H1-WT and Novolink, demonstrated positive signals in all of the 30 lesions, with 3+ reactivity seen in 28. The expression patterns were comparable with both of the latter methods in non-neoplastic crypt epithelial cells and colon cancer cells. In four cancerous lesions with 3+ reactivity with the standard EGFR PharmDx™ , both of the latter demonstrated very strong reactivity with cytoplasmic diffusion of the reaction product, and the modified EGFR PharmDx™ gave relatively high background staining.
Table 2.
Comparison among the standard and modified EGFR PharmDx™ and higy-sensitivity immunostaining for EGFR using DAK-H1-WT and Novolink
| Intensity score | Standard EGFR PharmDx™ | Modified EGFR PharmDx™ | High-sensitivity immunostaining | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Non-neoplastic crypt epithelial cells | Colorectal cancer cells | Non-neoplastic crypt epithelial cells | Colorectal cancer cells | Non-neoplastic crypt epithelial cells | Colorectal cancer cells | |
| 0 | 3 (10%) | 14 (47%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1+ | 23 (77%) | 6 (20%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2+ | 3 (10%) | 6 (20%) | 2 (7%) | 2 (7%) | 2 (7%) | 2 (7%) |
| 3+ | 1 (3%) | 4 (13%) | 28 (93%) | 28 (93%) | 28 (93%) | 28 (93%) |
0: negative, 1+: weak intensity, 2+: moderate intensity, 3+: strong intensity.
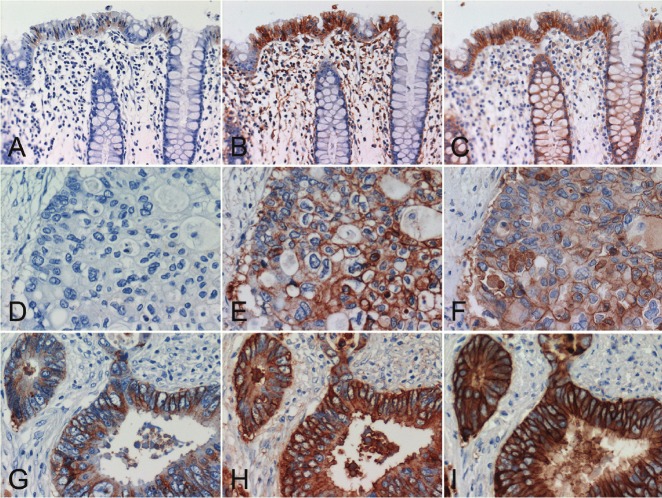
Figure 2.
Comparative study of two colon cancer cases using the standard and modified EGFR PharmDx™ and high-sensitivity EGFR immunostaining with DAK-H1-WT and Novolink. (A, D, G) standard EGFR PharmDx™, (B, E, H) modified EGFR PharmDx™ using CSAII as the secondary reagent, (C, F, I) high-sensitivity EGFR immunostaining with DAK-H1-WT and Novolink. (A, B, C) non-neoplastic crypt epithelial cells, (D, E, F, G, H, I) advanced colorectal carcinomas. The top panels (A-C) and middle panels (D-F) were sampled from the same case. In the normal region shown in the top panels (A-C), 1+ signals are obtained with the standard EGFR pharmDx™, and 3+ reactivities are appreciated many of non-neoplastic crypt epithelial cells in the modified EGFR PharmDx™ and high-sensitivity EGFR immunostaining. In the lesion indicated in the middle panels (D-F), no positivity is seen with the standard EGFR PharmDx™, while the modified EGFR PharmDx™ and high-sensitivity EGFR immunostaining result in strong reactivity in a large number of cancer cells. In the lesion demonstrated in the bottom panels (G-I), 3+ signals are obtained with the standard EGFR PharmDx™, and diffuse and very strong membrane plus cytoplasmic reactivities are seen with the modified EGFR PharmDx™ and high-sensitivity sequence. Stromal deposition of the reaction product is seen in case of the modified PharmDx™.
Discussion
Improvement of EGFR immunostaining has been reported using a variety of EGFR antibodies and secondary detection reagents [3,12-16]. In the present study, by comparing six different secondary detection reagents, we showed that the one-step polymer reagent, SSMAX, was inferior to the two-step polymer reagents, and that Novolink yielded the strongest reactivity. CSA II tended to show signal diffusion into the cytoplasm. Rocha et al. also recommended Novolink in estrogen receptor immunostaining for breast cancer [12]. However, Skaland et al. demonstrated EnVision™ FLEX+ being the most suitable for immunolocalization among five polymer reagents, including Novolink [13].
With EGFR PharmDx™, EGFR immunoreactivity was reportedly detected in 75% of stage IV colorectal adenocarcinomas [3]. In the present study, EGFR expression demonstrated by the standard EGFR PharmDx™ was observed in a bit more than half (16/30 = 53%) of the advanced colorectal cancer, and non-neoplastic crypt epithelial cells often showed weakly expression. In contrast, the modified EGFR PharmDx™, with the secondary reagent being simply replaced by CSAII, showed positive signals in all 30 cancer lesions, and the results were comparable with those obtained with the high-sensitivity EGFR immunostaining with DAK-H1-WT and Novolink. Most of them (28/30 = 93%) yielded 3+ reactivity. It should be of note that non-neoplastic crypt epithelial cells consistently showed strong membrane reactivity with both our high-sensitivity EGFR immunostaining and the modified EGFR PharmDx™. Consistent expression of EGFR on the plasma membrane of normal crypt epithelial cells has been described [14].
Burkley et al. reported that a monoclonal antibody, clone 31G7, slightly improved detection sensitivity when compared with EGFR PharmDx™ [15], while Bhargava et al. described that EGFR PharmDx™ and immunostaining with the same clone 31G7 gave the comparable results [16]. In non-small lung cancer, more than 80% of the lesions exhibited EGFR expression with both EGFR PharmDx™ and the immunostaining with 31G7 [17]. Derecskei et al. recommended the modification of EGFR PharmDx™ using microwave pretreatment [18]. It has been reported that fixatives affected the EGFR PharmDx™ reactivity of colorectal cancer [10]. Formalin overfixation may thus lead to false negativity.
We should discuss the fact that immunohistochemical evaluation of EGFR expression in colon cancer was proven not to be biologically meaningful. Frequent EGFR immunoreactivity in colon cancer was pointed out by Shia et al. [19], who also documented that EGFR gene amplification was observed only in 12% of primary colon cancer tissues. In early clinical trials, the response rates to anti-EGFR monoclonal antibody Cetuximab were 10-20% [20,21], and thereafter, it has been reported that activation of downstream effectors, especially KRAS [22] and BRAF [23] oncogene mutations, confers the resistance to anti-EGFR therapy. We thus reconfirmed herein that the immunohistochemical detection of EGFR in colon cancer was not clinically validated.
In conclusion, the sensitivity of the FDAapproved EGFR PharmDx™ was too low, often resulting in false negativity. Pathologists by a microscope often whisper a claim that the judging criteria are not reasonable since positive judgment is requested even when only weak and focal immunoreactivity is observed. Importantly, simple replacement of the secondary reagent in the kit with CSAII gave the very sensitive detection for EGFR immunoreactivity, and the results were comparable with those obtained with the high-sensitivity sequence with DAK-H1-WT, the different primary monoclonal, and Novolink as the secondary polymer reagent. The fact that with the improved methods, all 30 lesions of colorectal cancer showed clear positivity of EGFR should be re-evaluated in view of the therapeutic response in respective cancer patients. Our improved immunohistochemical sequences are readily applicable to daily diagnostic pathology services, in order to solve this point, we believe.
Acknowledgements
We thank Dako Japan for supplying the EGFR PharmDx™. Prof. Kotaro Maeda, M.D., Department of Surgery, Fujita Health University School of Medicine, Toyoake, Japan, kindly supplied us with surgical specimens of colorectal carcinoma. The skillful technical assistance by Ms. Mai Ito and Ms. Mika Maeshima, Department of Pathology, Fujita Health University School of Medicine, is cordially acknowledged.
The present work was supported by the Research Grant from Fujita Health University, 2011, where no specific grant number was given.
Competing interest statement
The authors no conflict of interest.
References
- 1.Kari C, Chan TO, Rocha de Quadros M, Rodeck U. Targeting the epidermal growth factor receptor in cancer: apoptosis takes center stage. Cancer Res. 2003;63:1–5. [PubMed] [Google Scholar]
- 2.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signaling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein NS, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: implications for a standardized scoring system. Cancer. 2001;92:1331–1346. doi: 10.1002/1097-0142(20010901)92:5<1331::aid-cncr1455>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 5.Ciardiello F, Tortora G. Epidermal growth factor receptor (EGFR) as a target in cancer therapy: understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur J Cancer. 2003;39:1348–1354. doi: 10.1016/s0959-8049(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 6.Ciardiello F, Bianco R, Damiano V, De Lorenzo S, Pepe S, De Placido S, Fan Z, Mendelsohn J, Bianco AR, Tortora G. Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res. 1999;5:909–916. [PubMed] [Google Scholar]
- 7.De Luca A, Selvam MP, Sandomenico C, Pepe S, Bianco AR, Ciardiello F, Salomon DS, Normanno N. Anti-sense oligonucleotides directed against EGF-related growth factors enhance anti-proliferative effect of conventional anti-tumor drug in human colon-cancer cells. Int J Cancer. 1997;73:277–282. doi: 10.1002/(sici)1097-0215(19971009)73:2<277::aid-ijc19>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Ciardiello F, Damiano V, Bianco R, Bianco C, Fontanini G, De Laurentiis M, De Placido S, Mendelsohn J, Bianco AR, Tortora G. Antitumor activity of combined blockade of epidermal growth factor receptor and protein kinase A. J Natl Cancer Inst. 1996;88:1770–1776. doi: 10.1093/jnci/88.23.1770. [DOI] [PubMed] [Google Scholar]
- 9.Mendelsohn J. Epidermal growth factor receptor as a target for therapy with antireceptor monoclonal antibodies. J Natl Cancer Inst Monogr. 1992:125–131. [PubMed] [Google Scholar]
- 10.Atkins D, Reiffen KA, Tegtmeier CL, Winther H, Bonato MS, Störkel S. Immunohistochemical detection of EGFR in paraffin-embedded tumor tissues: variation in staining intensity due to choice of fixative and storage time of tissue sections. J Histochem Cytochem. 2004;52:893–901. doi: 10.1369/jhc.3A6195.2004. [DOI] [PubMed] [Google Scholar]
- 11.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF American Society of Clinical Oncology College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 12.Rocha RM, Miller K, Soares F, Schenka N, Vassallo J, Gobbi H. Biotin-free systems provide stronger immunohistochemical signal in oestrogen receptor evaluation of breast cancer. J Clin Pathol. 2009;62:699–704. doi: 10.1136/jcp.2009.065326. [DOI] [PubMed] [Google Scholar]
- 13.Skaland I, Nordhus M, Gudlaugsson E, Klos J, Kjellevold KH, Janssen EA, Baak JP. Evaluation of 5 different labeled polymer immunohistochemical detection systems. Appl Immunohistochem Mol Morphol. 2010;18:90–96. doi: 10.1097/PAI.0b013e3181b0eaad. [DOI] [PubMed] [Google Scholar]
- 14.Maurer CA, Friess H, Kretschmann B, Zimmermann A, Stauffer A, Baer HU, Korc M, Büchler MW. Increased expression of erbB3 in colorectal cancer is associated with concomitant increase in the level of erbB2. Hum Pathol. 1998;29:771–777. doi: 10.1016/s0046-8177(98)90444-0. [DOI] [PubMed] [Google Scholar]
- 15.Buckley AF, Kakar S. Comparison of the Dako EGFR PharmDx kit and Zymed EGFR antibody for assessment of EGFR status in colorectal adenocarcinoma. Appl Immunohistochem Mol Morphol. 2007;15:305–309. doi: 10.1097/01.pai.0000213141.47277.bf. [DOI] [PubMed] [Google Scholar]
- 16.Bhargava R, Chen B, Klimstra DS, Saltz LB, Hedvat C, Tang LH, Gerald W, Teruya-Feldstein J, Paty PB, Qin J, Shia J. Comparison of two antibodies for immunohistochemical evaluation of epidermal growth factor receptor expression in colorectal carcinomas, adenomas, and normal mucosa. Cancer. 2006;106:1857–1862. doi: 10.1002/cncr.21782. [DOI] [PubMed] [Google Scholar]
- 17.Mathieu A, Weynand B, Verbeken E, Da Silva S, Decaestecker C, Salmon I, Demetter P. Comparison of four antibodies for immunohistochemical evaluation of epidermal growth factor receptor expression in non-small cell lung cancer. Lung Cancer. 2010;69:46–50. doi: 10.1016/j.lungcan.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Derecskei K, Moldvay J, Bogos K, Tímár J. Protocol modifications influence the result of EGF receptor immunodetection by EGFR PharmDx in paraffin-embedded cancer tissues. Pathol Oncol Res. 2006;12:243–246. doi: 10.1007/BF02893421. [DOI] [PubMed] [Google Scholar]
- 19.Shia J, Klimstra DS, Li AR, Qin J, Saltz L, Teruya-Feldstein J, Akram M, Chung KY, Yao D, Paty PB, Gerald W, Chen B. Epidermal growth factor receptor expression and gene amplification in colorectal carcinoma: an immunohistochemical and chromogenic in situ hybridization study. Modern Pathol. 2005;18:1350–1356. doi: 10.1038/modpathol.3800417. [DOI] [PubMed] [Google Scholar]
- 20.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 21.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 22.Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6:519–527. doi: 10.1038/nrclinonc.2009.111. [DOI] [PubMed] [Google Scholar]
- 23.Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S, Bardelli A. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J. Clin. Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]