Abstract
The cap is widely accepted to be the site of gravity sensing in roots because removal of the cap abolishes root curvature. Circumstantial evidence favors the columella cells as the gravisensory cells because amyloplasts (and often other cellular components) are polarized with respect to the gravity vector. However, there has been no functional confirmation of their role. To address this problem, we used laser ablation to remove defined cells in the cap of Arabidopsis primary roots and quantified the response of the roots to gravity using three parameters: time course of curvature, presentation time, and deviation from vertical growth. Ablation of the peripheral cap cells and tip cells did not alter root curvature. Ablation of the innermost columella cells caused the strongest inhibitory effect on root curvature without affecting growth rates. Many of these roots deviated significantly from vertical growth and had a presentation time 6-fold longer than the controls. Among the two inner columella stories, the central cells of story 2 contributed the most to root gravitropism. These cells also exhibited the largest amyloplast sedimentation velocities. Therefore, these results are consistent with the starch-statolith sedimentation hypothesis for gravity sensing.
Root gravitropism consists of gravity perception, signal transduction, and the growth response, which is manifested by differential growth across the elongation zone. Gravity perception in roots is generally believed to occur in the root cap (Sack, 1991), but it has been argued that perception might also be possible in other regions of the root (Poff and Martin, 1989). Previous attempts to prove that the root cap is the site of gravity perception involved surgical removal of the whole cap (Juniper et al., 1966) or small portions of the cap (Younis, 1954; Konings, 1968). These early studies implied a major role for the cap in root gravitropism, particularly the cells in the columella, the central region of the cap (Konings, 1968). In addition to its proposed role in gravity perception, the root cap has also been suggested as the site of initial development of auxin asymmetry, which is transmitted to the elongation zone during the gravity response (Konings, 1967, 1968; Moore and Evans, 1986; Hasenstein and Evans, 1988). Recently, it was shown that the apoplastic pH differences that form between the upper and lower flanks of gravistimulated roots can be abolished by surgically removing the cap (Monshausen et al., 1996). This provides evidence that a signal originating from the cap is transmitted to the elongation zone during gravistimulation.
Despite the wealth of information derived from the surgical approach, this method has one disadvantage: it does not allow the precise removal of defined cap cells (i.e. removing only columella cells versus removing only peripheral cap cells). The surgical approach was modified by Konings (1968) such that only small portions of pea root caps were removed prior to gravistimulation. Progressive elimination of the cap was based on distance from the root cap tip; therefore this approach did not allow the precise removal of single cells or even single cell layers. Although this study showed that removal of the part of the cap containing the columella caused the strongest inhibition of the gravitropic response, it was not possible to tell whether the peripheral cap cells that were removed together with the columella also contributed to the inhibition of curvature. Therefore, the relative contributions of individual cap cells to gravity perception and whether the columella cells are the only cells involved in gravity perception remain open questions.
There is other evidence pointing to the columella as the site of gravity perception. Behrens et al. (1985) reported changes in the membrane potential of Lepidium sativum columella cells when the roots were gravistimulated. No changes were observed in other cells of the cap after gravistimulation. Sievers et al. (1995) suggested that the bulk of these potential changes are actually in the columella apoplast. However, the functional significance of this finding is unclear, since similar membrane potential changes were seen in the elongation zone of gravistimulated mung bean roots (Ishikawa and Evans, 1990).
Cytological studies are also strongly suggestive of the role of the columella in gravity perception, since these are the only cells in the root that exhibit structural polarity with respect to gravity (Sack and Kiss, 1989; Sack, 1991). Columella cells contain starch-filled plastids, and the settling of these amyloplasts has been proposed to constitute the initial act of gravity perception in plants. This is known as the “starch-statolith hypothesis” (Sack, 1991), and numerous reports have been published supporting or refuting it (Caspar and Pickard, 1989; Salisbury, 1993; for review, see Sack, 1991). However, a recent study demonstrating that displacement of amyloplasts by high-gradient magnetic fields can cause gravitropic-like curvature in roots provides additional support for the starch-statolith hypothesis (Kuznetsov and Hasenstein, 1996).
Recently, laser ablation in Arabidopsis roots was used to study positional signaling among individual cells (van den Berg et al., 1995; Scheres et al., 1996). This system allows the selective elimination of single cells and therefore can be used to map the relative contribution of cap cells to the graviresponse with higher resolution than previous methods (Konings, 1968). The small number of cell layers and the simple pattern of cellular organization in the Arabidopsis root cap (Sack and Kiss, 1989; Dolan et al., 1993; Baum and Rost, 1996) make complete ablation mapping of cell function a feasible study. To test more directly the involvement of different cap cell types in root gravitropism, we used laser ablation to remove groups of cells from various positions in the cap, and, using a variety of parameters, we determined how this affected the ability of the root to respond to gravity.
Several researchers have quantified amyloplast sedimentation in presumptive statocytes of higher plants using both histological techniques that involved fixation of samples at intervals following gravistimulation (Sack et al., 1984, 1985; Sievers et al., 1989) and observation of amyloplast sedimentation rates in excised living tissues (Clifford and Barclay, 1980; Heathcote, 1981; Sack et al., 1986). Differences in statolith sedimentation rates between cells in different parts of the columella were reported in fixed L. sativum roots (Sievers et al., 1989). However, there have been no systematic attempts to investigate variations in the amyloplast sedimentation rate throughout the cap and explore correlations with the functional significance of each cell in gravitropism. Therefore, we have also constructed a map of amyloplast sedimentation velocities in the living root cap cells of intact Arabidopsis seedlings. We report that comparison of amyloplast-sedimentation patterns with the effects of different laser-ablation patterns shows that columella cells with the highest amyloplast-sedimentation velocities provide the greatest contribution to the root graviresponse. Our results provide both functional and correlative evidence that the inner, central columella cells provide the greatest contribution to gravity sensing in Arabidopsis roots.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of Arabidopsis thaliana ecotype Columbia were surface sterilized by immersing them successively in 95% ethanol and 10% bleach for 3 min each and then rinsing in sterile, distilled water. The seeds were then planted on a thin film of Phytagel (Sigma), and layered onto 22- × 22-mm cover glasses (no. 0, Thomas Scientific). The composition of the medium was essentially as that described by Legué et al. (1997). The seedlings and cover glasses were placed in 90-mm plastic Petri dishes, wrapped with Parafilm, and maintained in the vertical position at a constant photon flux density of 36 μmol m−2 s−1 at 22 to 24°C. Plants were used after 3 d, when the root was approximately 1 cm long.
Laser Ablation of Cap Cells and Confocal Microscopy
Seedlings with straight roots were selected, placed on the stage of an inverted microscope (Diaphot 300, Nikon), and viewed using a ×40, 1.2 numerical aperture, oil-immersion objective (Nikon). The microscope was equipped with a nitrogen laser (VSL-337ND, Laser Science, Newton, MA) capable of producing a nanosecond pulse of UV light at 337 nm with peak power of 85 kW. The root cap was imaged with a video camera (C2400 CRCD, Hamamatsu, Tokyo, Japan) attached to the camera port of the inverted microscope, and the output was directed to a video monitor (PVM-135, Sony, Tokyo, Japan). Alignment and other features of the laser system were described by Henriksen and Assmann (1997).
To visualize the distribution of ablated cells, roots were stained with 10 μm propidium iodide and imaged with a confocal microscope (LSM410, Zeiss). Propidium iodide is excluded from living cells but stains dead cells. Fluorescence from propidium iodide was excited with the 488-nm line of the argon ion laser using a 488-nm dichroic mirror, and emitted light was detected at 590 nm and selected using interference filters (Zeiss). Optical sections parallel to the root axis (Z sections) were obtained with a 1-μm interval between sections. Three-dimensional reconstruction was performed using the maximum projection likelihood software (Zeiss) by projecting a series of 30 to 40 optical sections after rotating about the Y axis with an angular increment of 8° between sections. Identification of the ablated cells with propidium iodide was done after the gravitropism experiments because prestaining the roots with propidium iodide partially inhibits the graviresponse (data not shown). Images from the confocal microscope were assembled using Photoshop 3.0 (Adobe Systems, Mountain View, CA) and printed on a dye-sublimation printer (Phaser II SDX, Tektronix, Wilsonville, OR).
Measurements of Curvature Kinetics, Presentation Time, and Deviation Angle
Since the laser system was attached to a standard inverted microscope, the vertically grown roots had to be ablated while lying on the horizontal stage. On the average it took about 2 min of placing the root horizontally to successfully generate the ablation patterns used for the gravitropism studies. To correct for the possible effects that this brief horizontal positioning of the roots could have on the results, the controls used for all of the experiments were roots with intact caps that were laid horizontally for the same time needed to ablate the cap cells. After the cell ablations seedlings (ablated and controls) were returned to their original vertical orientation and allowed to recover for 2 h.
To assess the effect of ablation on root gravitropism, we used three criteria: time course of curvature, presentation time, and deviation from vertical growth. For the time-course experiments, Petri dishes containing the roots were rotated 90° and root curvature was monitored every 20 min for 12 h. Roots that deviated more than 10° from the vertical prior to the 90° reorientation were not used. For growth-rate measurements, roots were maintained vertically and root length was measured every 20 min during a 12-h period. To determine the extent of deviation from vertical growth, roots (ablated and controls) were grown vertically for 5 h after the 2-h recovery period, and the angle of the root tip with respect to the gravity vector was measured (Kiss et al., 1989, 1996). To determine the presentation time, roots were given a brief (2–30 min) horizontal stimulation and then rotated on a 1-rpm clinostat. Curvature of the roots was measured after 3 h and plotted against the logarithm of the stimulation time. Presentation times were calculated as described by Kiss et al. (1996). As expected, when stimulation times were shorter than the calculated presentation time (e.g. 1 min for controls or 5 min for inner story ablations), no curvature could be detected. The minimum curvature that we could reliably measure was 3 to 4°.
All curvature and growth measurements were made from digitized images collected using a video camera (model C2400, Hamamatsu) and a 52-mm macrolens (Nikkor, Kogaku, Japan). Images of the root were captured using a frame grabber (LG-3, Scion, Frederick, MD) and a computer (Quadra 800, Apple Computer, Cupertino, CA) running image-acquisition software (IPLabs Spectrum, Signal Analytics, Vienna, VA). The minimum point-to-point resolution was 50 μm.
Measurement of Amyloplast Sedimentation
For measurement of amyloplast sedimentation, roots were mounted vertically on the stage of an epifluorescence microscope (Optiphot, Nikon) that had been mounted on its back so that the stage was vertical (Legué et al., 1997). This allowed us to keep the roots in their original vertical orientation. Roots were gravistimulated by rotating the stage 135° from the vertical while being continuously imaged. Cells were viewed using an ×40, 1.2 numerical aperture, fluor, oil-immersion objective (Nikon). Images of columella cells were captured every 15 s for 10 min, and the sedimentation of individual amyloplasts was digitally recorded and quantified using the camera imaging system described above (Hamamatsu). The minimum point-to-point resolution was about 1 μm. Separate plastids were easily distinguished and followed in digitized video images of sedimentation. Individual full-screen frames were then selected for measurement of amyloplast displacement. Amyloplast-sedimentation velocities were calculated for all stories and files of columella cells using the image-analysis software described above.
RESULTS
Cap Cell Ablation
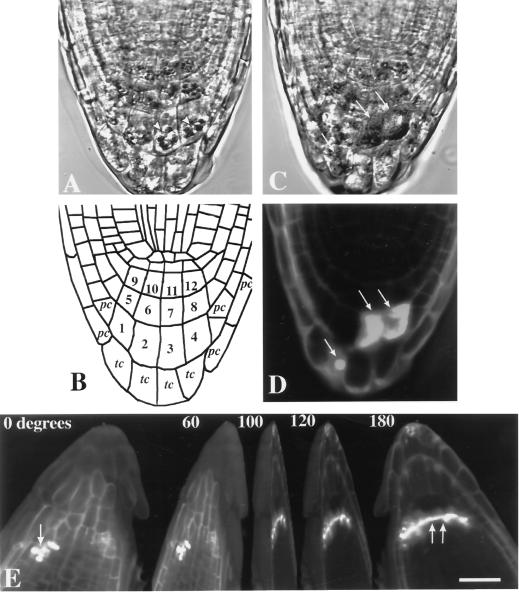
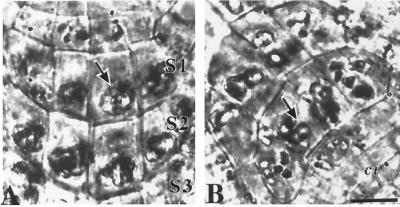
To provide a detailed analysis of the contribution of cap cells to Arabidopsis root gravitropism, we used laser ablation to remove groups of cells in the root cap and observed the root's response to gravistimulation. Transmission-detector images obtained from the confocal microscope of the root cap of a 3-d-old Arabidopsis seedling showed three horizontal stories and four vertical files of columella cells (Fig. 1A, cells 1–12 in Fig. 1B). A single story of tip cells (Fig. 1B, marked “tc”) formed the extreme apex of the cap. Although this was the typical arrangement of columella cells in the longitudinal view, some root caps were asymmetric or occasionally had only three vertical files and were not used for the experiments.
Figure 1.
A, Transmission detector image of the root cap of a 3-d-old Arabidopsis seedling. Amyloplasts (arrowheads) are clearly visible in the columella cells. B, Line diagram of the root cap depicted in A. In two dimensions, the columella cells (numbered) are typically organized into three horizontal stories and four vertical files. As a guide to the ablation experiments described in the succeeding figures, horizontal tiers are classified into three stories (S1–S3): S1, cells 9–12; S2, cells 5–8; and S3, cells 1–4. Vertical files are classified as flank columella cells, cells 1, 5, and 9 and 4, 8, and 12; and central columella cells, cells 2, 6, and 10 and 3, 7, and 11. tc, Tip cells; pc, peripheral cells. C, Ablation of S3 columella cells resulted in morphological distortion of the cells (arrows), whereas adjacent cells remained intact. D, Fluorescence image of the same root stained with propidium iodide, which enters the ablated cells (arrows) and is excluded by live cells. E, Rotational sequence of a three-dimensional confocal data set of an Arabidopsis root cap with the S1 columella cells and S3 peripheral cap cells ablated. Ablation was successful, as shown by the entry of propidium iodide (arrows). Note that the S1 ablations did not cause any damage to peripheral cap cells or adjacent stories and that peripheral cap cell ablations did not damage the inner cap cells. Bar = 25 μm.
For clarity, we have adopted some of the terminology of Sack and Kiss (1989), who classified columella cells into three horizontal stories: story 1 (S1), story 2 (S2), and story 3 (S3). In the current study, the columella cells closest to the quiescent center are referred to as S1, the second story as S2, and the third story, farthest from the meristem, as S3. The cells at the tip of the cap are referred to by Sack and Kiss (1989) as peripheral cells. Herein they are called tip cells; in this study, “peripheral cell” denotes the cap cells flanking the columella cells laterally (Fig. 1B, marked “pc”). We used the terms “flank” and “central” columella to distinguish between the outer and inner files of columella cells (Fig. 1B). Amyloplasts were clearly visible in S1 to S3 cells, whereas tip cells usually contained either shrunken plastids or were highly vacuolated with no visible plastids (Fig. 1A).
Four to five pulses of the nitrogen laser were sufficient to ablate the peripheral cells, tip cells, and S3 cells, whereas 5 to 10 pulses were necessary for successful ablation of S1 and S2 cells. Ablated cells showed obvious morphological distortions. The cytoplasm of ablated columella cells was completely disrupted, whereas no obvious effects on the cytoplasmic structure and distribution of amyloplasts of neighboring columella cells were observed (Fig. 1C). Successful ablation was further confirmed by a loss of staining with the viability stain fluorescein diacetate (data not shown) and by the entry of propidium iodide into the targeted cells. Propidium iodide is excluded by living cells (Fig. 1D; van den Berg et al., 1995). The precise and accurate ability of the laser system to remove selected cells was demonstrated by ablation of S1 columella cells without damage to the surrounding peripheral cap cells (Fig. 1E).
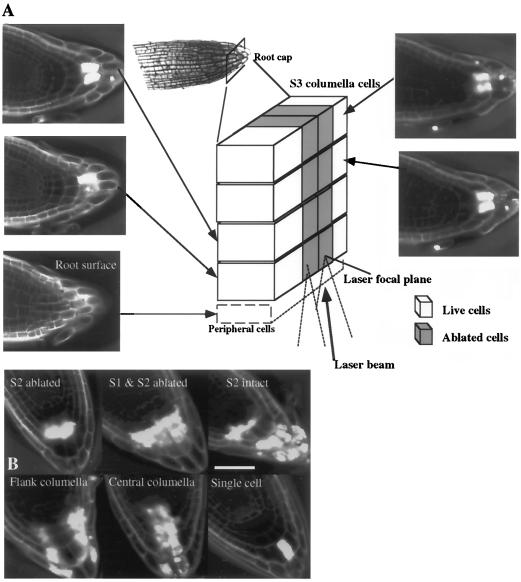
Although it was easy to resolve and target cells for ablation along the X and Y axes, we were not as confident in ablating individual columella cells along the Z axis. The potential problems are 2-fold. First, the defocused laser beam must still pass through all of the cells along the Z axis. However, the power density is sufficient to ablate cells only at the laser focal plane. It is important to note that we did not observe any changes in morphology in the cells that the defocused beam had passed through (Figs. 1E and 2A), but we were still concerned that this defocused beam might have effects on the nonablated cells. The second and more significant problem was the optical resolution along the Z axis. We could not consistently visualize a columella cell at a particular depth to ensure that we were aligned in the Z axis enough to avoid damage to columella cells directly above and below it. This problem was particularly true for S1 and S2 cells. Therefore, we ablated all columella cells throughout the depth of the cap (Z axis), as illustrated schematically in Figure 2A. Therefore, a single columella cell ablation in two dimensions is actually a total of four cells ablated one on top of another. A single-story ablation is actually a total of 16 ablated cells, which includes all columella cells in that transverse plane (Fig. 2B). Using this scheme we were able to generate patterns of story and file columella cell ablations without damage to cells in an adjacent file (Y axis) or story (X axis). Examples of some of the ablation patterns generated and used for the gravitropism studies described below are shown in Figure 2B.
Figure 2.
A, Schematic diagram of S3 columella cells illustrating the method used for columella cell ablations. After the target cells were ablated, roots were stained with propidium iodide and imaged with a confocal microscope, and optical sections (Z sections) were taken at the plane indicated by the arrows. The first section was taken at the plane of the peripheral cap cells (root surface). Exclusion of propidium iodide from the peripheral cap cells shows that these cells were not damaged, despite being in the path of the laser, as the power density of the laser was only strong enough to ablate cells at its focal plane. The laser was focused sequentially on all four Z axis planes of columella cells and the defined cells at the laser focal plane were ablated. The diagram illustrates two cells ablated in S3 at each focal plane for a total of eight cells (gray boxes). B, Examples of ablation patterns used for gravitropism studies. Bar = 50 μm.
Vertical Growth Rate
To ensure that ablation was not disrupting growth due to a wounding effect, the growth rate of vertically grown plants was measured during a 12-h period after the cap cells were ablated (Table I). Growth rates of control roots and roots with ablated cap cells were not significantly different, as determined by an ANOVA test (P = 0.964). In addition, amyloplast sedimentation velocities of columella cells adjacent to ablated cells were not different from the sedimentation velocities prior to ablation (data not shown).
Table I.
Growth rates of vertically grown Arabidopsis roots
| Cells Ablateda | Growth Rate | Cells Ablated | Growth Rate |
|---|---|---|---|
| μm h−1 | μm h−1 | ||
| Controls | 218 ± 18 (10) | S3 | 200 ± 11 (9) |
| Tip cells | 201 ± 9 (9) | S2 | 203 ± 18 (6) |
| Tip cells + S3 | 207 ± 14 (8) | S1 | 203 ± 7 (8) |
| Tip cells, S3 + S2 | 215 ± 22 (7) | Central columella | 202 ± 8 (10) |
| Tip cells, S3, S2 + S1 | 214 ± 13 (6) | Flank columella | 218 ± 14 (7) |
| S1 + S2 | 219 ± 9 (9) | Peripheral cap cells | 216 ± 7 (10) |
The growth rates of ablated and control roots were measured during a 12-h period. Growth rates were not significantly different as determined by ANOVA (P = 0.964). Values are means ± se. n, Number of roots measured (shown in parentheses). S1 to S3, Columella stories 1 to 3; controls, no cells ablated.
Time Course of Root Curvature
Because ablation did not affect the capacity for root growth, we next tested the effect of ablating cells of the root cap on the graviresponse. A primary parameter used to assess the effect of ablations on root gravitropism was the time course of root curvature. The rate of curvature during the initial 3 h of gravistimulation and the angle of curvature after 12 h are summarized in Table II.
Table II.
Rate and final angle of curvature of Arabidopsis roots
| Cells Ablateda | Rate of Curvatureb | Final Anglec |
|---|---|---|
| degrees h−1 | degrees | |
| Control | 13.49 ± 1.12 | 76.61 ± 2.59 |
| Tip cells | 13.97 ± 1.68 | 76.13 ± 3.62 |
| Tip/S3 | 7.95 ± 1.07 | 63.14 ± 3.07 |
| Tip/S3/S2 (S1 intact) | 3.89 ± 0.54 | 31.10 ± 6.16 |
| Tip/S3/S2/S1 | 0 | 8.13 ± 2.56 |
| S2/S1 | 2.20 ± 0.59 | 18.99 ± 6.43 |
| S3 | 9.58 ± 1.25 | 61.53 ± 2.43 |
| S2 | 8.94 ± 1.02 | 53.07 ± 2.89 |
| S1 | 7.32 ± 1.42 | 52.82 ± 4.85 |
| Tip/S2/S1 (S3 intact) | 2.44 ± 0.60 | 19.06 ± 5.92 |
| Tip/S3/S1 (S2 intact) | 5.31 ± 0.73 | 43.92 ± 3.23 |
| S1/S2/S3 (tip cells intact) | 0.40 ± 0.58 | 6.48 ± 2.29 |
| Peripheral cap cells | 14.01 ± 1.07 | 70.17 ± 2.18 |
| Central columella | 4.58 ± 0.60 | 42.66 ± 4.67 |
| Flank columella | 5.60 ± 0.83 | 73.5 ± 1.12 |
The indicated cells were ablated, and after a 2-h recovery period, the roots were rotated 90° from vertical. The resulting curvature during a 12-h period was recorded and analyzed. Values are means ± se, n ≥ 10 roots.
Rate represents average rate of curvature during the first 3 h of stimulation.
Final angle is the angle reached after 12 h of gravistimulation.
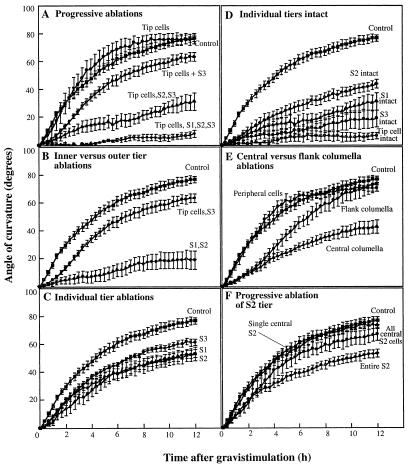
Initially, columella cells were progressively ablated story by story. Curvature kinetics of roots in which the tip cells had been ablated were similar to controls. Progressive reduction in root curvature was observed as more and more of the columella stories were ablated. Removal of the tip cells and all columella cells led to an almost complete inhibition of the graviresponse (Fig. 3A).
Figure 3.
Time course of curvature of roots with intact caps and roots with cap cells ablated. Downward curvature of the roots was measured for 12 h following a 90° horizontal reorientation. Note that a single central S2 cell shown in F is actually a total of four cells, two flank and two central columella cells. Each data point represents a mean ± se of at least 10 roots.
The experiment described above is similar to progressive microsurgical removal of portions of pea cap cells (Konings, 1968). However, this pattern of ablation did not allow us to clearly assign the effects of reduced curvature to individual stories or cells located in the interior region of the cap. The succeeding experiments capitalized on the ability of the laser ablation system to generate cell-removal patterns in the inner region of the cap, which is not possible with the microsurgical techniques (Fig. 1E). Roots with S1 and S2 ablated showed the strongest, although not complete, inhibition of curvature (Fig. 3B). The rate of curvature of these roots during the first 3 h of gravistimulation was only 2.20° h−1 compared with 13.49° h−1 for control roots and 7.95° h−1 for roots with tip cells and S3 cells ablated (Table II).
We next ablated individual stories. Whereas ablating tip cells had no effect on root curvature (Fig. 3A), ablating S1, S2, or S3 cells individually inhibited curvature (Fig. 3C). Roots with S3 cells ablated had the highest rate and final angle of curvature. Compared with roots with S3 cells ablated, curvature in roots with the S1 or S2 tiers ablated individually was slightly reduced (Fig. 3C).
To further resolve the contribution of individual stories to the graviresponse, all stories except one were ablated. The greatest curvature response was observed in roots with S2 cells intact, although this was significantly lower than in the roots with the entire cap intact (Fig. 3D). These roots curved at a rate of 5.31° h−1 during the first 3 h after gravistimulation and reached an angle of 43.92° after 12 h (Table II).
The laser system also allowed us to analyze the previously uncharacterized contributions of the flank and central files of columella cells. Ablating the central columella or the flank columella (Fig. 2B) caused the roots to curve at a slower rate. Curvature of roots with the central columella ablated was more strongly inhibited than roots with the flank columella ablated (Fig. 3E). We also ablated the central cells of S1 and S2 only and measured root curvature. Roots with the central cells of only S1 and S2 ablated showed the same curvature kinetics as roots with all central cells ablated (Figs. 2B and 3E). It was also possible to ablate the peripheral cap cells surrounding the columella without damaging the columella cells themselves. Roots with most of the peripheral cells ablated had the same rate and final angle of curvature as controls (Table II; Fig. 3E).
The ablation patterns used for the curvature experiments presented above involved removal of a fairly large number of cells. We also wanted to determine the minimum number of cells that could be ablated to cause an effect on root gravitropism. However, the optical resolution along the Z axis (discussed earlier) did not allow us to consistently ablate a single columella cell in the interior of S1 and S2 without damaging the columella cells directly above and below it. In roots in which we managed to successfully ablate a single S2 columella cell, there was no effect on curvature (data not shown). Ablating a single line of columella cells in S2 along the Z axis (i.e. a total of four cells: two flank and two central columella cells) also did not affect root curvature. A slight reduction in root curvature was observed when all central cells of S2 were ablated (Fig. 3F). There was no measurable reduction in root curvature when all flanking columella cells in S2 throughout the depth of the cap were ablated (data not shown).
Presentation Time and Deviation from Vertical Growth
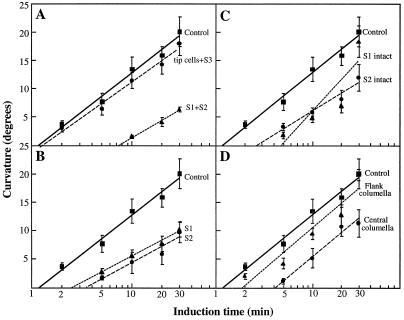
Roots were given a brief horizontal stimulation and allowed to develop curvature on a slowly rotating clinostat. Curvature was plotted against the logarithm of the stimulation time (Fig. 4), and the presentation times were calculated from the regression equation for y = 0° (Johnsonn and Pickard, 1979). Presentation times were 1.16 min for control roots, 1.28 min for roots with S3 and tip cells ablated, 7.13 min for roots with S1 and S2 ablated (Fig. 4A), 2.55 min for roots with S1 ablated, 3.53 min for roots with S2 ablated (Fig. 4B), 2.62 min for roots with S2 intact, and 4.85 min for roots with S1 intact (Fig. 4C). The presentation times of roots after ablating the flank and central columella cells were 1.91 and 4.07 min, respectively (Fig. 4D).
Figure 4.
Induction time of control Arabidopsis roots with intact caps and roots with columella cells ablated. After cell ablations, seedlings were kept vertical for 2 h, stimulated at 90° for a single 2- to 30-min period, and then rotated axially on a 1-rpm clinostat. After 3 h on the clinostat, the curvature was measured and plotted against the logarithm of the stimulation time. Presentation times were calculated from the regression equations for y = 0°. A, Inner versus outer story ablations. Presentation time was 1.16 min for control roots, 1.28 min for S3/tip cell ablations, and 7.13 min for S1/S2 cell ablations. B, Individual story ablations. Presentation time was 2.55 min for S1 cell ablations and 3.53 min for S2 cell ablations. C, Individual stories intact. The presentation time was 2.62 min for roots with only S2 cells intact and 4.85 min for roots with S1 cells intact. D, Central columella versus flank columella cell ablations. Presentation time was 4.07 min for roots with the central columella cells ablated and 1.91 min for roots with the flank columella cells ablated. Correlation coefficients for the regression lines are 0.99 (controls, tip and S3 cells, S1 and S2 cells, and S1 cells ablated), 0.98 (central), 0.97 (S2 cells intact), 0.96 (S2 cells ablated), 0.95 (flank), and 0.87 (S1 cells intact). Each data point represents a mean ± se of 15 to 30 roots.
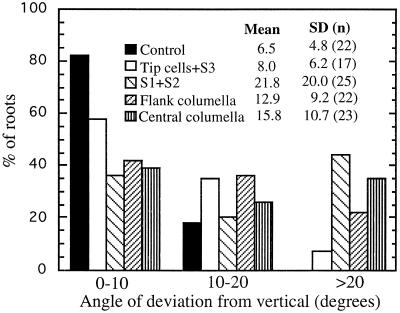
The extent of root deviation from vertical growth was also used as a measure of gravitropic sensitivity (Fig. 5; Kiss et al., 1989, 1996). When oriented vertically, most of the roots grew in a downward direction. Most of the roots with the cap intact (controls) grew vertically. The mean angle of divergence from the vertical was only 6.5°, and the sd of ± 4.8 was the lowest. Roots with tip cells and S3 ablated had a mean angle of divergence of 8.0° ± 6.18. A large percentage of roots with the inner (S1 and S2) columella cells ablated deviated significantly from vertical growth. The mean angle of divergence was 21.8 and the sd ±20.0° was about 4 times that of controls and roots with tip cells and S3 ablated. Ablating the flank or central columella cells also caused roots to deviate significantly from vertical growth. The mean angle of divergence was 12.9° for roots with the flank columella ablated and 15.8° for roots with the central columella cells ablated (Fig. 5).
Figure 5.
Extent of deviation from vertical growth of control roots, roots with S3 and tip cells ablated, roots with S1 and S2 cells ablated, roots with flank columella cells ablated, and roots with central columella cells ablated. Roots were maintained vertically and the curvature was measured after 5 h. Ablating the inner columella stories (S1 and S2 cells) caused the greatest deviation from vertical growth, as shown by the largest mean and sd.
Amyloplast Sedimentation
Using the vertical stage microscope described in an earlier report (Legué et al., 1997), we were able to collect digitized images of columella cells two cell layers under the peripheral cap cells. This was sufficient to resolve amyloplast sedimentation in living cells of the central columella as the root was rotated. The course of an individual amyloplast was followed through digitized movies of sedimentation before frames for actual measurements were selected. Individual frames from a video sequence of columella cells from a vertically oriented root and 5 min after a 135° reorientation are shown in Figure 6. Significant differences in amyloplast sedimentation velocity after reorientation were observed among columella cells of different files and stories (Table III). The central S2 columella cells displayed the highest sedimentation rate, and the central S1 cells displayed the next highest. The amyloplasts of the columella cells of the flanks of S2 and S1 usually sedimented as well but at a slower rate than the central cells (Table III).
Figure 6.
Digitized bright-field images of S1 and S2 columella stories collected from the vertical stage microscope and used for measurement of amyloplast (arrows) sedimentation velocities in a vertical root (A) and in a root 5 min after a 135° reorientation (B). Although amyloplasts in the columella cells of a vertical root appear clumped, individual amyloplasts could be resolved from actual video sequences of sedimentation. After the root was rotated horizontally, the course of an individual amyloplast was tracked through digitized video images of sedimentation before individual frames were selected to measure displacement. ci, Columella initials. Bar = 10 μm.
Table III.
Amyloplast sedimentation velocities of Arabidopsis S1 to S3 columella tiers
| Cell Typea | Velocityb | Sedimentationc |
|---|---|---|
| μm min−1 | % | |
| S1, central columella | 0.909 ± 0.059 (55) | 100 |
| S1, flank columella | 0.4960 ± 0.060 (54)2 | 66.67 |
| S2, central columella | 1.200 ± 0.059 (56) | 100 |
| S2, flank columella | 0.640 ± 0.058 (57)2 | 78.95 |
| S3, central columella | 0.085 ± 0.064 (47)1 | 14.89 |
| S3, flank columella | 0.072 ± 0.059 (56)1 | 8.93 |
Amyloplast sedimentation was measured over 5 min after 135° rotation of live roots on a vertical stage microscope. Values are means ± se. Values in parentheses are n values, the number of cells observed from 25 or more roots.
See Figure 1B for definition of cell type analyzed.
Sedimentation velocities (averages of all observed cells) are significantly different as determined by ANOVA/Tukey's Honestly Significant Difference test (P < 0.02) except as shown (1 and 2).
Percentages indicate proportions of cells with sedimentable amyloplasts.
The differences in sedimentation velocity between central and flank cells and between the different stories were highly significant (P < 0.02), as shown by ANOVA and Tukey's Honestly Significant Difference test. The behavior of the S3 amyloplasts was strikingly different from those of the inner stories (S1 and S2). Amyloplasts of the S3 cells occasionally sedimented. This was most common in caps in which the old tip cells had been recently sloughed off and the new S3 cells had more recently been S2. When sedimentation occurred, it was limited to a very short total distance (<5 μm), which was much less than the length of the cell. Saltation was also greatly reduced in these cells. In contrast, sedimentation of amyloplasts in S1 and S2 occurred over the entire length of the cell (>15 μm) and was correlated with the vigorous saltation of the plastids in these cells (Fig. 6).
DISCUSSION
Although it is generally accepted that the cap is the site of gravity perception in roots, the actual cells making up the gravity sensor have not been functionally defined. The columella cells are obvious candidates because of their structural polarity with respect to the gravity vector (Sack, 1991). In 3-d-old Arabidopsis seedlings, columella cells in the root cap are arranged in three horizontal stories and four vertical files. To dissect the relative contribution of these cellular arrays to root gravitropism, we removed selected cells by precise laser ablation and quantified the gravitropic response of the treated root.
There were earlier reports of decapping experiments to test directly the role of the root cap and columella cells in root gravitropism (for review, see Jackson and Barlow, 1981; Konings, 1995). Successive removal of cap cells with the laser system gave results confirming an earlier study with pea roots. Thus, progressive reduction in root curvature was observed as more and more of the pea root cap was cut away (Konings, 1968). Unlike surgical removal, the laser system is not limited to simple successive removal of outer cap cell layers but allows much more complex and precise patterns of cell elimination. With this technique we were able to target particular cells in the interior of the cap without damaging the surrounding tissue. More importantly, we were able to determine the contributions of small, precisely defined groups of columella cells to the gravity response in roots. This provides a new window on the functional heterogeneity of the columella cells in the root cap.
The data generated from the patterns of cap cell ablations provide direct evidence that the inner and central columella cells are the primary gravity-sensing cells in Arabidopsis roots. Roots in which the S1 and S2 columella cells had been ablated showed the greatest reduction in curvature after gravistimulation, had the longest presentation times, and deviated the most from vertical growth. The reduction in curvature elicited by S1 and S2 ablation alone did not resolve whether these cells were involved in gravity perception, because the curvature response comprises gravity perception, transmission of information, and differential growth (Moore and Evans, 1986). To address the role of S1 and S2 in perception, we measured the presentation times of roots after ablation. The presentation time or threshold time is defined as the minimum exposure time to a 1g field to induce a response (Sack, 1991) and has often been used as measure of gravitropic sensitivity in roots (Kiss et al., 1989, 1996; Legué et al., 1994).
Although ablating S3 caused a reduction in the rate and final angle of curvature, the presentation time and the percentage of roots that deviated from vertical growth were similar to that of control roots. Therefore, the similar presentation times of control and S3-ablated roots suggest that reduced root curvature in the S3-ablated roots is mainly due to the impaired transmission of the gravity signal and not to a major reduction in sensitivity to the gravity stimulus. The data also indicate that S3 ablations primarily affected the transduction phase of gravitropism rather than the capacity for differential growth, since growth rates of the S3-ablated roots and control roots were similar (Table I); however, this does not rule out the involvement of S3 cells in gravity perception. However, these results show that the contribution of S3 to gravity perception is much less than S1 and S2, since roots with only the S3 intact were still capable of curving, although at significantly reduced rates (Fig. 3D).
Having established that the inner columella stories (S1 and S2) contributed the most to gravity perception, we next attempted to resolve which among these two stories had a greater contribution. Surprisingly, the reduction in kinetics of curvature was similar between roots with either S1 or S2 ablated (Fig. 3C). These data alone suggest that each story contributes equally to gravitropism. However, analysis of presentation time resolved differences in the contribution of these two inner stories to the graviresponse. The presentation time of roots with S2 ablated was longer (3.53 min) compared with roots with S1 ablated (2.55 min). In addition, roots in which all stories except S2 were ablated caused the least reduction in curvature (Fig. 3D), and the presentation time of these roots was shorter compared with roots with only S1 intact (Fig. 4C). Therefore, the S2 columella cells appear to contribute the most to gravity sensing in Arabidopsis roots.
By cutting off the apical 0.2 mm of pea root caps, Konings (1968) was able to show that tip cells in pea roots are not involved in root gravitropism. Our ablation of all tip cells confirms this observation. However, it remained unclear from Konings' experiments whether the peripheral cells surrounding the columella laterally contribute to the graviresponse. Our data provide direct evidence that these cells have no role in root gravitropism, since ablating these cells did not affect root curvature (Fig. 3E).
A potential drawback of laser ablation is that it could generate wound-induced responses in the root (Meyer and Weisenseel, 1997), and this wounding effect might have been superimposed on our analysis of the effects on gravistimulation. To identify such nonspecific effects, the elongation of ablated and control vertically growing roots was measured. The growth rates of vertical roots with various patterns of cap cells ablated and of control roots were both about 200 μm h−1, which were not significantly different (Table I). Therefore, as found in the early decapping experiments (Juniper et al., 1966; Konings, 1968), if there was a wound response due to the cell ablations, it was not sufficient to alter the growth rate. Additionally, ablation of the peripheral cells (which involved destruction of a substantial number of cells and hence was most likely to induce a wound response) did not alter the kinetics of the root curvature in response to gravity.
There are reports that removal of the cap from maize roots results in growth stimulation (Pilet, 1972; Wilkins and Wain, 1974). One probable reason that we did not see growth stimulation in roots with ablated cap cells is because all growth and curvature measurements were made only after a 2-h recovery period. The stimulation of root growth in decapped maize roots is only transient and occurred only during the initial 3 h after decapping (Pilet, 1972; Wilkins and Wain, 1974). Therefore, it is possible that we may have missed transient growth stimulation that could have occurred during the 2-h recovery period. However, Konings (1968) also did not see any promotion of root growth in pea roots, despite taking hourly growth measurements immediately after decapping. Therefore, the promotive effect of decapping on root growth may not be true for all species.
We were still concerned that other cellular functions that could influence the gravity-perception mechanisms might be more subtly altered by the ablations, even though growth rate was not affected. To check this, we measured the sedimentation velocities of amyloplasts of columella cells surrounding the ablated cells, and these were not different from rates in unablated caps. Vigorous saltations also continued in cells adjacent to the ablated cells, which is indicative of the viability of nonablated cells. These results suggest that the cell ablations caused no nonspecific effects at either the cellular or whole-organ level that could have interfered with the interpretation of the results.
The spatial distribution of the in vivo amyloplast sedimentation correlated well with the functional roles of the different cap cell types as determined by the laser-ablation studies. In general, cells in the tip and the peripheral cap cells of Arabidopsis contained no amyloplasts. Very occasionally (<5%), these cells contained amyloplasts, but they were small and did not sediment. When these tip cells and peripheral cap cells were ablated, root curvature was not affected, and this is consistent with the starch-statolith hypothesis, which predicts that cells with nonsedimenting amyloplasts are of little or no significance to gravity perception in wild-type organs. The highest sedimentation velocities were observed in the cells of S2 and, as mentioned earlier, ablating S2 had the greatest effect on reducing gravitropism. This correlation also held for flank and central columella files. Amyloplast sedimentation velocities were significantly greater in central than in flank columella cells, and ablation of central columella files had a more significant effect on curvature and presentation time than flanking columella cell ablation (Figs. 3E and 4D).
Since the S3 cells alone were still able to generate a slight root curvature (Fig. 3D), but S3 cells had low amyloplast-sedimentation activity (Table III), it is probable that substantial amyloplast sedimentation is not absolutely required for gravity detection, as has been previously suggested (Jackson and Barlow, 1981; Sack, 1991). The plastids in the S3 columella cells may be bound in a net of actin filaments that play a role in signal transduction (White and Sack, 1990; Sievers et al., 1995) and, thus, although unable to sediment freely, may still be able to exert force on cellular receptors through this actin network. It is not clear whether the lack of free sedimentation is a direct cause of reduced gravitropic signal strength in S3 cells or simply a coincidence. The root cap is a dynamic developmental system. Cells produced by the meristem traverse a developmental gradient from S1 to S3 columella and then differentiate to secretory tip cells before being sloughed from the cap (Sack and Kiss, 1989; Sack, 1991; Baum and Rost, 1996). This gradient is most clearly visible in the differences in amyloplast size, which increases from S1 to S3 and then decreases rapidly, resulting in shrunken or absent amyloplasts in the tip cells.
Other cellular changes that accompany the progressive differentiation into secretory tip cells might be offset in time from changes in plastid size and could be more or equally important for graviperception and signal strength. We would predict multiple modifications of cellular structure and components as a cell entered the graviperceptive state; one of these appears to be alterations to the cytoskeleton that allow free amyloplast sedimentation (Baluska et al., 1997). It could be argued that the free sedimentation observed in the columella is simply a side effect of other physiological processes and is therefore of no functional importance. However, our observations of the striking correlation between sedimentation velocities of particular cells and the effect of the removal of these cells on gravitropic curvature suggest a functional role of sedimentation in gravity perception.
In summary, we have used laser ablation as a tool to test directly the functional roles of columella cells in the response of Arabidopsis roots to gravity. The rate of amyloplast sedimentation correlates strongly with their role in the graviresponse and thus is consistent with the starch-statolith model of gravity perception. Although all columella cells play a role in the graviresponse, we have determined that the central cells of S2 provide the greatest contribution to gravitropism in Arabidopsis roots.
ACKNOWLEDGMENTS
We thank Dr. Sarah M. Assmann for use of the laser-ablation system, which was funded by a grant to S.M.A. from the National Aeronautics and Space Administration/National Science Foundation Network for Research on Plant Sensory Systems (grant no. MCB-9416039). We also thank Dr. Fiona Armstrong for technical assistance with the use and proper alignment of the laser-ablation system and Bruce Link for access to the clinostat.
Abbreviation:
- ANOVA
analysis of variance
Footnotes
This work was supported by the National Science Foundation (grant no. IBN 95-13991) and by a Plant Responses to the Environment: Biochemical Bases, Physiological Responses, and Ecological Consequences training grant (no. NSF DBI-9413204 to J.M.F.)
LITERATURE CITED
- Baluska F, Kreibaum A, Vitha S, Parker JS, Barlow PW, Sievers A. Central root cap cells are depleted of endoplasmic microtubules and actin microfilament bundles: implications for their role as gravity-sensing statocytes. Protoplasma. 1997;196:212–223. doi: 10.1007/BF01279569. [DOI] [PubMed] [Google Scholar]
- Baum SF, Rost TL. Root apical organization in Arabidospis thaliana. I. Root cap and protoderm. Protoplasma. 1996;192:178–188. [Google Scholar]
- Behrens HM, Gradmann D, Sievers A. Membrane-potential responses following gravistimulation in roots of Lepidium sativum L. Planta. 1985;163:463–472. doi: 10.1007/BF00392703. [DOI] [PubMed] [Google Scholar]
- Caspar T, Pickard BG. Gravitropism by a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity-sensing. Planta. 1989;177:185–197. [PubMed] [Google Scholar]
- Clifford PE, Barclay GF. The sedimentation of amyloplasts in living statocytes of the dandelion flower stalk. Plant Cell Environ. 1980;3:381–386. [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Cellular organisation of the Arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- Hasenstein KH, Evans ML. Effects of cations on hormone transport in primary roots of Zea mays. Plant Physiol. 1988;86:890–894. doi: 10.1104/pp.86.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote DG. The geotropic reaction and statolith movements following geotropic stimulation of mung bean hypocotyls. Plant Cell Environ. 1981;4:131–140. [Google Scholar]
- Henriksen GH, Assmann SM. Laser-assisted patch clamping: a methodology. Pflugers Arch-Eur J Physiol. 1997;433:832–841. doi: 10.1007/s004240050352. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML. Gravity-induced changes in intracellular potentials in elongating cortical cells of mung bean roots. Plant Cell Physiol. 1990;31:457–462. [PubMed] [Google Scholar]
- Jackson MB, Barlow PW. Root geotropism and the role of growth regulators from the cap: a reexamination. Plant Cell Environ. 1981;4:107–123. [Google Scholar]
- Johnsonn A, Pickard B. The threshold stimulus for geotropism. Physiol Plant. 1979;45:315–319. [Google Scholar]
- Juniper BE, Gorves S, Landau-Schachar B, Audus LJ. Root cap and the perception of gravity. Nature. 1966;209:93–94. [Google Scholar]
- Kiss JZ, Hertel R, Sack FD. Amyloplast are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta. 1989;95:314–329. [PubMed] [Google Scholar]
- Kiss JZ, Wright JB, Caspar T. Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol Plant. 1996;97:237–244. doi: 10.1034/j.1399-3054.1996.970205.x. [DOI] [PubMed] [Google Scholar]
- Konings H. On the mechanism of the transverse distribution of auxin in geotropically exposed pea roots. Acta Bot Neerl. 1967;16:161–176. [Google Scholar]
- Konings H. The significance of the root cap for geotropism. Acta Bot Neerl. 1968;17:203–211. [Google Scholar]
- Konings H. Gravitropism of roots: an evaluation of progress during the last three decades. Acta Bot Neerl. 1995;44:195–223. doi: 10.1111/j.1438-8677.1995.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Kuznetsov OA, Hasenstein KH. Intracellular magnetophoresis of amyloplast and induction of root curvature. Planta. 1996;198:87–94. doi: 10.1007/BF00197590. [DOI] [PubMed] [Google Scholar]
- Legué V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Cytosolic free calcium in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–800. doi: 10.1104/pp.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legué V, Vilaine F, Tepfer M, Pérbal G. Modification of the gravitropic response of seedling roots of rapeseed (Brassica napus) transformed by Agrobacterium rhizogenes A4. Physiol Plant. 1994;91:559–566. [Google Scholar]
- Meyer AJ, Weisenseel MH. Wound-induced changes of membrane potential, endogenous currents, and ion fluxes in primary roots of maize. Plant Physiol. 1997;114:989–998. doi: 10.1104/pp.114.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Zieschang HE, Sievers A. Differential proton secretion in the apical elongation zone caused by gravistimulation is induced by a signal from the root cap. Plant Cell Environ. 1996;19:1408–1414. doi: 10.1111/j.1365-3040.1996.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Moore R, Evans ML. How roots perceive and respond to gravity. Am J Bot. 1986;73:574–587. [PubMed] [Google Scholar]
- Pilet PE. Root cap and root growth. Planta. 1972;106:169–171. doi: 10.1007/BF00383996. [DOI] [PubMed] [Google Scholar]
- Poff KL, Martin HV. Site of graviperception in roots: a re-examination. Physiol Plant. 1989;76:451–455. doi: 10.1111/j.1399-3054.1989.tb06218.x. [DOI] [PubMed] [Google Scholar]
- Sack FD. Plant gravity sensing. Int Rev Cytol. 1991;127:193–252. doi: 10.1016/s0074-7696(08)60695-6. [DOI] [PubMed] [Google Scholar]
- Sack FD, Kiss JZ. Root cap structure in wild type and in a starchless mutant of Arabidopsis. Am J Bot. 1989;76:454–464. [PubMed] [Google Scholar]
- Sack FD, Suyemoto MM, Leopold AC. Kinetics of amyloplast sedimentation in gravistimulated maize coleoptiles. Planta. 1984;161:459–464. [PubMed] [Google Scholar]
- Sack FD, Suyemoto MM, Leopold AC. Amyloplast sedimentation kinetics in gravistimulated maize roots. Planta. 1985;165:295–300. [PubMed] [Google Scholar]
- Sack FD, Suyemoto MM, Leopold AC. Amyloplast sedimentation and organelle saltation in living corn columella cells. Am J Bot. 1986;73:1692–1698. [PubMed] [Google Scholar]
- Salisbury F. Gravitropism: changing ideas. Hortic Rev. 1993;15:233–277. [Google Scholar]
- Scheres B, Mckhann H, van den Berg C, Willemsen V, Wolkenfelt H, de Vrieze G, Wiesbeck P. Plant Soil. 1996;187:97–105. [Google Scholar]
- Sievers A, Kruse S, Kuo-Huang L-L, Wendt M. Statoliths and microfilaments in plant cells. Planta. 1989;179:275–278. doi: 10.1007/BF00393699. [DOI] [PubMed] [Google Scholar]
- Sievers A, Sondag C, Trebacz K, Hejnowicz Z. Gravity induced changes in intracellular potentials in statocytes of cress roots. Planta. 1995;197:392–398. doi: 10.1007/BF00202662. [DOI] [PubMed] [Google Scholar]
- van den Berg C, Willemsen V, Hage W, Weisbeek P, Scheres B. Cell fate in Arabidopsis root meristem determined by directional signaling. Nature. 1995;378:62–65. doi: 10.1038/378062a0. [DOI] [PubMed] [Google Scholar]
- White RG, Sack FD. Actin microfilaments in presumptive statocytes of root caps and coleoptiles. Am J Bot. 1990;77:17–26. [PubMed] [Google Scholar]
- Wilkins H, Wain RL. The root cap and control of root elongation in Zea mays L. seedlings exposed to white light. Planta. 1974;121:1–8. doi: 10.1007/BF00384000. [DOI] [PubMed] [Google Scholar]
- Younis AF. Experiments on the growth and geotropism of roots. J Exp Bot. 1954;5:357–372. [Google Scholar]