Abstract
Cardiac embolism, primarily from atrial fibrillation (AF), is implicated in a quarter of all ischemic strokes. In the setting of AF, contraindications to traditional therapies can create a clinical dilemma when choosing an agent for secondary stroke prophylaxis. Newer horizons in the medical and surgical management of AF have helped us choose from a wide variety of available therapies, the best possible management. In this article, we review the current trends in AF management including newer oral anticoagulants as well as surgical devices from a neurologist's view.
Keywords: Anticoagulation, atrial fibrillation, atriclip, reversal of anticoagulation
INTRODUCTION
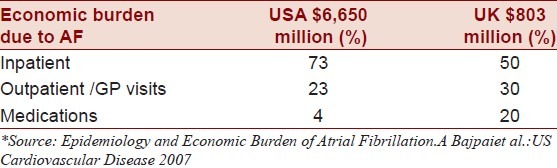
Atrial fibrillation (AF) is recognized as the most common cardiac arrhythmia, accounting for a lifetime risk of about 25%.[1–3] It presents a 5–6 fold increased stroke risk and accounts for at least one in every seven ischemic strokes. The common conception is that highest risk of embolic stroke is in those who develop thrombus formation in the left atrial appendage (LAA). The burden of disease rests on the elderly, as half of all patients with AF are over the age of 75.[4] Given the dramatic increase in the aging population in the United States, it has been estimated that disease prevalence will be more than double by 2050, which might be equivalent to 5.6–12.1 million adults.[4,5] This increase in AF will contribute an additional 170,000 annual strokes, the equivalent of nearly 25% of the current stroke incidence, including significant burden on the economy [Table 1]. The risk of AF-related stroke mortality can be up to 24% in those aged 80–89 years. In the setting of AF, oral anticoagulation (OA) has proven to reduce the risk of ischemic stroke by 60% when compared to placebo and 52% fewer strokes when compared to aspirin. However, first generations OA (such as warfarin) are not well accepted by patients, families, and even clinicians often because the risk of hemorrhage is often anecdotally exaggerated. In addition, the frequency of necessary monitoring of blood levels and the interaction of drugs with other medications that work on the same enzyme complexes make these medications too difficult to manage in deserving patients. The aforementioned issues lead to underutilization of first-generation anticoagulants in clinical practice. RecentFDA-approved OA and advancements in novel surgical therapeutics are undoubtedly shifting the paradigm of the management of AF.
Table 1.
Cost factor related to AF*
Classification
Several different classification systems have been proposed for AF, but none have fully accounted for all disease elements. Recently, the AHA, ACC, and ESC recommended the following classification scheme aimed for simplification and clinical applicability. Once multiple episodes of AF are experienced, it is deemed recurrent AF, which is further subcategorized as “paroxysmal AF” (terminates within seven days) “persistent AF” (sustained longer than seven days), or “permanent AF” (sustained longer than one year) Figure 1. The term “lone AF” has been loosely defined and poorly studied, but is often applied to patients under the age of 60 without any evidence of cardiopulmonary disease, and as such these patients typically do not necessarily carry the burden of increased mortality and thromboembolic risk.[6]
Figure 1.

Classification of Recurrent AF: Recurrent AF can be categorized as Paroxysmal, Persistent, or Permanent
Pathophysiology
The pathophysiology of AF remains incompletely understood,[7] but it is widely accepted that it requires both a substrate and a trigger [Figure 2]. The substrate refers to cardiac tissue that is susceptible to re-entry electrical activity, and the trigger refers to the source of the ectopic foci.[8] Electrical remodeling begins soon after arrhythmia onset and is characterized by a shortening of the atrial refractory period. Structural remodeling is then followed if arrhythmia occurs weeks to months. Both of these processes (electrical and structural) result in contractile remodeling that may facilitate AF persistence.
Figure 2.
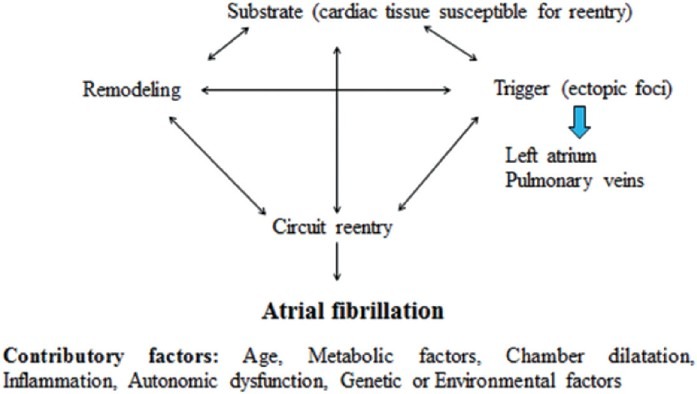
Pathophysiology of AF: The complex interactions of different factors playing role in the pathophysiology of AF
On the other hand, the mechanism underlying AF in patients with heart failure seems to be somewhat different. Fibrosis and loss of muscle mass arefound to be increased in the atria, predisposing to the occurrence of AF. The fibrosis seems to be due to up regulation of the renin–angiotensin–aldosterone system and dysregulation of intracellular calcium homeostasis. It is unclear whether the fibrosis is a cause or consequence of AF, but it may result in heterogeneous conduction characteristics that make AF more likely.
The vast majority of ectopic foci are present in the left atrium and pulmonary veins, and it has been reported that up to 94% of foci occur within the pulmonary veins.[9,10] The transition of tissue between the pulmonary vein endothelium and the left atrial (LA) epicardium represents an area of two adjacent cell types with very different electrical properties. This junction may potentiate the development of AF.[11] AF is a multifactorial process, which among many possible causes, may be the result of age-related structural and metabolic changes, chamber dilatation, inflammatory processes, and autonomic dysfunction. Population studies have shown that genetic and environmental factors influence progression of AF, but causation is left to be determined.[9] Studies postulate inflammation as a one of the predisposing factor for AF, which is supported by myocardium biopsy showing inflammatory infiltrates, myocyte necrosis, and fibrosis in patients with AF.[12,13]
Effects of AF on brain: Apart from the risk of cardio embolism, AF can affect the brain through other mechanisms. The OA medications used in primary stroke prevention including newer anticoagulants can cause hemorrhagic strokes. The brain perfusion is impaired in patients with AF as evidenced by CT perfusion in stroke imaging. AF refractory medical treatment show signs of brain hypoperfusion and cognitive impairment, which can be reversed by improving cardiac function by ablation and pacing.[14]
Clinical risk stratification
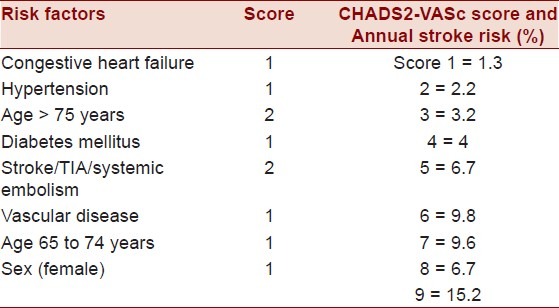
Several scoring systems have been proposed to assess stroke and transient ischemic attack (TIA) risk in AF. These scores are designed to predict clinical outcome and provide a framework to assess appropriateness of intervention. The CHADS2 score is the most widely used scoring system in clinical practice. In the CHADS2 score, one point is assigned for the presence of each of the following: history of congestive heart failure (CHF), history of hypertension (HTN), age ≥ 75 years, diabetes, and two points are assigned for history of TIA or stroke. A higher CHADS2 score correlates with increasing stroke risk, as presented in Table 2, as well as increasing risk of sludge and/or thrombus formation in left atrium and LAA.[15] This scoring system was recently modified, and the CHA2DS2-VASc score now accounts for additional stroke risk factors in AF by assigning one point for age 65–75, female gender, and history of vascular disease, while age ≥75 was increased to two points.[16] The ESC recently recommended the application of the CHA2DS2-VASc score when further risk stratification is indicated, particularly for patients with low and medium stroke risk, with a CHADS2 score of 0 or 1.[17]
Table 2.
CHADS2 annual stroke risk[16]
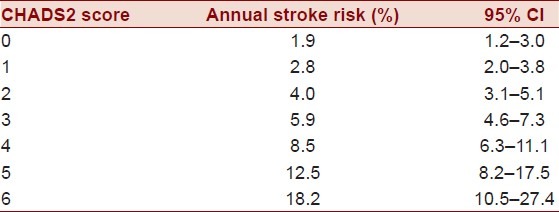
The CHADS2 or the newer CHADS2-VASc scores are useful clinical tools in determining the appropriateness of initiating anticoagulation therapy. A score of 0 in either scoring regime indicates low risk of future stroke and TIA. Such patients are managed with an oral aspirin regimen. A score of 1 in either scoring regime indicates moderate risk, in which case either aspirin or long-term OA is appropriate [raise target International Normalized Ratio (INR) to 2.0–3.0], which depends on individual patient preference. A score of 2 or greater is considered high risk in either scoring system, and OA is then recommended. In order to maximize sensitivity of the clinical prediction tool, the CHADS2-VAScscore should be applied if the patient is considered low or intermediate risk [Table 3]. OA will be discussed in greater detail in the “Management” section of this review.
Table 3.
CHADS2-VASC score and annual stroke risk
Management
Rate and rhythm control
Rhythm control does not seem to have any additional protection against stroke.[18,19] Most antiarrhythmic drugs (AADs) only provide sinus rhythm maintenance about 25% of the time after 12 months of use. As a result, pharmacotherapy in AF is targeted at rate control.[8,20] In the absence of complicating features, rate control is achieved with dilitazem, verapamil, or a beta blocker. Digoxin has a role in the treatment of AF when there is co-existent heart failure. In patients with poor left ventricular function, amiodarone may be used. Intravenous medications, such as procainamide, are available for rate control in AF in the attempt for cardioversion.[21]
Once rate control and hemodynamic stability areachieved and secondary causes of AF have been evaluated, the clinician must address the potential need for cardioversion. Most patients with new onset symptomatic AF warrants an attempt of either pharmacological or electrical cardioversion. Patients with an elevated bleeding risk should not be cardioverted if anticoagulation cannot be initiated prior to or following the return to normal sinus rhythm (NSR). Additionally, there are many patients in whom the risk of cardioversion outweighs the benefit, specifically elderly asymptomatic patients with a larger number of comorbidities.[21]
In the management of AF, it is imperative that the clinician identify situations indicating the need for immediate cardioversion. Pharmacological or electrical cardioversion is indicated in the following scenarios: electro physiologic or clinical evidence of ischemia, any evidence of organ hypo perfusion, severe manifestations of heart failure, or presence of pre-excitation pathway.[21] In these situations, the hemodynamic stability that is provided by return to NSR outweighs the thromboembolic risk. And although prompt initiation of heparin is of the upmost importance, it should not delay cardioversion. However, if new onset AF has been present for greater than 48 h, transesophageal echocardiogram (TEE) is recommended prior to cardioversion because of the increased risk of LA thrombus. In the setting of a LA thrombus or inability to perform TEE, anticoagulation with heparin for at least three weeks prior to cardioversion is indicated to allow for resolution of thrombus. The goal of anticoagulation prior to cardioversion is to prevent the embolic shed from preexisting thrombus. Additionally, even if AF has been present for less than 48 h, delayed cardioversion with anticoagulation may also be indicated especially if there is a history of thromboembolic events or if there is a significant history of heart failure, associated mitral valve disease, or cardiomyopathy.[21,22]
Rate control is commonly employed by the clinical Neurologist, and it is important to remember that rate control is aimed at preventing AF complications by avoiding a rapid ventricular response. Rate control medications achieve this goal by regulating impulses at the AV node, but these medications do no directly treat the underlying arrhythmia. Rhythm control, though less often employed by the neurologist, is aimed at a different aspect of AF. It is designed to treat the arrhythmia itself, rendering it a seemingly attractive treatment option, but its efficacy in stroke prevention is poor, hence its limited utility with respect to a Neurologist's perspectives.
Anticoagulation
The clinical goal in the management of AF is to reduce the risk of cardio embolic events. This can be achieved by chronic anticoagulation which prevents thrombus formation in the LAA, the most favored location of thrombi formation.
Historically, chronic anticoagulation has been the mainstay in the treatment of AF among patients with moderate-to-high risk of subsequent stroke and TIA (based on aforementioned clinical risk stratification). It is recommended that low-risk patients and those with contraindications to anticoagulation be managed with 81–325 mg Aspirin daily.[21] The warfarin related annual risk of a fatal bleed is 0.6%, risk of major bleed is 3%, and risk of combined (major or minor)bleedis 9.6%. This represents a fivefold increase risk as compared to those without anticoagulation.[23]
The AFFIRM trial first reported improved survival and reduced stroke risk with rate control combined with anticoagulation versus rhythm control. Chronic anticoagulation is usually achieved with Warfarin and a target INR of 2-3.[21] With the frequency of AF expected to double by 2050, there has been a large push by the medical industry to find alternatives to Warfarin that are cost effective, clinically practical, simple for patients, and free of frequent monitoring requirements.[24] Newer oral anticoagulants have since been introduced as attractive alternatives to Warfarin.
Most notably in October 2010, dabigatran (Pradaxa), a direct thrombin inhibitor, received FDA approval for the treatment of stroke prevention in AF. Its major advantage is the lack of INR monitoring that is normally required with Warfarin. Dabigatran etexilate is a prodrug of dabigatran, independent of CYP-450 and a potent nonpeptide molecule that reversibly inhibits thrombin by binding to the active site of the thrombin molecule. The half-life of dabigatran has been estimated to be 12–14 h.[25]
In the RE-LY study, compared to warfarin, dabigatran 150 mg orally twice a day dose was found to decrease the risk of stroke or systemic embolism, with a relative risk reduction of 34% and an absolute risk reduction of 0.58% with no increase in the risk of major bleeding and a decrease in the risk of hemorrhagic stroke.[26] On the other hand, dabigatran 110 mg twice a day dose was noninferior to warfarin for stroke prevention and was associated with a decreased risk of major bleeding and hemorrhagic stroke except for major gastrointestinal bleeding which was more common with dabigatran 150 mg twice daily than with warfarin.[27] Dosing is renally adjusted to account for decreased clearance among patients with severe renal impairment. When converting patients from warfarin to dabigatran, discontinue warfarin and then begin dabigatran twice daily therapy when the INR is below 2.0.
Rivaroxaban (Xarelto), is an oral competitive factor Xa inhibitor which targets factor Xa, a common factor in both the extrinsic and intrinsic coagulation pathway.[25] It was recently (2011) approved for stroke prevention in nonvalvular atrial fibrillation. The landmark trial ROCKET-AF demonstrated that it was as effective as Warfarin in reducing stroke while carrying a similar overall bleed risk and decreased risk of intracranial and fatal bleeds.[28] Like dabigatran, the recommended dose of Rivaroxaban is adjusted in patients with renal impairment, but dosing is once daily. When switching from Warfarin to Rivaroxaban, one must discontinue warfarin and then start Rivaroxaban once INR is below 3.
Apixaban is another oral factor Xa inhibitor awaiting FDA approval that has been compared to both ASA and warfarin for stroke prevention in AF. In a randomized controlled trial of patients considered unsuitable for warfarin therapy apixaban 5 mg orally twice a day dose was more effective than Aspirin for prevention of stroke or systemic embolism, with a 50% relative riskreduction and a 2.1% absolute risk reduction in the primary outcome and no observed increase in the risk of major bleeding.[29] A reduced dose of apixiban (2.5 mg twice a day) was used for individuals aged over 80 years or with a serum creatinine greater than 1.5 mg/dL.
In another randomized controlled trial, apixiban 5 mg twice daily was more effective than warfarin in reducing the risk of stroke or systemic embolism, with a relative risk reduction of 21% and an absolute risk reduction of 0.33% and a decrease in the risk of major bleeding and hemorrhagic stroke.[30]
Some limitations in the use of the newer oral anticoagulants are the inability to monitor its effect with lab tests (such as INR) and dosing concerns in the setting of renal impairment generating some uncertainly with respect to physiologic impact, which cannot be measured. Of further clinical concern is the lack of reversibility of newer oral anticoagulants. These pose a clinical dilemma, particularly when patients on newer OA present as an ischemic stroke and consideration are given to administer intravenous thrombolysis. The current concept which is mainly from expert opinion is, if patients are on newer anticoagulation and has been off the medication for 2 days then it might be safe to receive thrombolysis in acute ischemic stroke situation. However, newer OA medications provide the clinician an opportunity to initiate them in people who are reluctant to take older anticoagulants due to the complexities discussed earlier, for primary or secondary stroke prophylaxis. This can contribute not only to greater disease control, but greater patient compliance and satisfaction.
Clinical questions encountered by neurologist in AF patients
Is there a need for anticoagulation patients with AF?
The use of prophylactic anticoagulation for AF achieves about a one third reduction of disabling stroke and other major vascular events as compared to anti-platelet agents. Still, only half of all patients with AF are adequately anti-coagulated.[31–33] Compliance compromises adequate anticoagulation in many cases, but still many eligible candidates refuse anticoagulation because of fear of hemorrhage or hemorrhagic conversion of the infarct. The fear of initiating anticoagulation in elderly with fall risk might be exaggerated.[34] Too often, patients refuse anticoagulation because of poor understanding about the benefits.[35]
What are the predictors of bleeding due to anti-coagulation?
There are a few clinical predictors which may guide clinicians in deciding to initiate or restart anticoagulation. One of these predictors is “HAS-BLED,” which has been recently incorporated into the European society of cardiology guidelines for management of AF. This scoring system assigns 1 point for each risk factor like hypertension, abnormal renal function, abnormal liver function, stroke history, bleeding history, labile INR, age ≥75, aspirin/NSAID use, and drug/alcohol use. If the HAS-BLED score is greater than the pateint's CHADS2 score, bleeding risk outweighs the potential benefit of OA,[15,36] but this clinical scoring tool needs further validation.[37]
Is bridging with heparin necessary when OA is initiated?
A common controversy that faces the clinical neurologist is the question of using heparin or low molecular weight heparin (LMWH) as a bridge during the initiation of Warfarin therapy, until a therapeutic INR is achieved. Clinical trials have not identified the most effective strategy to bridge with heparin or a heparinoid, but risk stratification with a clinical predictor like the CHADS2 score or HAS-BLED score might play a role in selecting the patients that may benefit from bridging therapy. Bridging therapy is also an issue in the perioperative period, and some authors have suggested that high risk AF patients undergoing surgery can bridge with heparin in a safe and effective manner, but this needs further clarification.[38] The risk of bleeding in any particular procedure must be carefully considered as this risk might help in selecting a heparin bridge versus continuation of OA.[38]
Not uncommonly, the Neurologist cares for a patient who develops a thrombotic state in spite of therapeutic INR, in which case, an increase in target INR or the addition of an anti-platelet agent is recommended. Still, the clinician must consider the higher bleeding risk associated with these changes, and this must be communicated to the patient.[39]
Is it a good idea to combine dual anti-platelets with anticoagulation?
Another controversial issue encountered routinely that of dual antiplatelet therapy as an adjuvant to warfarin in cardiac stents and coronary artery disease patients. This particular issue, like many discussed in this article, involves the collaboration of Cardiology, Cardiothoracic Surgery, and Neurology. Benefit of anticoagulation versus risk of bleeding is considered in a multidisciplinary manner, and there are an only few consensus and observational studies addressing this issue[39] and newer anticoagulants or surgical therapy discussed below might play a role in resolving this controversy.
What to do in cases of intra-cerebral hemorrhage associated with anticoagulation?
Another common clinical issue is the AF patients presenting with intracranial hemorrhage (ICH) associated with medications described above. Hemorrhages are a common neurological emergency with the use of unfractionated heparin (UH) and can be managed by promptly stopping the UH infusion, as the half-life of IV heparin is 60–90 minutes. If bleeding is not life threatening, then watchful waiting may be employed. If further therapy is necessary, beyond stopping UH, Protamine sulfate has proven an effective antidote. Approximately 1mg of protamine sulfate will neutralize 100-U of UH. Infusion should not exceed 5mg/min due to risk of bronchoconstriction, hypotension, and anaphylaxis. Maximum dose of Protamine sulfate is 50 mg per 10 min, but may be repeated if required. A higher incidence of severe allergic reaction including anaphylaxis may exist with prior Protamine exposure or exposure to Protamine containing products (e.g. NPH insulin) or history of fish allergy. There is no role for FFP in the reversal of UH. For bleeding during a continuous infusion of UH; Protamine dosing is 1mg/100 U UH given to neutralize all UH given within the last hour + one half the dose of heparin given during the preceding hour + one-quarter of the dose of heparin given in the hour prior to that.[40,41]
In cases of low molecular weight heparin (LMWH) associated ICH, there is no proven method to fully reverse the anticoagulation. The half-life is 4–8 h, so some authors recommend 1mg of protamine for every 1mg of LMWH given in the last 4 h, but efficacy is unproven. Recombinant factor VIIa can be considered in the management of intractable bleeding.[40,41]
Fondaparinux has a, half-life of 14 h, and again there is no specific antidote so activated recombinant factor VIIa (rVIIa) can be considered in the management of intractable bleeding.[40,41]
The warfarin related ICH or life threatening bleeding comprises 12%–14% of all intracranial hemorrhages. Mortality doubles in patients with ICH on OA. When the INR is greater than 1.7, there are few options. One cocktail includes vitamin K 10 mg with prothrombin complex concentrate (PCC) 30u/kg and 2 units of FFP. Alternatively, a cocktail of vitamin K 10 mg, fresh frozen plasma (FFP) 15ml/kg, and rVIIa 40 μg/kg may be used.[17,42,43] The use of PCC, which contains factors II, IX, and X complex, may carry an increased thrombotic risk and is therefore contraindicated in disseminated intravascular coagulation.
Newer oral anticoagulants pose a particular challenge when bleeding complications present. In cases of dabigatran-related ICH, no specific antidote exists and activated charcoal can be used if ingestion within 2 h. More likely, rVIIa 60–90 μg/kg or PCC 50 u/kg may be used in an attempt to reverse anticoagulation reference not valid. In cases of Rivaroxaban associated ICH, there is no specific antidote and the half-life of the drug is 5–9 h. PCC can be considered in the management of intractable bleeding with a recommended dose of 50u/kg.[44]
When do you consider surgical therapy in management of AF?
Recent guidelines[17] suggest that ablation of the AV node and cardiac resynchronization therapy (CRT) to control heart rate should be considered when the heart rate cannot be controlled with medical therapy. Also catheter ablation should be tried for paroxysmal AF and persistent symptomatic AF who have previously failed antiarrhythmic therapy. However, catheter ablation of AF may be considered prior to AAD therapy in symptomatic patients despite adequate rate control with paroxysmal symptomatic AF but no significant underlying heart disease.
Alternatively, surgical ablation is an option in patients planned to undergo other cardiac surgeries in low to moderate symptomatic patients and low-risk asymptomatic patients. There is a role for minimally invasive surgical ablation in symptomatic AF patients without concomitant cardiac surgery after they fail catheter ablation. Various surgical techniques employed in AF are discussed in the next section.
Surgical management
Various surgical techniques have been developed in the last 25 years playing a complementary role in the treatment of AF. The Maze procedure (or Cox-Maze III), has established itself as the gold standard for surgical approaches in patients with AF.[45,46] Basic principle is to create multiple right and LA incisions. The incisions are organized in such a manner that they not only interrupt reentrant circuits but also simultaneously guide electrical impulses to the AV node. The Maze procedure also includes removal of LAA and isolation of the pulmonary veins by incision. Total procedure time is less than 60 min in the hands of an experienced practitioner.[45,46] Given the exposure provided by this open approach additional interventions such as valvular repair or bypass can be achieved during the same procedure. Of course the nature of the procedure also requires cardiopulmonary bypass and median sternotomy. The Maze procedure has been successful at long-term elimination of AF with 93% success rate at 8.5 year follow-up.[45] Thoracoscopic approaches to the Maze procedure have been introduced to reduce the invasive nature of the procedure. While introducing higher surgical costs, this approach offers decreased length of stay and decreased post-operative recovery period, with an efficacy similar to the traditional MAZE procedure.[47]
While the traditional MAZE procedure is performed with physical interruption of electrical pathways by incisions, a number of novel energy sources such as laser, ultrasound, and radiofrequency have since been employed to create tissue scarring more rapidly and safely than “cutting and sewing.” Still given the variable nature of lesions applied through this approach, efficacy data havebeen difficult to interpret.[48] Radiofrequency ablation is now available as an additional ablative technique that has been used in conjunction with the traditional Maze approach, often referred to as the modified Maze procedure, or Maze IV.[49,50] Radiofrequency is also available as a standalone procedure that can be performed through a thoracoscopic approach or catheter-based approach.
Catheter-based radiofrequency ablation with the intent to achieve NSR by eradicating the faulty electrical pathways in a manner that is far less invasive than sternotomy or thoracoscopy is also available. Many consider catheter-based ablation to be minimally invasive and highly efficacious, but the data suggest a low rate of success of about 30% from a single procedure and 80% with repeat procedures.[49,51]
Another commonly used technique is cryoablation. Cryoablation can be employed through both a catheter-based approach or in conjunction with the Maze or minimally invasive Maze procedure. In cryoablation, tissue is cooled with helium or argon to low temperatures (–50 to –75°C) in order to induce targeted scarring. As compared to other ablative techniques, cryoablation prevents vascular/collagen damage and less peripheral tissue damage.[51]
High-frequency ultrasound, another ablative technique, scars cardiac tissue in a trans-mural fashion through hyperthermic lesions. This can be performed through a catheter-based approach in a quick manner that offers the potential for wall visualization given the nature of the technology. However, this approach carries the risk of delivering hyperthermic stress to adjacent tissues. Esophageal or mediastinal injury has been reported following ultrasound and radiofrequency ablation.[52]
Microwave ablation has been similarly used to produce thermal injury to cardiac tissue and is capable of producing a trans-mural lesion when it is applied to the epicardial surface via thoracoscopic approach. However, both microwave- and laser-based ablation have been taken off from the market in recent years due to poor efficacy.
Management of left atrial appendage in AF
The LAA is the site of majority (91%) of thrombi in patients with nonrheumatic AF. In fact, 98% of arterial thrombi were found in the LAA through TEE trials involving 1181 patients.[53] This led to the technique of LAA occlusion also as a mean to reducing the risk of stroke in AF. LAAOS was the first randomized study evaluating LAA occlusion in patients undergoing other cardiac surgery, and it was demonstrated that occlusion can be performed without increasing surgical time, bleeding, or other surgical complications.[54] It was noted during LAAOS that successful complete occlusion by suture can be technically challenging, and accordingly a number of epicardial clip devices have since been introduced.
The Atriclip is one such clip that was recently evaluated with regard to safety and effectiveness[Figure 3].[55] The Atriclip was approved by FDA to be applied epicardially via a median sternotomy approach at the time of mitral valve or bypass surgery however; surgeons have begun to use the clip off label via thoracoscopic port access procedures. The LAA Atriclip system consists of a titanium core frame with nitinol springs on each end and is covered with a polyester fabric sleeve. The device is currently available in a number of sizes to account to variability in LAA size. When closed, the clip applies uniform pressure over the length of the twoparallel branches to ensure consistent, secure occlusion of the LAA. This device can be easily deployed during other cardiac procedures that are being performed through a sternotomy. In fact, The ACC and AHA have recommended that LAA ligation be performed during the course of valvular surgery, and the Atriclip provides one viable option by which to achieve this goal.[56] In the pivotal trial CT scan follow-up provided proof of absence of LAA thrombus, clip stability, and demonstrated LAA closure in 98% of patients at 6 month follow-up.[55]
Figure 3.
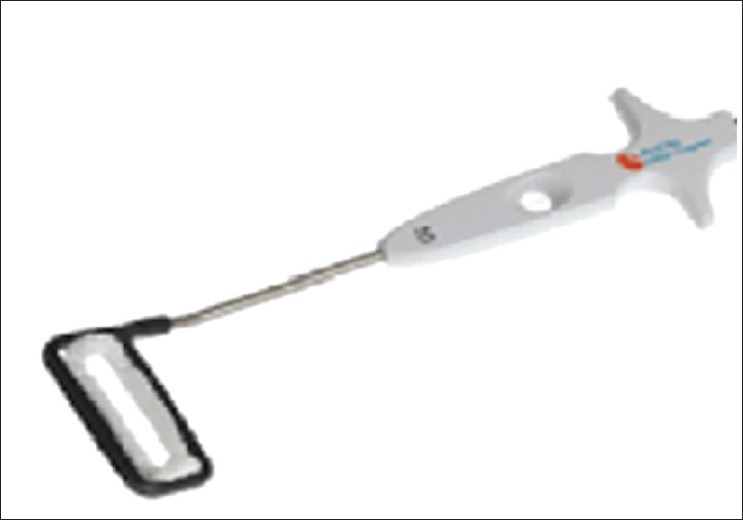
Atriclip: Titanium core frame with nitinol springs on each end and is covered with a polyester fabric sleeve
Role of minimally invasive surgery and catheter approach
Recent trend is minimally invasive approach via mini thoracotomy for isolating the pulmonary vein, exclusion of the LAA, and extensive ablation of the ganglionic plexuses and the ligament of Marshall in a single procedure.[57]
LAA occlusion can also be achieved by a percutaneous catheter deployment of devices [PLAATO and WATCHMAN [Figures 4 and 5], via a femoral vein approach with trans septal puncture. The PLAATO device consists of a nitinol cage coated with an impermeable polytetrafluoroethylene that is sealed within the LAA rendering thrombus formation obsolete. The WATCHMAN device is deployed via a similar percutaneous approach, but consists of a nitinol frame that is coated with a permeable polytetrafluoroethylene membrane. This frame self-expands when deployed within the LAA, but unlike the PLAATO device, it remains permeable to blood, so the device will eliminate thrombus formation once endotheliazed. Until that time, about 45 days following the procedure, the risk of thrombus formation and dislodgement remains and conventional prophylaxis is required. This approach is minimally invasive, but carries its own surgical risks including pleural effusion, pericardial effusion, cardiac-tamponade, hemo-thorax, deep vein thrombosis (DVT), or air emboli. But as compared to the conventional surgical approach, there is a reduction in recovery time and bleeding risk, with additional advantage of eliminating the need for long-term anticoagulation.[58]
Figure 4.
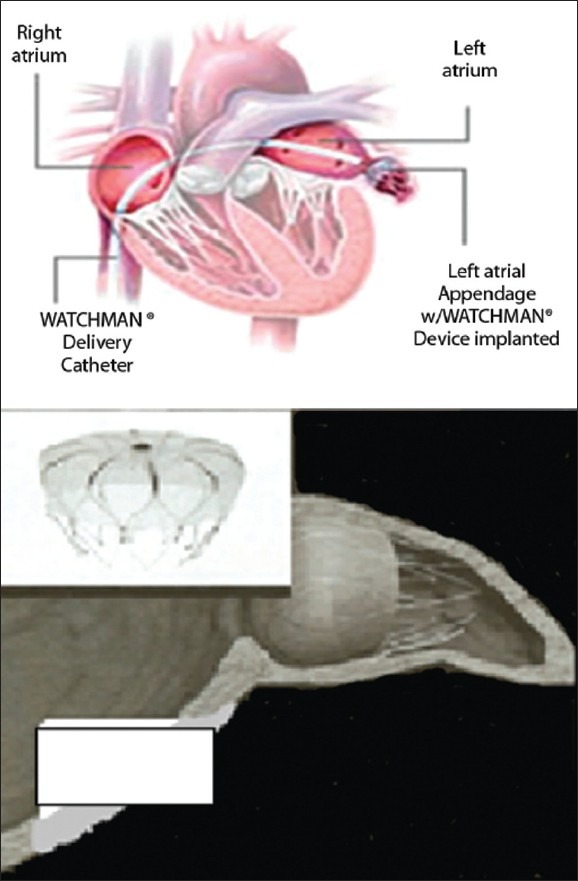
Deployment of WATCHMAN Devices Watchman devices
Figure 5.
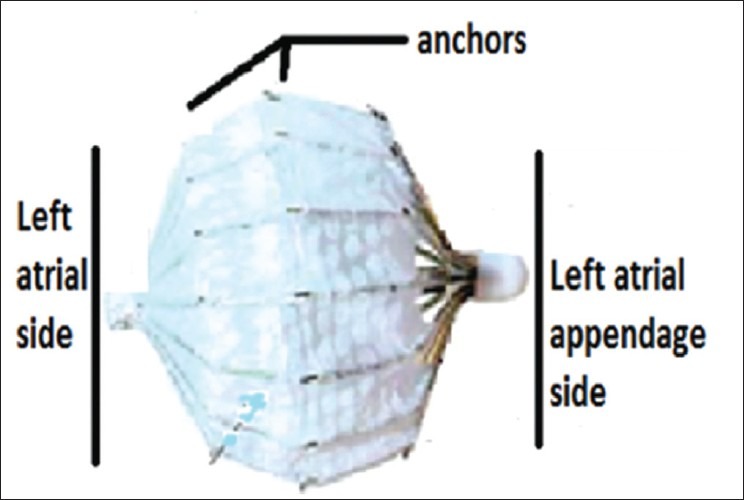
PLAATO device: Nitinol cage coated with an impermeable polytetrafluoroethylene that is sealed within the LAA rendering thrombus formation obsolete
The PROTECT AF trial was a multicenter prospective randomized study that sought to document the effectiveness of the WATCHMAN implant when compared to control patients solely on long-term warfarin. The primary endpoint included hemorrhagic or ischemic stroke, cerebrovascular death, systemic embolism, and documented TIA. Primary safety endpoint included life-threatening bleeds requiring transfusion. Control patients were maintained at an INR between 2 and 3 and were followed at 45 days and 6, 12, 24, 36, 48, and 60 months. Patients that underwent the WATCHMAN implantation had INR below 2 and were prescribed aspirin 81mg at least 1 day before procedure. These patients were back on therapeutic levels of warfarin until a 45-day post-op TEE showed LAA occlusion, suggesting lack of flow through the implant and jet flow of less than 3 mm around the device. Warfarin was then discontinued, and clopidogrel 75 mg and ASA 325 mg were then started for the duration of the trial. Initial experience with WATCHMAN device published in April 2007 noted promising results with 66 patients undergoing implantation. TEE at 45 days showed 54 of 58 devices successfully blocking the LAA. Patients were followed for a mean of 740 days and despite discontinuation of anticoagulation, no strokes were documented. Of note two cases of device embolization were reported, both later retrieved. Thus, preliminary results showed initial feasibility and safety.[58]
An article in the Lancet (Aug 2009) reported the results of 707 eligible patients. Over 1065 patient-years follow up; primary efficacy was 3.0 per 100 patient years in the WATCHMAN group and 4.9 per 100 patient years in the Warfarin control group. This resulted in the statistically significant result of noninferiority of more than 99.9%. In regards to primary safety events, more events occurred in the WATCHMAN implant group 7.4 per 100 patient-years vs. 4.4 per 100 patient-years in the control group. Authors note that these safety events were a result of peri-procedural complications.[59] The noninferiority of the WATCHMAN device sheds light that LAA occlusion may be an alternate option to Warfarin therapy. However, the FDA has not yet approved release of the Watchman.
CONCLUSION
AF carries a significant risk of cerebral embolism;therefore,anticoagulation plays a vital role in mitigating this risk. There are contraindications to traditional anticoagulants, but clinicians can now turn to newer options. Recently, FDA-approved alternative anticoagulants, which act by direct thrombin and factor Xa inhibition, have demonstrated comparable results to warfarin. Numerous surgical options are also available. Minimally invasive procedures and catheter-based approaches hold promise as tools for the future of AF control. Apart from additional protection from embolism, these procedures may also play a role in patients that are ineligible for anticoagulation. Major studies are underway in this area and results are awaited.
ACKNOWLEDGMENTS
Publication of this article was funded in part by the University of Florida Open-Access Publishing Fund.
Footnotes
Source of Support: Publication of this article was funded in part by the University of Florida Open-Access Publishing Fund.
Conflict of Interest: None declared.
REFERENCES
- 1.Broderick JP, Phillips SJ, O’Fallon WM, Frye RL, Whisnant JP. Relationship of cardiac disease to stroke occurrence, recurrence, and mortality. Stroke. 1992;23:1250–6. doi: 10.1161/01.str.23.9.1250. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, et al. Framingham Heart Study. Lifetime risk for developing congestive heart failure: The Framingham Heart Study. Circulation. 2002;106:3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: The Framingham Heart Study. Circulation. 2004;110:1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 5.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: The Rotterdam study. Eur Heart J. 2006;27:949–53. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 6.Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR, Jr, Ilstrup DM, et al. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. 1987;317:669–74. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- 7.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 8.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–26. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 9.Falk RH. Atrial fibrillation. N Engl J Med. 2001;344:1067–78. doi: 10.1056/NEJM200104053441407. [DOI] [PubMed] [Google Scholar]
- 10.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 11.Nathan H, Eliakim M. The junction between the left atrium and the pulmonary veins. An anatomic study of human hearts. Circulation. 1966;34:412–22. doi: 10.1161/01.cir.34.3.412. [DOI] [PubMed] [Google Scholar]
- 12.Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–49. doi: 10.1093/eurheartj/ehi645. [DOI] [PubMed] [Google Scholar]
- 13.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–4. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 14.Efimova I, Efimova N, Chernov V, Popov S, Lishmanov Y. Ablation and pacing: Improving brain perfusion and cognitive function in patients with atrial fibrillation and uncontrolled ventricular rates. Pacing Clin Electrophysiol. 2012;35:320–6. doi: 10.1111/j.1540-8159.2011.03277.x. [DOI] [PubMed] [Google Scholar]
- 15.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 16.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 17.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 18.Sherman DG, Kim SG, Boop BS, Corley SD, Dimarco JP, Hart RG, et al. National Heart, Lung, and Blood InstituteAFFIRM Investigators. Occurrence and characteristics of stroke events in the Atrial Fibrillation Follow-up Investigation of Sinus Rhythm Management (AFFIRM) study. Arch Intern Med. 2005;165:1185–91. doi: 10.1001/archinte.165.10.1185. [DOI] [PubMed] [Google Scholar]
- 19.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 20.Nattel S, Hadjis T, Talajic M. The treatment of atrial fibrillation. An evaluation of drug therapy, electrical modalities and therapeutic considerations. Drugs. 1994;48:345–71. doi: 10.2165/00003495-199448030-00003. [DOI] [PubMed] [Google Scholar]
- 21.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: Full text: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:651–745. doi: 10.1093/europace/eul097. [DOI] [PubMed] [Google Scholar]
- 22.Collins LJ, Silverman DI, Douglas PS, Manning WJ. Cardioversion of nonrheumatic atrial fibrillation. Reduced thromboembolic complications with 4 weeks of precardioversion anticoagulation are related to atrial thrombus resolution. Circulation. 1995;92:160–3. doi: 10.1161/01.cir.92.2.160. [DOI] [PubMed] [Google Scholar]
- 23.Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: Clinical epidemiology, prediction, and prevention. Am J Med. 1993;95:315–28. doi: 10.1016/0002-9343(93)90285-w. [DOI] [PubMed] [Google Scholar]
- 24.Gums JG. Place of dabigatran in contemporary pharmacotherapy. Pharmacotherapy. 2011;31:335–7. doi: 10.1592/phco.31.4.335. [DOI] [PubMed] [Google Scholar]
- 25.Maan A, Padmanabhan R, Shaikh AY, Mansour M, Ruskin JN, Heist EK. Newer Anticoagulants in Cardiovascular Disease: A Systematic Review of The Literature. Cardiol Rev. 2012:255–65. doi: 10.1097/CRD.0b013e3182503e2d. [DOI] [PubMed] [Google Scholar]
- 26.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 27.Ganetsky M, Babu KM, Salhanick SD, Brown RS, Boyer EW. Dabigatran: Review of pharmacology and management of bleeding complications of this novel oral anticoagulant. J Med Toxicol. 2011;7:281–7. doi: 10.1007/s13181-011-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 29.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 30.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 31.Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;3:CD006186. doi: 10.1002/14651858.CD006186.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Srivastava A, Hudson M, Hamoud I, Cavalcante J, Pai C, Kaatz S. Examining warfarin underutilization rates in patients with atrial fibrillation: Detailed chart review essential to capture contraindications to warfarin therapy. Thromb J. 2008;6:6. doi: 10.1186/1477-9560-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tentschert S, Parigger S, Dorda V, Bittner K, Unterbuchschachner D, Greisenegger S, et al. Vienna Stroke Study Group. Recurrent vascular events in patients with ischemic stroke/TIA and atrial fibrillation in relation to secondary prevention at hospital discharge. Wien Klin Wochenschr. 2004;116:834–8. doi: 10.1007/s00508-004-0259-x. [DOI] [PubMed] [Google Scholar]
- 34.Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med. 1999;159:677–85. doi: 10.1001/archinte.159.7.677. [DOI] [PubMed] [Google Scholar]
- 35.Hylek EM, D’Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice: The challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37:1075–80. doi: 10.1161/01.STR.0000209239.71702.ce. [DOI] [PubMed] [Google Scholar]
- 36.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: The HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–80. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 37.Loewen P, Dahri K. Risk of bleeding with oral anticoagulants: An updated systematic review and performance analysis of clinical prediction rules. Ann Hematol. 2011;90:1191–200. doi: 10.1007/s00277-011-1267-3. [DOI] [PubMed] [Google Scholar]
- 38.Garwood CL, Hwang JM, Moser LR. Striking a balance between the risks and benefits of anticoagulation bridge therapy in patients with atrial fibrillation: Clinical updates and remaining controversies. Pharmacotherapy. 2011;31:1208–20. doi: 10.1592/phco.31.12.1208. [DOI] [PubMed] [Google Scholar]
- 39.Broukhim M, Halperin JL. Stroke prevention in the high-risk atrial fibrillation patient: Medical management. Curr Cardiol Rep. 2011;13:9–17. doi: 10.1007/s11886-010-0148-z. [DOI] [PubMed] [Google Scholar]
- 40.Beshay JE, Morgan H, Madden C, Yu W, Sarode R. Emergency reversal of anticoagulation and antiplatelet therapies in neurosurgical patients. J Neurosurg. 2010;112:307–18. doi: 10.3171/2009.7.JNS0982. [DOI] [PubMed] [Google Scholar]
- 41.Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. American College ofChest Physicians. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):141S–159S. doi: 10.1378/chest.08-0689. [DOI] [PubMed] [Google Scholar]
- 42.Holland L, Warkentin TE, Refaai M, Crowther MA, Johnston MA, Sarode R. Suboptimal effect of a three-factor prothrombin complex concentrate (Profilnine-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion. 2009;49:1171–7. doi: 10.1111/j.1537-2995.2008.02080.x. [DOI] [PubMed] [Google Scholar]
- 43.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. FAST Trial Investigators. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–37. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 44.Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: A randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–9. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 45.Cox JL, Schuessler RB, Lappas DG, Boineau JP. An 8 1/2-year clinical experience with surgery for atrial fibrillation. Ann Surg. 1996;224:267–75. doi: 10.1097/00000658-199609000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarthy PM, Gillinov AM, Castle L, Chung M, Cosgrove D., 3rd The Cox-Maze procedure: The Cleveland Clinic experience. Semin Thorac Cardiovasc Surg. 2000;12:25–9. doi: 10.1016/s1043-0679(00)70013-x. [DOI] [PubMed] [Google Scholar]
- 47.Szalay ZA, Skwara W, Pitschner HF, Faude I, Klovekorn WP, Bauer EP. Midterm results after the Mini-Maze procedure. Eur J Cardiothorac Surg. 1999;16:306–11. doi: 10.1016/s1010-7940(99)00208-0. [DOI] [PubMed] [Google Scholar]
- 48.Sirak J, Jones D, Sun B, Sai-Sudhakar C, Crestanello J, Firstenberg M. Toward a definitive, totally thoracoscopic procedure for atrial fibrillation. Ann Thorac Surg. 2008;86:1960–4. doi: 10.1016/j.athoracsur.2008.07.066. [DOI] [PubMed] [Google Scholar]
- 49.Cheema A, Vasamreddy CR, Dalal D, Marine JE, Dong J, Henrikson CA, et al. Long-term single procedure efficacy of catheter ablation of atrial fibrillation. J Interv Card Electrophysiol. 2006;15:145–55. doi: 10.1007/s10840-006-9005-9. [DOI] [PubMed] [Google Scholar]
- 50.Wolf RK, Schneeberger EW, Osterday R, Miller D, Merrill W, Flege JB, Jr, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg. 2005;130:797–802. doi: 10.1016/j.jtcvs.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 51.Comas GM, Imren Y, Williams MR. An overview of energy sources in clinical use for the ablation of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2007;19:16–24. doi: 10.1053/j.semtcvs.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Ninet J, Roques X, Seitelberger R, Deville C, Pomar JL, Robin J, et al. Surgical ablation of atrial fibrillation with off-pump, epicardial, high-intensity focused ultrasound: Results of a multicenter trial. J Thorac Cardiovasc Surg. 2005;130:803–9. doi: 10.1016/j.jtcvs.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 53.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–9. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 54.Healey JS, Crystal E, Lamy A, Teoh K, Semelhago L, Hohnloser SH, et al. Left Atrial Appendage Occlusion Study (LAAOS): Results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005;150:288–93. doi: 10.1016/j.ahj.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 55.Ailawadi G, Gerdisch MW, Harvey RL, Hooker RL, Damiano RJ, Jr, Salamon T, et al. Exclusion of the left atrial appendage with a novel device: Early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011;142:1002–9. doi: 10.1016/j.jtcvs.2011.07.052. 1009 e1. [DOI] [PubMed] [Google Scholar]
- 56.Gillinov AM. Advances in surgical treatment of atrial fibrillation. Stroke. 2007;38(2 Suppl):618–23. doi: 10.1161/01.STR.0000247934.04848.79. [DOI] [PubMed] [Google Scholar]
- 57.Han FT, Kasirajan V, Wood MA, Ellenbogen KA. Minimally invasive surgical atrial fibrillation ablation: Patient selection and results. Heart Rhythm. 2009;6(12 Suppl):S71–6. doi: 10.1016/j.hrthm.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 58.Sick PB, Schuler G, Hauptmann KE, Grube E, Yakubov S, Turi ZG, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007;49:1490–5. doi: 10.1016/j.jacc.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 59.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet. 2009;374:534–42. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]


