Abstract
Background:
Coronary artery disease is mainly caused by atherosclerosis and its complications. Platelets and their activity have an important role in initiation of atherosclerotic lesions and coronary thrombus formation. Larger platelets are enzymatically and metabolically more active and have a higher potential thrombotic ability as compared with smaller platelets.
Aims:
To study the changes in platelet volume indices and platelet count in acute myocardial infarction, stable coronary artery disease and compare them with controls to assess their usefulness in predicting coronary events.
Materials and Methods:
This was a comparative study of 128 subjects; 39 patients with acute myocardial infarction (AMI), 24 patients with stable coronary artery disease (SCAD) and 65 controls. Venous sample were drawn from AMI subjects on admission (within 4 hours of chest pain) and collected in standardized EDTA sample tubes. Platelet count and volume indices were assayed within 30 minutes of blood collection, using Sysmex KX21-N autoanalyzer. Venous samples were also drawn from SCAD on who were admitted for angiography and subject attending routine checkups.
Results:
The mean platelet volume was significantly higher in patients with AMI (9.65 ± 0.96) as compared to SCAD (9.37 ± 0.88) and controls (9.21 ± 0.58). The best cut-off values for MPV when predicting AMI and SCAD in patients were 9.25 fl (sensitivity 56.4%; specificity 45.9%) and 9.15 fl (sensitivity 54.2%; specificity 42.23%), respectively.
Conclusions:
Measurements of MPV may be of some benefit in detecting those patients at higher risk for an AMI and CAD.
Keywords: Acute myocardial infarction, coronary artery disease, mean platelet volume
INTRODUCTION
Acute coronary syndromes (ACS) are a set of signs and symptoms due to the rupture of a plaque and are a consequence of platelet-rich coronary thrombus formation. The thrombus leads to partial or complete coronary artery occlusion, which, in turn, leads to myocardial ischemia and various clinical manifestations ranging from unstable angina (UA) to acute myocardial infarction (AMI).
Platelets are heterogeneous blood elements with diverse sizes and densities. Platelet activation is a hallmark of ACS. In addition to aggregation, platelets modulate important pathophysiological processes including inflammation and coagulation. It has been shown that platelet size, when measured as mean platelet volume (MPV), is a marker of platelet function and is positively associated with indicators of platelet activity. An increased MPV, an indicator of larger and more reactive platelets, has been associated with myocardial damage in ACS and has been found to be predictive of an unfavorable outcome among survivors of AMI.[1,2] Aggregation also depends on platelet count. There are reports that systemic inflammation plays a role in development and progression of coronary heart disease. Previous studies have documented ethnic differences in MPV level.[3–6] Association of higher MPV values with ACS has been mostly studied among Caucasian patients.[7] A few reports have revealed larger MPV values in Indian patients with ACS compared with healthy controls.[8] However, there are less reports in comparison with stable coronary artery diseases. We currently lack understanding of the predictive accuracy of MPV for spectrum of CAD. Our aim was to study MPV and other platelet volume indices (PVI) in AMI and stable CAD and compare them with age- and sex-matched controls and to find predictive value of MPV in spectrum of CAD.
MATERIALS AND METHODS
This hospital-based case control study was designed to assess whether MPV and other PVI show variation in the spectrum of CAD. The study protocol was approved by the Institutional Review Board of the hospital, and written inform consent was obtained from the patients. Sample size was calculated based on standard error obtained in pilot study. Sample size was calculated to allow detection of a 30% difference in MPV between different groups and with α of .05 and power of .80. Total 128 subjects were recruited and studied in three groups. Group 1A and 1B had patients of coronary artery disease and Group 2 had healthy controls. Group 1A had patients with AMI on admission. Group 1B had patients of coronary artery disease who had AMI at least 5 weeks prior and admitted for angiography without chest pain. Following patients were excluded from the study: Patients with severe hepatic or renal impairment, patients taking oral anticoagulation medicine (but Group 1B patients were thrombolysed and all were on anti-platelet therapy), myeloproliferative disorders and malignancy. The enrolment period was between September 2010 and April 2011.
In Group 1A patients, before administration of anti-coagulants and anti-platelet drugs, we collected blood samples within 6 hours on arrival at casualty into tubes containing EDTA who were subsequently diagnosed having AMI. For measurement of platelet count (PLC), mean platelet volume (MPV), platelet distribution width (PDW), platelet large cell ratio (P-LCR) and plateletcrit (PCT), samples were analyzed within 30 minutes after collection with Sysmex KX21-N automated flow meter. Blood samples of Group B were collected on the day of admission and were analyzed. Group C subjects came for routine check-up and their blood samples were collected in the outpatient department.
AMI was diagnosed based on the following criteria: detection of rise or fall in cardiac biomarker Trop I or CKMB with at least one value above 99th percentile of upper limit together with evidence of myocardial ischemia based on at least one of the following. 1) symptoms of ischemia, 2) ECG changes indicative of new ischemia, 3) development of pathological Q wave in the ECG, 4) imaging evidence of new loss of viable myocardium or a new regional wall motion abnormality. Group 1B patients were diagnosed based on the following criteria: Evidence of AMI at least 5 weeks prior to admission. Their case reports showed 1) development of pathological Q wave in the ECG and 2) imaging evidence of new loss of viable myocardium or a new regional wall motion abnormality.
DATA ANALYSIS
Results were presented as mean ± SD or frequency (percentage) as appropriate. One-way analysis was used for statistical analysis of categorical variables with comparisons of P < 0.05 between Group 1A, 1B and 2. Group 1A and Group 1B were included in CAD group (Group 1) and compared with control Group 2 using independent t test. To determine the accuracy and respective best cut-off values of MPV for predicting AMI/SCAD, the receiver operating characteristic (ROC) curves and their corresponding areas under the curve (AUC) were used. A P value of <0.05 was considered statistically significant. A common statistical package (SPSS 16.0) was used to perform all statistical tests.
RESULTS
During the 8 months of study, 128 individuals were selected for the study (17 females, 111 males). AMI was diagnosed in 39 patients (Group 1A). Total 24 patients who had AMI at least 5 weeks prior to getting admitted for angiography were enrolled (Group 1B); 65 individuals were selected from the outpatient department who were attending for routine check-up (Group 2).
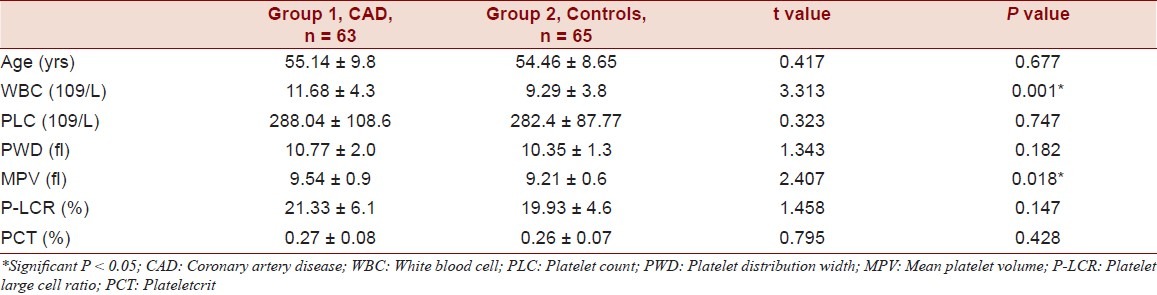
There was no significant difference between age and sex among the 3 groups. (Table 1) Increased MPV, PLC, P-LCR and PCT were observed in AMI Group compared to SCAD and control group (P 0.025). (Table 2) MPV levels were significantly raised where other platelet indices were not raised significantly. Increased MPV, PLC, P-LCR and PCT were observed in CAD group compared to control group. MPV levels were significantly raised (P = 0.018) whereas other platelet indices were not raised significantly. Significantly increased WBC count was observed in AMI compared to SCAD and control groups (P = 0.004). But, increase in platelet count was insignificant. ROC curve of MPV when predicting AMI in patients was constructed and AUC was found to be 0.620 (95% CI) statistically significant (P = 0.031). Additionally, AUC of the MPV in predicting SCAD in patients was 0.483 (95% CI) statistically insignificant (P = 0.800). The best cut-off values for MPV when predicting AMI and SCAD in patients were 9.25 fl (sensitivity 56.4%; specificity 45.9%) and 9.15 fl (sensitivity 54.2%; specificity 42.23%), respectively.
Table 1.
Comparison of platelet volume indices in all the cases
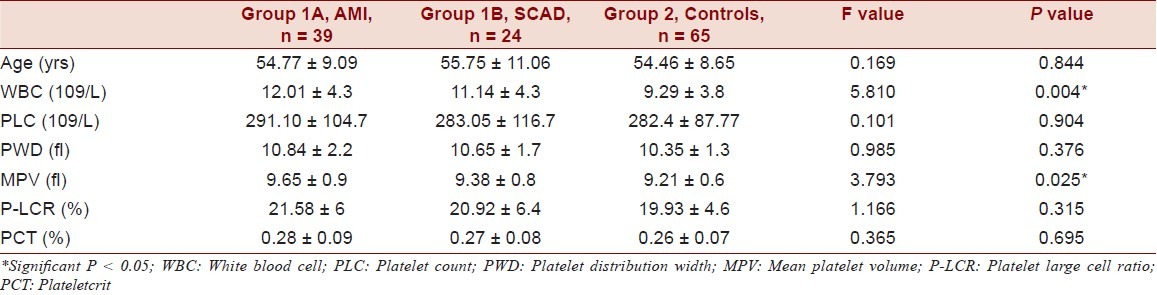
Table 2.
Comparison of platelet volume indices in CAD and controls
DISCUSSION
We observed increased platelet volume indices among AMI group compared to SCAD and controls. Specifically, MPV levels were significantly higher among patients with AMI compared to controls. The MPV comparison between these groups showed borderline significance. Future research with a larger sample size is needed to clarify this issue.
Our study has certain limitations. Sample size was small. The relatively low number of included subjects was due to the study design attempting to limit the influence of several co-variables. We could not measure Trop I and CKMB in all the patients, which can have predictive value in ACS so that we could have compared the predictive value of MPV. There are potential confounding factors of MPV. It has been shown that MPV values vary between different ethnicities; furthermore, medications and illness also influence this value. For example, obesity, smoking, aging and diabetes increase MPV values, but aspirin, clopidogrel and inflammatory bowel disease decrease MPV values.[9–13] Chu et al. observed stepwise decrease in MPV in subjects with chest pain in AMI, UA and non-cardiac chest pain.[14] Yilmaz et al observed a stepwise decrease in MPV between MI, UA and stable coronary artery disease among patients in Turkey.[15] Lippi et al. reported that Italian patients with ACS had significantly higher MPV values than patients without ACS.[16] Our results, like those of previous studies, demonstrated that MPV can be predictive of AMI, though it was not significant statistically for SCAD. However, in those studies, blood sampling was done either within 24 hours of onset of chest pain or at a time not specified except for the study by Chu et al. A few reports published have revealed a larger MPV in Indian patients with ACS compared with healthy controls or patients with stable coronary heart disease.[8] However, those studies are all retrospective reviews of laboratory data, and the time between blood sampling and ACS events is not specified. To our knowledge, we are the first in the literature to study this issue in Indian population that is, within 4 hours of onset of chest pain. Automated cell counters in modern hospital laboratories have made MPV measurement routinely available. Thus, this effortless laboratory test can be added value to diagnosis of spectrum of CAD.
The clinical implications of our findings are multiple. First, a multi-marker approach to the diagnosis of AMI, combining markers reflecting different pathophysiology, has been shown to be clinically helpful. Second, we have found that signs of platelet activation seem to occur as early as 6 hours after onset of chest pain in patients with AMI. MPV had been proven to be a prognostic factor for angiographic reperfusion and 6-month mortality in patients with AMI treated with primary percutaneous intervention.[17] Martin et al. have shown that the MPV, when measured 6 months after AMI, is an independent risk factor for recurrent MI.[18] The differences in MPV between those with MI and healthy controls have been found to persist after 6 weeks.[19] To substantiate these findings, we have also observed persistently increased MPV in Group 1B, that is, even after 5 weeks of myocardial infarction MPV was persistently high in our subjects. Taking all these findings together, MPV reflects an atherosclerothrombic tendency in the human body. Future studies including the use of MPV in a risk stratification system to predict MI or ACS as well as in response to intervention are worthy of consideration.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kiliçli-Camur N, Demirtunç R, Konuralp C, Eskiser A, Başaran Y. Could mean platelet volume be a predictive marker for acute myocardial infarction? Med Sci Monit. 2005;11:387–92. [PubMed] [Google Scholar]
- 2.Endler G, Klimesch A, Sunder-Plassmann H, Schillinger M, Exner M, Mannhalter C, et al. Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. Br J Haematol. 2002;117:399–404. doi: 10.1046/j.1365-2141.2002.03441.x. [DOI] [PubMed] [Google Scholar]
- 3.Farkouh ME, Smars PA, Reeder GS, Zinsmeister AR, Evans RW, Meloy TD, et al. A clinical trial of a chest-pain observation unit for patients with unstable angina. Chest Pain Evaluation in the Emergency Room (CHEER) Investigators. N Engl J Med. 1998;339:1882–8. doi: 10.1056/NEJM199812243392603. [DOI] [PubMed] [Google Scholar]
- 4.Schull MJ, Vermeulen MJ, Stukel TA. The risk of missed diagnosis of acute myocardial infarction associated with emergency department volume. Ann Emerg Med. 2006;48:647–55. doi: 10.1016/j.annemergmed.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Lee TH, Rouan GW, Weisberg MC, Brand DA, Acampora D, Stasiulewicz C, et al. Clinical characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room. Am J Cardiol. 1987;60:219–24. doi: 10.1016/0002-9149(87)90217-7. [DOI] [PubMed] [Google Scholar]
- 6.Puleo PR, Meyer D, Wathen C, Tawa CB, Wheeler S, Hamburg RJ, et al. Use of a rapid assay of sub forms of creatine kinase MB to diagnose or rule out acute myocardial infarction. N Engl J Med. 1994;331:561–6. doi: 10.1056/NEJM199409013310901. [DOI] [PubMed] [Google Scholar]
- 7.Bath PM, Butterworth RJ. Platelet size: Measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7:157–61. [PubMed] [Google Scholar]
- 8.Ranjith MP, Divya R, Mehta VK, Krishnan MG, KamalRaj R, Kavishwar A. Significance of platelet volume indices and platelet count in ischaemic heart disease. J Clin Pathol. 2009;62:830–3. doi: 10.1136/jcp.2009.066787. [DOI] [PubMed] [Google Scholar]
- 9.Demirtunc R, Duman D, Basar M. Effects of doxazosin and amlodipine on mean platelet volume and serum serotonin level in patients with metabolic syndrome: A randomised, controlled study. Clin Drug Investig. 2007;27:435–41. doi: 10.2165/00044011-200727060-00006. [DOI] [PubMed] [Google Scholar]
- 10.Coban E, Ozdogan M, Yazicioglu G, Akcit H. The mean platelet volume in patients with obesity. Int J Clin Pract. 2005;59:981–2. doi: 10.1111/j.1742-1241.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 11.Hekimsoy Z, Payzin B, Ornek T, Kandoğan G. Mean platelet volume in Type 2 diabetic patients. J Diabetes Complications. 2004;18:173–6. doi: 10.1016/S1056-8727(02)00282-9. [DOI] [PubMed] [Google Scholar]
- 12.Kario K, Matsuo T, Nakao K. Cigarette smoking increases the mean platelet volume in elderly patients with risk factors for atherosclerosis. Clin Lab Haematol. 1992;14:281–7. doi: 10.1111/j.1365-2257.1992.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 13.Kapsoritakis AN, Koukourakis MI, Sfiridaki A, Potamianos SP, Kosmadaki MG, Koutroubakis IE, et al. Mean platelet volume: A useful marker of inflammatory bowel disease activity. Am J Gastroenterol. 2001;96:776–81. doi: 10.1111/j.1572-0241.2001.03621.x. [DOI] [PubMed] [Google Scholar]
- 14.Chu H, Chen WL, Huang CC, Chang HY, Kuo HY, Gau CM, et al. Diagnostic performance of mean platelet volume for patients with acute coronary syndrome visiting an emergency department with acute chest pain: The Chinese scenario. Emerg Med J. 2011;28:569–74. doi: 10.1136/emj.2010.093096. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz MB, Cihan G, Guray Y, Guray U, Kisacik HL, Sasmaz H, et al. Role of mean platelet volume in triaging acute coronary syndromes. J Thromb Thrombolysis. 2008;26:49–54. doi: 10.1007/s11239-007-0078-9. [DOI] [PubMed] [Google Scholar]
- 16.Lippi G, Filippozzi L, Salvagno GL, Montagnana M, Franchini M, Guidi GC, et al. Increased mean platelet volume in patients with acute coronary syndromes. Arch Pathol Lab Med. 2009;133:1441–3. doi: 10.5858/133.9.1441. [DOI] [PubMed] [Google Scholar]
- 17.Huczek Z, Kochman J, Filipiak KJ, Horszczaruk GJ, Grabowski M, Piatkowski R, et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;46:284–90. doi: 10.1016/j.jacc.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 18.Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991;338:1409–11. doi: 10.1016/0140-6736(91)92719-i. [DOI] [PubMed] [Google Scholar]
- 19.Martin JF, Plumb J, Kilbey RS, Kishk YT. Changes in volume and density of platelets in myocardial infarction. Br Med J (Clin Res Ed) 1983;287:456–9. doi: 10.1136/bmj.287.6390.456. [DOI] [PMC free article] [PubMed] [Google Scholar]


