Abstract
Background:
Hyperhomocysteinemia has recently been identified as a risk factor for coronary artery disease. Some genetic variants (such as C677T polymorphism) are postulated in this regard. We studied the relation between hyperhomocysteinemia and the above genetic variant and risk of coronary artery disease (CAD) and also the number of involved vessels.
Materials and Methods:
From a total of 90 patients, 45 showed angiographically documented CAD and 45 had clinical manifestations of CAD but a negative angiography. Blood homocysteine level and C677T polymorphism were evaluated by Enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) respectively.
Results:
Homocysteine level was significantly higher in the case group (P < 0.001) but no correlation was found between its level and extent of CAD. More homozygote cases of C677T allele were detected in the case group which was not related to the extent of CAD either.
Conclusion:
Presence of hyperhomocysteinemia increases the risk of CAD but does not predict the extent of it.
Keywords: Hyperhomocysteinemia, genotype, risk factor
INTRODUCTION
It has been identified that elevated plasma homocysteine level is associated with a greater risk of coronary artery disease.[1,2] Molecular defects (C677T Polymorphism) in the methylen tetrahydrofolate reductase (MTHFR) gene might be associated with hyperhomocysteinemia.[2]
Homocysteine, produced as an intermediate product in methionine metabolism, is a sulfur-containing amino acid with three key enzymes contributing to its metabolism: cystathionine β synthase, methyltetrahydrofolate homocysteine methyltransferase, and MTHFR. Molecular defects in each of these enzymes including MTHFR may be associated with a high homocysteine level. An elevated homocysteine level is recently discovered as an independent risk factor for coronary artery disease and premature atherosclerosis.[1]
This study was performed to investigate the correlation between the elevated plasma homocysteine level and its genotypes as a risk factor for coronary artery disease and the extent of atherosclerotic changes (single, two- or three-vessel disease).
MATERIALS AND METHODS
On the basis of clinical manifestations or noninvasive test results that suspicious of coronary artery disease, we selected 90 patients (65 men and 25 women, with 25--78 years of age), who underwent coronary angiography. These patients were admitted to Ekbatan Hospital, Hamedan, Iran (between December 2008 and August 2009). To rule out the dependency of homocysteine level on renal failure, folic acid consumption, multivitamin therapy and postmenopausal hormone replacement therapy, these items were considered as exclusion criteria. Coronary angiography was performed in all subjects and according to the results of diagnostic coronary angiography, the subjects were divided into two groups (case and control). Subjects in the case group were divided into three subgroups on the basis of the number of stenotic coronary arteries. Indeed, case group was selected among the patients whose coronary arteries had 70% stenosis in one, two or three of left anterior descending, left circumflex, and right coronary arteries. Case and control groups were matched in terms of conventional risk factors of CAD.
Fasting venous blood sample was drawn, plasma homocysteine level was measured by ELISA and genotype was analyzed by PCR methods for all of the 90 enrolled patients. Plasma was immediately separated and stored at –20 °C until measurement of total homocysteine. Homocysteine was measured with an enzyme linked immunosorbent (ELISA) method (DRG Instruments GmbH, Marburg, Germany). The PCR process includes several steps: denaturation where DNA strands are melted and separated, annealing where the primers anneal to template DNA, and extension where the enzyme works to elongate the DNA strands. These steps are carried out 20–35 times in a thermal cycler to produce many replicates of the DNA template.
Then, we analyzed the distribution of C677T mutation in MTHFR gene as the most common hereditary risk factor leading to an elevated homocysteine level. The MTHFR is an important enzyme in the homocysteine metabolism and catalyzes the conversion of 5,10-methylenetetrahydrofolate into 5-methyltetrahydrofolate, the predominant circulating form of folate. Three MTHFR genotypes for C677T mutation (cc, tt, and ct) were assessed by PCR method. Thereafter, the relation between C677T genotype of MTHFR and severity of coronary artery disease was assessed by a linear trend test. Quantitative data were analyzed and expressed as mean ± standard deviation (SD).
All of the patients filled out a complete informed consent and the study was approved in all aspects by ethics committee of Hamedan University of Medical Sciences.
RESULTS
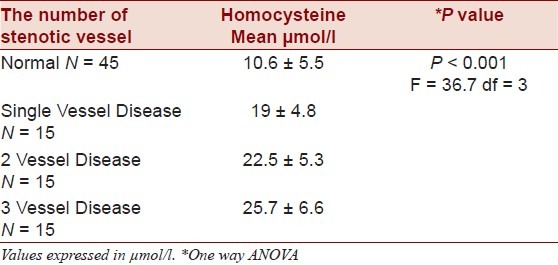
Plasma homocysteine level was 22.3 ± 6.1 and 10.6 ± 5.5 in case and control subjects, respectively. Mean homocysteine level in case group, was significantly higher than control group (one-way ANOVA method, P < 0.001). There was no significant difference between homocysteine level in three subgroups of cases in terms of the number of stenotic coronary arteries (P < 0.001, F = 36.7, df = 3, 19 ± 4.8, 22.5 ± 5.3, 25.7 ± 6.6, respectively) [Table 1].
Table 1.
The mean and SD of plasma Homocysteine levels in three subgroups of single vessel, two-vessel and three-vessel disease
The frequency of homozygote genotypes (cc, tt) in three subgroups of single vessel, two-vessel and three-vessel disease among 45 subjects of the case group were 38.2%, 35.3%, and 26.5 %, respectively, whereas the heterozygote genotype (ct) were 27.3%, 27.3%, and 45.5%, respectively.
Distributions of c allele in 45 case subjects in three subgroups in terms of the number of stenotic coronary arteries as single, two- and three-vessel disease were 38.6%, 35.7%, and 25.7%, respectively. When genotype frequency was compared among the patients with different numbers of stenotic coronary arteries, the frequency of homozygote genotypes was not significantly higher in patients with 3VD (26.5%) than in patients with single or two vessel disease (38.2% and 35.3%, respectively, P = 0.49) [Table 2].
Table 2.
The comparison of genotype's (cc, ct, tt) distribution in case group (in terms of the number of stenotic vessels)
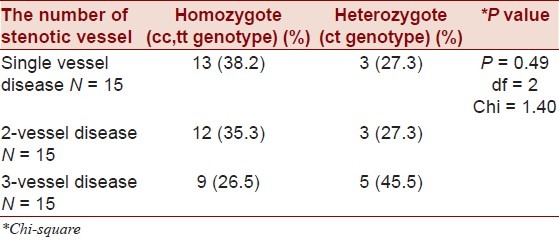
When homozygote genotype was compared with heterozygote in each affected subgroup, the majority of patients belonged to homozygote category (single vessel disease: 38.2% versus 27.5%, two-vessel disease: 35.3 versus 27.3%, three-vessel disease: 26.5% versus 45.5%).
Frequency of c allele in these three mentioned subgroups was 38.6%, 35.7% and 25.7% respectively, whereas frequency of t allele in these three mentioned subgroups was 33.3%, 33.3% and 13.2%, respectively.
DISCUSSION
The main purpose of this study was to investigate the role of elevated plasma homocysteine level as a risk factor for coronary artery disease and the most common genetic mutation (C677T in MTHFR gene) causing hyperhomocysteinemia. Our results confirmed that plasma homocysteine level was significantly higher in subjects with at least one stenotic coronary artery, which is comparable to that in Framingham's study.[3]
Molecular defects in genes encoding enzymes involved in homocysteine metabolism may account for hyperhomocysteinemia as an independent risk factor for coronary artery disease. The most common polymorphism (C677T) in the MTHFR gene might be associated with hyperhomocysteinemia and coronary artery disease in some populations. This form of study has not been performed in Iran.
Allele C677T – MTHFR homozygous individuals show a significantly higher risk for coronary artery disease.
Some previous studies demonstrated that elevated plasma homocysteine level was in a close association with premature atherothrombosis leading to cardiovascular events.[4] Recent studies showed that a high homocysteine level was an independent prognostic index for the development of atherosclerosis in dyslipidemic patients.[3–5]
Besides, hyperhomocysteinemia was recently believed to have a key role in premature atherosclerosis and also coronary artery disease.[1,2,4,6]
High homocysteine level is related to high blood pressure in the general population,[4,6] in diabetics,[7] and probably in the patients with multivessel disease.[8] It seems to be a positive correlation between plasma homocysteine level and other known modifiable risk factors of coronary artery disease, especially smoking and hypertension.[9–11]
According to the some studies elevated HC level was an independent risk factor for deep venous thrombosis, new stroke and abortion.[11–13]
Active management of hyperhomocysteinemia decreases the mortality rate, revascularization necessity and non-fatal myocardial infarction.[14,15]
Although the precise mechanism of hyperhomocysteinemia as an atherogenic factor was not elucidated, various in vitro studies have been proposed.[2]
Homocysteine has direct toxic effects on cultured endothelial cells which can be prevented by catalyses.[16–18]
Free radical production during hyperhomocysteinemia plays a major role in endothelial dysfunction.[19–21]
Furthermore, homocysteine induces cyclin D and cyclin A expression and stimulates vascular smooth muscle cells proliferation.
A large body of data links hyperhomocysteinemia and folate status with oxidant stress.[22] In addition to a moderate hyperhomocysteinemia observed in the coronary artery disease, a significant higher levels of the oxidized LDL (ox-LDL) were found among these patients.[23]
It also enhances endothelial cell associated factor 5 activity[24] and inhibits thrombomodulin surface expression,[25] protein C activation,[26] tissue-type plasminogen activator binding,[26] and anticoagulant heparin sulfate expression in endothelial cells. It also shows an increase in thromboxane A2 formation in platelets.
ACKNOWLEDGMENT
The authors would like to thank Farzan Institute for Research and Technology for technical assistance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Marinou K, Antoniades C, Tousoulis D, Pitsavos C, Goumas G, Stefanadis C. Homocysteine: A risk factor for coronary artery disease? Hellenic J Cardiol. 2005;46:59–67. [PubMed] [Google Scholar]
- 2.Morita H, Taguchi J, Kurihara H, Kitaoka M, Kaneda H, Kurihara Y, et al. Genetic polymorphism of 5,10- methylen tetrahydrofulate reductase (MTHFR)as a risk factor for coronary artery disease. Circulation. 1997;95:2032–6. doi: 10.1161/01.cir.95.8.2032. [DOI] [PubMed] [Google Scholar]
- 3.Bostom AG, Rosenberg H, Silvershat H. None fasting plasma total homocysteine incidence in elderly persons: The Framingham Study. Ann Intern Med. 1999;131:352–5. doi: 10.7326/0003-4819-131-5-199909070-00006. [DOI] [PubMed] [Google Scholar]
- 4.Meshino J. Prevention of heart disease in women, Folic acid and homocysteine. Dyn Chiropractic. 2003;21:04. [Google Scholar]
- 5.Ueland PM, Refsum H, Bradstterom L. Plasma homocysteine and cardiovascular disease. In: Francis RB Jr, editor. Atherosclerotic Cardiovascular disease, haemostasis, and endothelial function. New York: Dekker; 1992. pp. 183–236. [Google Scholar]
- 6.Kaufman J. Homocysteine and cardiovascular outcomes in kidney disease. Nephrol Rounds. 2004;2:1–7. [Google Scholar]
- 7.Chao CL, Kou TL, Lee YT. Effects of methionine -induced hyperhomocysteinemia on endothelium dependent vasodilatation and oxidative status in healthy adults. Circulation. 2000;101:485–90. doi: 10.1161/01.cir.101.5.485. [DOI] [PubMed] [Google Scholar]
- 8.Araki A, Sako Y. Plasma sulfhydryl containing amino acids in patients with cerebral infarction and in hypertensive subjects. Atherosclerosis. 1989;79:139–46. doi: 10.1016/0021-9150(89)90118-4. [DOI] [PubMed] [Google Scholar]
- 9.Refsum A, Ueland PM. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Mailnow MR, Levenson J, Giral P. Role of blood pressure, uric acid on plasma homocysteine concentration. Atherosclerosis. 1995;114:175–83. doi: 10.1016/0021-9150(94)05481-w. [DOI] [PubMed] [Google Scholar]
- 11.Agardeh CD, Agardeh E, Andersson A. Lack of association between plasma homocysteine levels and microangiopathy in type 1 diabetes mellitus. Scand J Clin Lab Invest. 1994;54:637–41. doi: 10.3109/00365519409087544. [DOI] [PubMed] [Google Scholar]
- 12.Aronow WS, Ahu G, Gutstein H. Increased plasma homocysteine is an independent predictor of new atherothrombotic brain infarction in older persons. Am J Cardiol. 2005;86:585–6. doi: 10.1016/s0002-9149(00)01025-0. [DOI] [PubMed] [Google Scholar]
- 13.Bostom AG, Shemin D, Verhoef F. Elevated fasting plasma homocysteine levels and cardiovascular disease outcomes in maintenance dialysis patients. Arterioscler Thromb Vasc Biol. 1997;17:2554–8. doi: 10.1161/01.atv.17.11.2554. [DOI] [PubMed] [Google Scholar]
- 14.Pancharutini N, Lewis CA, Sauberlich HE. Plasma homocyst(e)ine, folate, and vitamin B-12 concentration and risk for early onset coronary artery disease. Am J Clin Nutr. 1994;59:940–8. doi: 10.1093/ajcn/59.4.940. [DOI] [PubMed] [Google Scholar]
- 15.Wall RT, Harlan JM, Harker LA, Striker GE. Homocysteine -induced endothelial cell injury in vitro: A model for the study of vascular injury. Thromb Res. 1980;18:113–21. doi: 10.1016/0049-3848(80)90175-9. [DOI] [PubMed] [Google Scholar]
- 16.Starkebaum G, Harlan JM. Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine. J Clin Invest. 1986;77:1370–6. doi: 10.1172/JCI112442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudman NP, Hicks C, Lynch JF, Wilcken DE, Wang J. Homocysteine thiolactone disposed by human arterial endothelial cells and serum in vitro. Arterioscler Thromb. 1991;11:663–70. doi: 10.1161/01.atv.11.3.663. [DOI] [PubMed] [Google Scholar]
- 18.Aronow W. Homocysteine. The association with atherosclerotic vascular disease in older persons. Geriatrics. 2003;58:22–4. 27-8. [PubMed] [Google Scholar]
- 19.Chambers JC, Ueland PM, Obeid OA. Improved vascular endothelial function after oral B vitamins. Circulation. 2000;102:2479–83. doi: 10.1161/01.cir.102.20.2479. [DOI] [PubMed] [Google Scholar]
- 20.Chambers JC, Mc Gregor A, Jean-Marie J. Demonstration of rapid on set vascular endothelial dysfunction after hyperhomocysteinemia: An effect reversible with vitamin C therapy. Circulation. 1999;99:1156–60. doi: 10.1161/01.cir.99.9.1156. [DOI] [PubMed] [Google Scholar]
- 21.Rodgers GM, Kane WH. Activation of endogenous factor V by a homocysteine–induced vascular endothelial cell activator. J Clin Invest. 1986;77:1909–16. doi: 10.1172/JCI112519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman M. Hypothesis: Hyperhomocysteinemia is an indicator of oxidant stress. Med Hypotheses. 2011;77:1088–93. doi: 10.1016/j.mehy.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Nakbi A, Koubaa N, Ben Hamda K, Hammami S, Attia N, Boumiza R, et al. Association between oxidative stress parameters and inflammation markers according to the gravity of the acute coronary syndrome. Tunis Med. 2011;89:621–6. [PubMed] [Google Scholar]
- 24.Lentz SR, Sadler JE. Inhibition of thrombomodulin surface expression and protein c activation by the thrombogenic agent homocysteine. J Clin Invest. 1991;88:1906–14. doi: 10.1172/JCI115514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodgers GM, Conn MT. Homocysteine, an atherogenic stimulus, reduces protein C activation by arterial and venous endothelial cells. Blood. 1990;75:895–901. [PubMed] [Google Scholar]
- 26.Hajjar KA. Homocysteine-induced modulation of tissue plasminogen activator binding to its endothelial cell membrane receptor. J Clin Invest. 1993;91:2873–9. doi: 10.1172/JCI116532. [DOI] [PMC free article] [PubMed] [Google Scholar]


