Abstract
Rhabdomyolysis is an uncommon but life-threatening adverse effect of simvastatin therapy. A 73-year-old male on chronic simvastatin therapy received azithromycin for acute bronchitis. He presented with weakness of all extremities with a significant increase in creatinine phosphokinase levels and acute kidney injury. Simvastatin was stopped and supportive therapy with intravenous saline and bicarbonate was initiated. The serum creatinine and creatine phosphokinase returned to baseline in the next 7 days. Two months later, simvastatin was resumed without any recurrence of symptoms. Our case report highlights the rare description of rhabdomyolysis caused by a drug interaction between simvastatin with azithromycin.
Keywords: Azithromycin, cytochrome 3A4, rhabdomyolysis, simvastatin
INTRODUCTION
Therapy with 3-hydroxy-3-methylglutarlyl-coenzyme A reductase inhibitors (statins) is often limited by muscular adverse effects such as myalgia, elevated serum creatine phosphokinase (CPK), and acute kidney injury from rhabdomyolysis.[1] The majority of statins including simvastatin are metabolized by the cytochrome P (CYP) 3A4 enzymes and inhibition of these enzymes increase the serum concentration of statins and hence increase the risk of myopathy. Although macrolide antibiotics clarithromycin and erythromycin are metabolized by CYP3A4 and result in decreased clearance of statins and increased risk of rhabdomyolysis,[2] azithromycin weakly inhibits CYP3A4 enzymes.[2] However, coadministration of azithromycin with chronic simvastatin therapy in our patient led to rhabdomyolysis. We now explore the potential mechanisms for this interaction and suggest measures for prevention.
CASE REPORT
A 73-years-old obese Caucasian male with chronic kidney disease due to idiopathic interstitial nephritis, severe gout, polymyalgia rheumatica, type 2 diabetes mellitus, hypertension, and hyperlipidemia was admitted with severe weakness and pain in his arms and legs for 5 days. His regular medications included allopurinol 100 mg daily, prednisone 5 mg daily, labetalol, bumetanide, amlodipine, simvastatin 80 mg daily, and glargine insulin. One week earlier oral azithromycin 500 mg on the first day followed by 250 mg daily for the next 4 days was given to treat acute bronchitis. He developed acute kidney injury with a serum creatinine of 3.8 mg/dl (baseline 1.7 mg/ dl), blood urea nitrogen of 200 mg/dl (baseline 50 mg/dl), and rhabdomyolysis with a CPK level of 11,240 u/L (reference range 0–310 u/L). Simvastatin was immediately discontinued. He received intravenous hydration with saline and bicarbonate to alkalinize the urine to a pH between 7 and 8. Serum creatinine returned to baseline in parallel with the CPK over the next 7 days without hemodialysis. The marked muscle weakness improved and returned to baseline in 3 weeks. Two months after the onset of symptoms, he resumes on simvastatin 40 mg daily initially and later increased to 80 mg daily without recurrence of myalgia or weakness confirming the temporal association of the drug interaction between simvastatin and azithromycin.
DISCUSSION
The true incidence of statin-induced rhabdomyolysis caused by drug interactions remains elusive since these interactions are reported on a voluntary basis.[3] Rowan et al.[3] used the adverse effect reporting system of the Food and Drug Administration Medwatch to calculate interactions between simvastatin and CYP3A4 inhibitors. A total of 118 cases from 1991 to July 2001 were reported with a rate of 38.4 cases per 10 million prescriptions of simvastatin.[3] Strandell et al. reviewed the World Health Organization Adverse drug reaction database (Vigibase) and reported four cases of rhabdomyolysis in patients receiving azithromycin with simvastain (overlap in one case), or with atorvastatin (six cases, 4 overlap), or with cervistatin (two cases).[2]
The risk of statin-induced myopathy is increased by advanced age, diabetes mellitus, low body mass index, hypothyroidism, alcohol consumption, and concurrent use of other medications that may alter the pharmacokinetics of statins. Indeed, rates seem to best correlate with higher statin doses and higher plasma concentrations.[4] After near complete absorption into portal blood, lipophillic statins (especially simvastatin) go through a high first pass effect. Statins enter hepatocytes by active transporters such as organic anion transporting polypeptide (OATP). In the hepatocyte [Figure 1], most statins except pravastatin (only partly metabolized by CYP3A4) may either be metabolized by the CYP enzymes[5] (simvastatin and lovastatin by CYP3A4 and 3A5, atorvastatin by CYP3A3 and CYP3A4, and fluvastatin by CYC2C9) or secreted into the bile by multiple efflux transporters such as multidrug resistance protein 1 (P glycoprotein), multidrug resistance associated protein 2 (MRP2), and breast cancer resistance protein (BCRP).[5]
Figure 1.
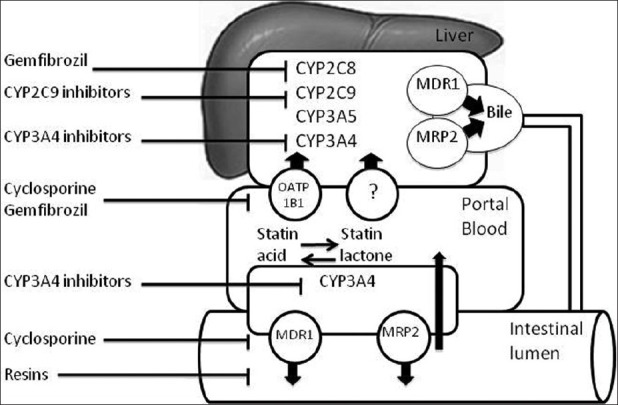
Schema of pharmacokinetics of Statins. CYP: cytochrome, MDR1: multidrug resistance protein 1 (P-glycoprotein), MRP2: multidrug resistance associated protein 2, OATP: organic anion transporting polypeptide
Potent inhibitors of CYP3A4 (including clarithromycin and erythromycin) cause frequent clinically significant interactions [Table 1]. Azithromycin, a newer subclass of macrolide antibiotic, due to insertion of an extra nitrogen atom,[6] interferes weakly with CYP3A4 in vitro. In vivo, it is reported to cause rhabdomyolysis with lovastatin,[1] simvastatin,[2] atorvastatin,[2] and cervistatin.[2] Polymorphism of CYP3A4 isoenzymes can account for up to a 10–20 fold interindividual variablility in CYP3A4 catalytic activity and this offers a possible explanation for variable inhibition of these enzymes by azithromycin. In an in vivo model of cynomolgus monkeys, administration of midazolam (another CYP3A4 substrate) with multiple doses of azithromycin (15 mg/kg) on days 1–3 increased area under the plasma concentration–time curve of midazolam 2.0 fold.[7] Moreover, polymorphisms of the CYP3A5 gene may also affect interindividual variability of simvastatin disposition. In young healthy adults,[8] mean area under the plasma concentration curve over time for simvastatin in CYP3A5*1/*1 carriers (4.94 +/- 2.25 ng × h/mL) was significantly lower than CYP3A5*3/*3 carriers (16.35 +/- 6.37 ng × h/mL; P = 0.013).[8]
Table 1.
The commonly used substrates, inhibitors, and inducers of CYP3A4 enzyme systems (top) and P-glycoprotein transport protein (bot tom)
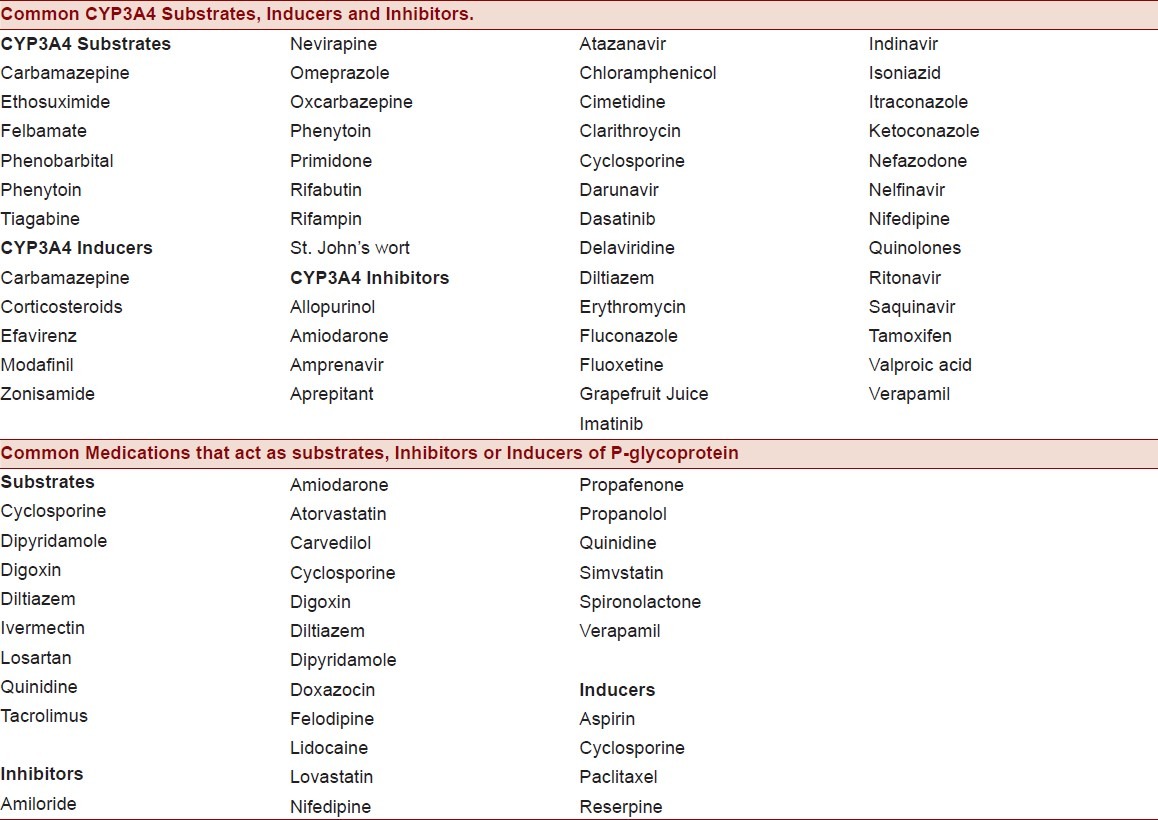
Azithromycin may also inhibit P-glycoprotein mediated drug disposition into bile. Indeed, azithromycin was strongly associated (odds ratio 3.71) with development of digoxin toxicity[9] in a population-based study. These findings were consistent with the earlier work of Eberl and colleagues,[10] who studied the inhibitory potential of macrolides (including azithromycin) in an in vitro model of P-glycoprotein-mediated digoxin transport using Caco-2 cells. This is especially important since similar to digoxin, simvastatin is a high-affinity substrate for multidrug efflux transporter P-glycoprotein, and efflux inhibition by azithromycin increases the risk of simvastatin toxicity by reducing energy-dependent simvastatin transport from enterocytes into the intestinal lumen (effectively increasing bioavailability). Similarly, azithromycin inhibits the hepatobiliary excretion of drugs that are substrates for P-glycoprotein and MRP2.[11]
OATP1B1 is a genetically polymorphic influx transporter expressed on the sinusoidal membrane of human hepatocytes and mediates hepatic uptake of xenobiotics. A common single-nucleotide variation (coding DNA c.521T > C, protein p. V174A, rs4149056) in the SLCO1B1 gene encoding OATP1B1 decreases the transporting activity of OATP1B1, resulting in markedly increased plasma concentrations of many statins, particularly of active simvastatin acid (3.2 fold) enhancing the risk of statin-induced myopathy.[12]
In our patient, several mechanisms may have played a role. The high dose of simvastatin (80 mg) along with azithromycin may have predisposed him to rhabdomyolysis due to one or more polymorphisms of the hepatic enzymes/transporters and/or inhibition of P-glycoprotein and MRP-2 proteins. The temporal association of the onset of rhabdomyolysis early after the use of azithromycin in our patient and the subsequent re-exposure of our patient to simvastatin for 1.5 years without rhabdomyolysis confirms the clinically significant drug interaction between these two agents. Our study underscores the recent FDA alert to ban the 80 mg dose of simvastatin unless patients have been already using it for 1 year or more. Since the SHARP[13] trial studying ezetimibe with simvastatin showed reduction in major atherosclerotic events, the number of patients with chronic kidney disease prescribed simvastatin will likely increase. We suggest caution while using azithromycin with simvastatin, as there are no worldwide safety data available. In addition, while co-administering azithromycin with other P-glycoprotein substrates [Table 1], patients should be carefully monitored for unexpected muscle weakness, pain, tenderness and fatigue.
CONCLUSION
Our patient experienced rhabdomyolysis associated with a drug interaction between azithromycin and simvastatin. His underlying chronic kidney disease, advanced age, and high dose of the simvastatin likely predisposed him to myopathy. Although azithromycin remains the safest choice among macrolides in patients using statins, life-threatening complications may occur including severe rhabdomyolysis and acute kidney injury. Patients using both drugs should be warned of this interaction and carefully monitored for signs of muscle toxicity.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gruden JW, Fisher KA. Lovastatin induced rhabdomyolysis possibly associated with clarithromycin and azithromycin. Ann Pharmacother. 1997;31:859–63. doi: 10.1177/106002809703100710. [DOI] [PubMed] [Google Scholar]
- 2.Strandell J, Bate A, Hägg S, Edwards IR. Rhabdomyolysis a result of azithromycin and statins: An unrecognized interaction. Br J Clin Pharmacol. 2009;68:427–34. doi: 10.1111/j.1365-2125.2009.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowan C, Brinker AD, Nourjah P, Chang J, Mosholder A, Barrett JS, et al. Rhabdomyolysis reports show interaction between simvastatin and CYP 3A4 inhibitors. Pharmacoepidemiol Drug Saf. 2009;18:301–9. doi: 10.1002/pds.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krum H, Marin J. Cytochrome P 450 interactions within the HMG-CoA reductase inhibitor class: Are they clinically relevant? Drug Saf. 2003;26:13–21. doi: 10.2165/00002018-200326010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Asberg A. Interaction between cyclosporine and lipid lowering drugs. Implications for organ transplant recipients. Drugs. 2003;63:367–78. doi: 10.2165/00003495-200363040-00003. [DOI] [PubMed] [Google Scholar]
- 6.Amacher DE, Schomaker SJ, Retsema JA. Comparison of the effects of new azalide antibiotic, azithromycin, and erythromycin estolate on rat liver cytochrome P-450. Antimicrob Agents Chemother. 1991;35:1186–90. doi: 10.1128/aac.35.6.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogasawara A, Negishi I, Kozakai K, Kume T. In vivo evaluation of drug-drug interaction via mechanism-based inhibition by macrolide antibiotics in cynomolgus monkeys. Drug Metab Dispos. 2009;37:2127–36. doi: 10.1124/dmd.109.028969. [DOI] [PubMed] [Google Scholar]
- 8.Kim KA, Park PW, Lee OJ, Kang DK, Park JY. Effect of polymorphic CYP3A5 genotype on the single-dose simvastatin pharmacokinetics in healthy subjects. J Clin Pharmacol. 2007;47:87–93. doi: 10.1177/0091270006295063. [DOI] [PubMed] [Google Scholar]
- 9.Gomes T, Mamdani MM, Juurlink DN. Macrolide-induced digoxin toxicity: A population-based study. Clin Pharmacol Ther. 2009;86:383–6. doi: 10.1038/clpt.2009.127. [DOI] [PubMed] [Google Scholar]
- 10.Eberl S, Renner B, Neubert A, Reisig M, Bachmakov I, König J, et al. Role of p-glycoprotein inhibition for drug interactions: Evidence from in vitro and pharmacoepidemiolo-gical studies. Clin Pharmacokinet. 2007;46:1039–49. doi: 10.2165/00003088-200746120-00004. [DOI] [PubMed] [Google Scholar]
- 11.Asakura E, Nakayama H, Sugie M, Zhao YL, Nadai M, Kitaichi K, et al. Azithromycin reverses anticancer drug resistance and modifies hepatobiliary excretion of doxorubicin in rats. Eur J Pharmacol. 2004;484:333–9. doi: 10.1016/j.ejphar.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: A genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63:157–81. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- 13.Sharp Collaborative Group. Study of Heart and Renal Protection (SHARP): Randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J. 2010;160:785–94. doi: 10.1016/j.ahj.2010.08.012. [DOI] [PubMed] [Google Scholar]


