Abstract
We present a case of vascular purpura revealing an intra-cardiac left-sided thrombus complicating an end-stage dilated cardiomyopathy. Vascular purpura main etiologies encompass the wide specturm of vasculitides and microvascular-occlusion syndromes. Among them, cardiac embolism represents an unusal but potentially severe etology.
Keywords: Cardiogenic shock, cardiomyopathy, dilated, leukocytoclastic, purpura, vasculitis
INTRODUCTION
With an estimated prevalence of 1:2500, dilated cardiomyopathy (DCM) is the third most common cause of heart failure and the most frequent cause of heart transplantation. DCM is the most common subtype of cardiomyopathy, according to a recent classification.[1] The onset of DCM is often progressive; however, it might also be revealed by acute complications resulting from cardioembolic events.[1]
Here, we present a case of vascular purpura revealing an intracardiac left-sided thrombi complicating end-stage DCM. Vascular purpura main etiologies encompass a wide spectrum of vasculitides and microvascular occlusion syndromes. Among them, cardiac embolism represents an unusual but potentially severe etiology.
CASE REPORT
A 48-year-old patient was admitted for disseminated vascular purpuric lesions. His medical history included diabetes mellitus and hypertension. He was a nonsmoker and reported alcohol consumption of 30 g per day.
One month before admission, he had consulted for cough and chest pain without fever. A chest X-ray revealed a left upper lobe infiltrate. He received an antibiotic treatment for 2 weeks with no clinical and radiological improvement. NYHA class IV dyspnea and lower limb edemas progressively appeared. He subsequently developed painless rapidly extending purpuric lesions of lower limb extremities. Upon hospital admission, his blood pressure was 95/78 mmHg, heart rate 121/min, temperature 36.3°C, and SpO2 100%. There was no focal deficit or meningeal syndrome. Skin examination revealed extensive purpuric and infiltrated lesions spreading over the four limbs, with a predominant distal topography. The feet, ankles, and legs were more severely involved with bullous lesions leading to rapidly extensive and painful necrosis [Figure 1].
Figure 1.
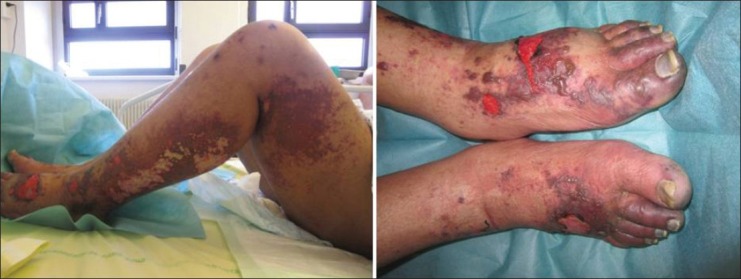
Extensive infiltrated and necrotic purpura of a leg. Note the peripheral edema due to congestive heart failure
Cardiological exam revealed signs of congestive heart failure including lung crackles, jugular venous distension, hepatojugular reflux, hepatomegaly, and lower limbs edema. EKG was normal. Chest X-ray still showed infiltrate in the left upper lobe [Figure 2a]. Laboratory tests showed mild anemia (Hemoglobin 10.4 g/dL), normal renal function (glomerular filtration rate 65 mL/min), mild hepatic cytolysis (SGOT = 2N), mixed hyperbilirubinemia (total bilirubin 34 μmol/L) without cholestasis, increased NT-proBNP (6737 ng/L), and negative troponin T. Blood coagulation tests were normal. Blood cultures were negative. Auto-immunity tests, including antinuclear factors, antineutrophil cytoplasmic antibodies, antiphospholipid antibodies, and cryoglobulinemia, were all negative. A broad spectrum empiric antibiotic therapy was promptly initiated as purpura fulminans and infective endocarditis were suspected.
Figure 2.
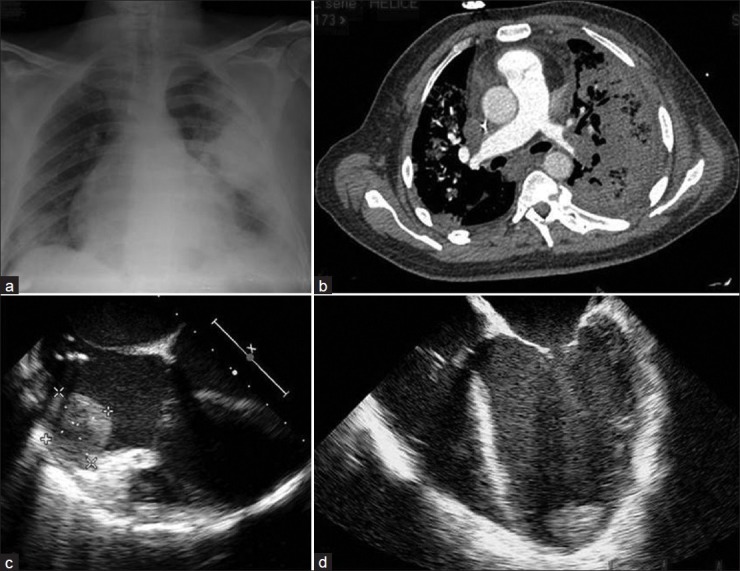
(a) Radiologic cardiomegaly is associated with a focal left upper lobe infiltrate. (b) CT-scan angiography revealing a pulmonary embolism with lung infarction. (c and d) Echocardiography image displaying two large left ventricular thrombi
Two cutaneous biopsies revealed numerous thrombi inside the postcapillary venules of the dermis. One of the specimen [Figure 3a] showed cutaneous infarction involving the epidermis. In hypoderma, arteriolis were free of vasculitis and thrombi. The other [Figure 3b] exhibited characteristic findings of a leucocytoclastic vasculitis, with infiltration of the perivascular zone with degenerating neutrophils, edema fluid, and fibrin deposits. Direct immunofluorescence was negative in all specimens.
Figure 3.
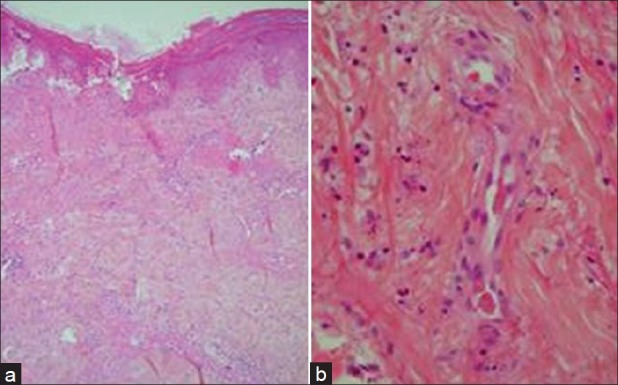
H and E staining of two skin biopsy specimens. The first specimen (a) left side, magnification ×10 shows a cutaneous infarction involving the epidermis. In hypoderma, arterioli were free of vasculitis and thrombi. On the second one (b) right side, magnification ×40, a perivascular infiltration by degenerating neutrophils is associated with edema fluid and fibrin deposits within the vessel, consistent with leucocytoclastic vasculitis
Shock with encephalopathy, oliguria, and lactic acidon's occurred 12 h following hospital admission, and required ICU referral. A transthoracic and a trans-esophageal echocardiography were performed and revealed a severe DCM with global hypokinesia, very low left ventricle ejection fraction (measured at 15%), and low cardiac output. A large left ventricular apical thrombus, together with a right atrial thrombus, was evidenced [Figures 2c and d]. Coronarography was normal. A chest CT scan angiography revealed bilateral proximal pulmonary embolism and alveolar consolidation of the upper and the lower pulmonary lobes compatible with lung infarction [Figure 2b].
Mental status deterioration led to intubation and mechanical ventilation. Lactic acidosis (lactatemia = 10 mmol/L) worsened despite increasing doses of vasopressors. Central extra-corporeal membrane oxygenation (ECMO) was implanted due to refractory cardiogenic shock. Hemodynamics and oxygenation were immediately improved. However, heart function failed to improve after ECMO implantation. A streptococcal bloodstream infection occurred 5 days after. The patient died on day 15 following hospital admission.
DISCUSSION
We report an exceptional observation of extensive vascular purpura revealing an end-stage DCM complicated by multiple systemic emboli. Purpura fulminans and antiphospholipid syndrome were rapidly ruled out due to negativity of blood cultures and autoantibodies. Autoimmunity tests were not in favor of a systemic vasculitis. Diagnostic overlap between vasculitis and heterogenous disorders capable of simulating similar skin lesions[2] might explain that systemic embolisms as well as in situ thrombotic disorders can be revealed by rapidly extensive purpura mimicking cutaneous vasculitides.[3,4]
Cutaneous microembolizations from intracardiac thrombi was the final diagnosis; the left-sided thrombi spread into the systemic circulation and led to more distal embolisms, in contrast with a right-sided thrombi resulting in pulmonary embolisms with lung infarction. The etiology of DCM remained unsettled in this case since genetic tests were not performed. DCM might have been favored by an excessive chronic alcohol consumption. In contrast, hypertension and diabetes unlikely contributed to this patient's heart disease, given the lack of left ventricle hypertrophy and the absence of an ischemic heart disease.
The association between heart disease and cutaneous vasculitis has first been described in atheromatous patients, with numerous descriptions of cholesterol embolization syndrome.[5–7] In the current case, however, coronary arteries were normal, and no cholesterol plaques were found on CT scan, suggesting that only the severe DCM and associated intracardiac thrombi were responsible for the vascular purpura.
The onset of DCM is often insidious, presenting with progressive dyspnea and peripheral edemas. Acute complications such as cardiogenic shock, sudden death, and arrhythmias can also be observed although less frequently. Cardioembolic events, including cerebral stroke and peripheral arterial embolism, are common and usually result in the occlusion of medium and large arteries. Distal microembolization mimicking leukocytoclastic vasculitis is a much more uncommon presentation of cardiac embolism.
Our observation suggests that vascular purpura resulting from systemic microembolization of an intracardiac thrombus can be an unusual presentation of end-stage DCM. Early diagnosis is of critical importance owing to the need for a specific cardiac management, which may include an anticoagulant treatment. A trans-thoracic echocardiography should be routinely considered in patient presenting with a nonthrombocytopenic purpura, once the most common etiologies have been ruled out.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: A position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–6. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 2.Carlson JA, Chen KR. Cutaneous pseudovasculitis. Am J Dermatopathol. 2007;29:44–55. doi: 10.1097/01.dad.0000245195.35106.3c. [DOI] [PubMed] [Google Scholar]
- 3.Grau R. Pseudovasculitis: Mechanisms of vascular injury and clinical spectrum. Curr Rheumatol Rep. 2002;4:83–9. doi: 10.1007/s11926-002-0028-7. [DOI] [PubMed] [Google Scholar]
- 4.Lie JT. Vasculitis simulators and vasculitis look-alikes. Curr Opin Rheumatol. 1992;4:47–55. [PubMed] [Google Scholar]
- 5.Chesney TM. Atheromatous embolization to the skin. Am J Dermatopathol. 1982;4:271–3. doi: 10.1097/00000372-198206000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Harland CC, Kilby PE. Vasculitis in association with atherosclerosis. Br J Dermatol. 1992;126:90–1. doi: 10.1111/j.1365-2133.1992.tb08413.x. [DOI] [PubMed] [Google Scholar]
- 7.Alegre VA, Winkelmann RK. Necrotizing vasculitis and atherosclerosis. Br J Dermatol. 1988;119:381–4. doi: 10.1111/j.1365-2133.1988.tb03232.x. [DOI] [PubMed] [Google Scholar]


