Abstract
Background & objectives:
Extensively drug resistant tuberculosis (XDR-TB) has become a new threat for the control of TB in many countries including India. Its prevalence is not known in India as there is no nation-wide surveillance. However, there have been some reports from various hospitals in the country.
Methods:
We have reviewed the studies/information available in the public domain and found data from 10 tertiary care centres in 9 cities in India.
Results:
A total of 598 isolates of XDR Mycobacterium tuberculosis have been reported in the studies included. However, the reliability of microbiological methods used in these studies was not checked and thus the XDR-TB data remained invalidated in reference laboratories.
Interpretation & conclusions:
Systematic surveillance and containment interventions are urgently needed.
Keywords: Extensively drug-resistant tuberculosis, India, MDR-TB, XDR-TB
The term ‘extensively drug-resistant tuberculosis’ was coined in 2006 by scientists of the Centers for Disease Control and Prevention (CDC), USA, based on the World Health Organization's (WHO) guidelines for management of drug-resistant tuberculosis (TB)1. Multidrug-resistant tuberculosis (MDR-TB) is caused by Mycobacterium tuberculosis resistant to rifampicin and isoniazid, the two main first line antimicrobials2. When MDR M. tuberculosis has additional resistance to a fluroquinolone and a second line injectable antibiotic (i.e. amikacin, kanamycin or capreomycin), it is designated extensively drug-resistant (XDR)1,2. Although clinical treatment failure is indicative of drug resistance, the diagnosis of MDR- and XDR-TB requires the isolation of bacterium and antimicrobial drug susceptibility testing (DST). Therefore, the probability and sensitivity of XDR-TB case-detection in a community are dependent on the coverage and quality of microbiological support services for the management of TB.
Against this backdrop it is very important to know where India stands with the emergence of XDR-TB, in terms of both the burden and the geographic spread. WHO has recognized 58 countries, including India, in which XDR-TB has been detected3. The number of XDR-TB formally reported by India's Revised National TB Control Programme (RNTCP) to WHO is just one4. That particular case was actually detected sometime between 1999 and 2003 in Chennai (Tamil Nadu State)4. Currently surveys are under way in Ahmedabad (Gujarat State) and Chennai to measure the frequency of XDR organisms among MDR5.
Since 2006 there have been many papers published in peer reviewed journals from both public and private sector institutions with their data on XDR-TB. These data have been generated from their mycobacteriology laboratories that have been performing 1st and 2nd line mycobacterial DST for many years. Though accreditation is available for 1st line mycobacterial DST, there is none currently available for 2nd line mycobacterial DST. Therefore, the reported XDR-M. tuberculosis isolates in India have not been validated. However, since a large number of such reports have been published in peer-reviewed journals, there is a need to take the situation seriously for its public health implications.
Material & Methods
Our strategy was to search PubMed database and use Google search engine to identify papers qualifying the terms ‘extensively drug-resistant tuberculosis’ or ‘XDR tuberculosis’ and ‘India’. In addition, the data presented at the Round Table Conference on “Challenges of MDR/XDR Tuberculosis in India”, organized by the Ranbaxy Science Foundation in New Delhi, on December 12, 2008 were also included6. Annual Report of Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, Kerala, was also searched for information on the number of XDR-TB isolates detected as work on molecular biology of MDR and XDR-TB was being done at this Centre7.
Results
A total of 16 publications (14 papers, one meeting abstract and one institutional annual report) were identified (Table). Even though the term XDR was coined only in 2006, M. tuberculosis conforming to the definition of XDR had been detected in isolates obtained during the years from 1997 to 2007 as shown in Fig. 1. However, the reports were published since 2006.
Table.
Number of MDR M. tuberculosis isolates and number and proportion of XDR isolates, India.
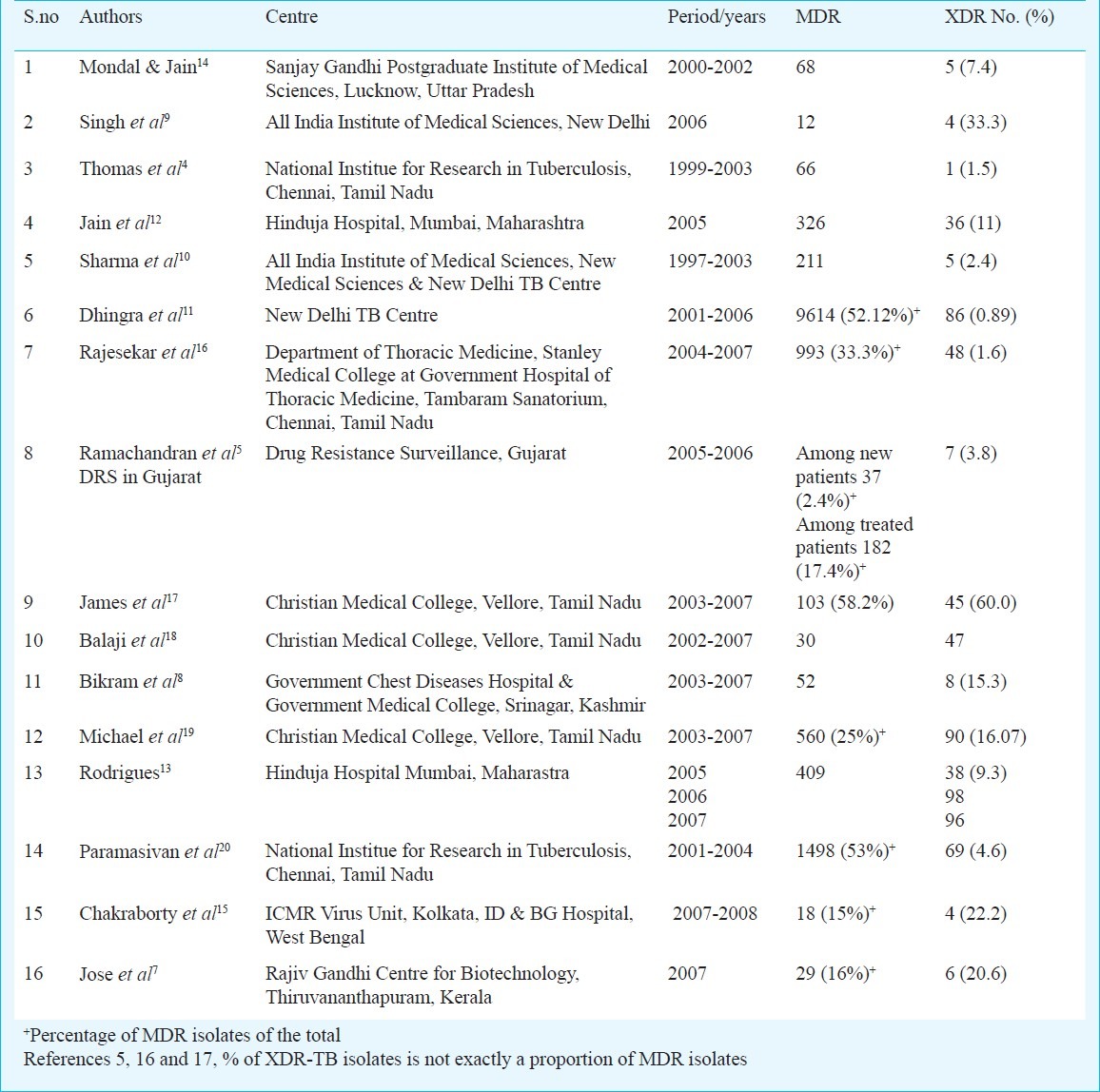
Fig. 1.
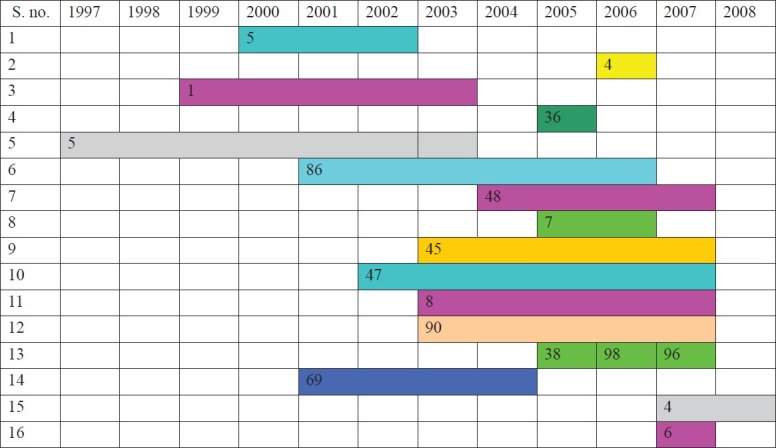
The years during which M. tuberculosis isolates were screened for drug sensitivity and MDR and XDR isolates were detected. Information on the exact year(s) in which XDR isolates were identified was not available in the papers from most centres. The numbers indicate the reported XDR M. tuberculosis isolates.
The geographic distribution of centres that have reported XDR-TB is shown in Fig. 2. These are in the States of Kashmir8, Delhi9–11, Maharashtra12,13, Uttar Pradesh14, West Bengal15, Gujarat5, Tamil Nadu4,16–20 and Kerala7.
Fig. 2.
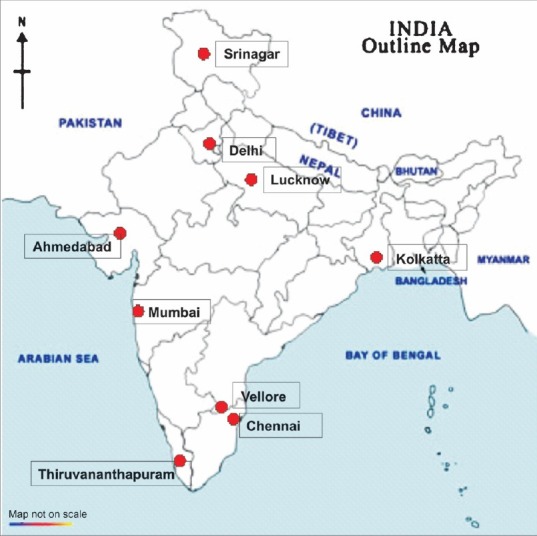
Map of India showing the location of nine cities from where the publications on XDR-TB have emerged. The circles are placed at approximate geographic locations. Except in Delhi with two centres, every other city had only one centre reporting XDR-TB. In Tamil Nadu State there are two centres, Chennai and Vellore.
The total number of cases of XDR-TB reported in these 16 publications was 694. There were three reports from Vellore (Tamil Nadu); two reported 45 and 47 cases with focus on clinical aspects17,18 and one reported 90 isolates from the microbiology laboratory19. Since we did not attempt to screen clinical case records to count the number of overlapping cases, we assume that the microbiology laboratory report would have included the clinical cases. Similarly there were two reports from New Delhi TB centre10,11 with overlapping dates, with the possibility of duplication of 2-4 cases. After discounting all potential duplications a total of 598 cases of XDR-TB have been documented.
This review covered all available sources of information on XDR-TB, but not all reports showed the proportion of XDR organisms among MDR ones9,18,21. Thus, in some reports the MDR organisms appeared to be under-represented in comparison with the numbers of reported XDR organisms18.
Discussion
There were limitations in our approach to describe the distribution of XDR-TB. We did not explore the reliability of microbiological methods in various laboratories. The microbiological diagnosis of XDR-TB data included here remain not validated by reference laboratories. National Institute for Tuberculosis Research (NITR) in Chennai is RNTCP's Supranational Reference Centre (designated by WHO) and it participates in international external quality assessment for microscopy, culture and DST22. In turn, NITR monitors the quality of laboratories in the country on behalf of RNTCP. Two laboratories that reported XDR-TB (New Delhi TB Centre and Christian Medical College at Vellore) are accredited by RNTCP for culture and DST. The Vellore laboratory was the nodal centre for the in-country external quality assessment scheme of the Indian Association of Medical Microbiologists22. Hinduja Hospital, Mumbai, is accredited by College of American Pathologists13.
These reports have originated from 10 tertiary care centres in nine cities, distributed widely in the country. Therefore, the data have no representative value for epidemiological assessment. Many hospitals that diagnose and treat TB may use the recommended sputum smear-examination and/or chest X-ray without culturing the bacterium. Many laboratories with culture facilities for M. tuberculosis may not conduct DST even for first line anti-TB drugs to diagnose MDR-TB. When MDR organisms are detected, DST for second line drugs is unlikely to be conducted, being cumbersome and expensive. Thus the true prevalence of XDR-TB remains unknown.
The diagnosis of XDR-TB has enormous implications both for the individual and the community. The choice of anti-TB drugs is limited and the ones available are too expensive and too reactogenic24. Treatment outcome is mostly disappointing and case-fatality rate (CFR) is very high - in one report the CFR was 51 per cent within a month of diagnosis25. When transmitted to new hosts the organisms will remain XDR, hence progressive primary TB and eventual secondary TB (after the period of latent infection), if occur, will inevitably be due to XDR-TB. Therefore, there is an urgent need to prevent secondary transmission at all costs, barring the curtailment of human rights beyond ethical limits. The tension between individual freedom of movement in society and public health need for its restriction has to be addressed and resolved. These considerations call for urgent national policy and guidelines and innovative design for early diagnosis and case management of XDR-TB.
A national registry of XDR-TB will allow every institution to report cases as soon as they are detected. The bacilli isolated from each case should be collected and verified in a reference laboratory. Therefore, a number of reference laboratories should be established and networked so that the facility is readily accessible. A treatment protocol should be designed and applied under RNTCP supervision on every diagnosed case of XDR-TB. While on treatment, precautions necessary to prevent transmission to members of family and to healthcare workers in contact should be applied. The elements of such precautions need to be urgently defined. There is also an urgent need for effective infection-control measures within clinics and hospitals. Prevention of transmission through reducing the airborne shedding and inhalation inoculation can be achieved by measures to reduce aerosol production and circulation in room air. This must be implemented in every hospital co-ordinated by hospital infection control committees. There has been a report of death of a healthcare worker with XDR-TB, most likely hospital-acquired26.
In order to inhibit the development of MDR and XDR-TB, better diagnostic algorithm needs to be designed and popularized27,28. Based on carefully crafted clinical criteria every case of suspected pulmonary TB should have at least one sputum culture for M. tuberculosis in order to substantially improve the diagnostic sensitivity and to allow DST well ahead of clinical treatment failure in case the organism is MDR27,28. This will require massive expansion of laboratory capacity throughout the country29. Every MDR isolate should be further screened for XDR organisms in reference laboratories. The currently recommended sputum smears for detection of acid fast bacilli (AFB) by Zeihl-Neilsen staining should be supplemented with a more sensitive method using fluorescent microscopy29.
Treatment of TB by community-based medical practitioners often do not conform the recommended regimen; also many patients interrupt or discontinue treatment. This sets the stage for emergence of MDR- and XDR-TB in India30. Many patients with MDR-TB have been documented to have non-standard treatment regimens in the past18 or had repeatedly defaulted on treatment. Such patients should be identified, counselled and restrained with assistance from family and employers - balancing rights of the individual and safety of society.
Although HIV infection does not by itself increase the probability of developing anti TB drug resistance, the co-morbidity with MDR- or XDR-TB is life threatening to HIV positive people. One study9 had looked at the prevalence of XDR-TB in HIV infected patients and found that among 54 patients, 12 (22%) had MDR-TB and among them 4 (33.3%) had XDR-TB. All four died within 3 months of diagnosis.
Addressing XDR-TB in India will be a formidable challenge. The strategy of RNTCP has been to minimize the development of MDR-TB by standardized drug regimens and consequently reduce the emergence of XDR bacilli. We suspect that the major cause of emergence of XDR-TB is the widespread practice of non-standard drug regimens in the private sector healthcare settings. Guidelines to fully integrate DOTS and DOTS-Plus (diagnosis and treatment of MDR-TB) have already been brought out by RNTCP30. The target is to detect and treat at least 30,000 cases of MDR-TB annually, free of charge, from 2012-2013 onwards31. Unless TB treatment in private sector is effectively regulated, the problems of MDR- and XDR-TB will remain largely unrecognized.
In conclusion, reports from just nine cities across India document the presence of XDR-TB in India. There is an urgent need for country-wide surveillance of MDR- and XDR-TB. Massive expansion of quality-assured mycobacteriology laboratories and strict guidelines and protocols are essential for diagnosis and treatment of XDR-TB. Practical and effective infection-containment measures and facilities for intensive counselling of TB patients need to be established. Educating professionals on these elements and school and college students and the public on prevention of TB must also become part of national TB control efforts.
XDR-TB in India
This write up on extensively drug resistant tuberculosis in India - a review by Drs Joy Sarojini Michael and T. Jacob John is an interesting attempt to focus on a problem which generates very diverse actions among people, scientific leaders, clinicians and public health personnel. As these reports are based on various tertiary care hospitals, these cannot be extrapolated to estimate the burden of the drug resistance in the community. Secondly, there are very few accredited laboratories for second line of TB drugs in India and as such there can be problems about the interpretation and credibility of the profiles reported by many investigators. Nevertheless, one cannot ignore that the problem needs to be addressed by giving it due importance. However, this should not lead to scare as a very small proportion of MDR isolates has been generally found to be XDR. Many Institutions of Government of India including those of ICMR have been working hard to provide the services and augment the capabilities to accurately diagnose the resistance. Several international agencies are also playing their part. I am sure the infrastructure to accurately diagnose different types of the resistance to second line drugs and management of drug resistant cases will be strengthened over a period of time. Till then the readers should read these reports with caution knowing fully well the limitations but get ready to improve as per the needs to do better.
V.M. Katoch
Chairman, Editorial Board, IJMR, Director-General, I.C.M.R. & Secretary, Department of Health Research, Government of India, New Delhi 110 029, India
vishwamohan_katoch@yahoo.co.in
References
- 1.Centers for Disease Control and Prevention. Revised definition of extensively drug resistant tuberculosis. Morb Mortal Weekly Rep. 2006;55:1176. [Google Scholar]
- 2.World Health Organization. Extensively drug resistant tuberculosis (XDR TB): recommendations for prevention and control. Wkly Epidemiol Rec. 2006;81:430–2. [PubMed] [Google Scholar]
- 3.Multidrug and extensively drug-resistant tuberculosis: global report on surveillance and response. Geneva, Switzerland: World Health Organization; 2010. World Health Organization. [Google Scholar]
- 4.Thomas A, Ramachandran R, Rehaman F, Jaggarajamma K, Santha T, Selvakumar N, et al. Management of multidrug resistance tuberculosis in the field: Tuberculosis Research Centre experience. Indian J Tuberc. 2007;54:117–24. [PubMed] [Google Scholar]
- 5.Ramachandran R, Nalini S, Chandrasekar V, Dave PV, Sanghvi AS, Wares F, et al. Surveillance of drug-resistant tuberculosis in the state of Gujarat, India. Int J Tuberc Lung Dis. 2009;13:1154–60. [PubMed] [Google Scholar]
- 6.Sood OP, Sharma SK. Proceedings of Round Table Conference Series No. 22. New Delhi, India: Ranbaxy Science Foundation; 2008. Dec, Challenges of MDR/XDR Tuberculosis in India. 2008. [Google Scholar]
- 7.Jose L, Mundayoor M, Ajaykumar R. Annual Report of the Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram. Thiruvananthapuram: RGCB; 2007. p. 220. [Google Scholar]
- 8.Bikram DS, Hassan G, Kadri SM, Qureshi W, Kamili MA, Singh H, et al. Multidrug-resistant and extensively drug resistant tuberculosis in Kashmir, India. J Infect Dev Ctries. 2010;4:19–23. doi: 10.3855/jidc.669. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Sankar MM, Gopinath K. High rate of extensively drug-resistant tuberculosis in Indian AIDS patients. AIDS. 2007;21:2345–7. doi: 10.1097/QAD.0b013e3282f125c9. [DOI] [PubMed] [Google Scholar]
- 10.Sharma SK, George N, Kadhiravan T, Saha PK, Mishra HK, Hanif M, et al. Prevalence of extensively drug-resistant tuberculosis among patients with multi-drug resistant tuberculosis: a retrospective hospital-based study. Indian J Med Res. 2009;130:392–5. [PubMed] [Google Scholar]
- 11.Dhingra VK, Malik S, Hanif M, Arora VK. XDR tuberculosis: A report from the New Delhi tuberculosis centre, India. J Coll Physicians Surg Pak. 2009;19:133–5. [PubMed] [Google Scholar]
- 12.Jain S, Rodrigues C, Mehta A, Udwadia ZF. Proceedings of the American Thoracic Society International Conference, May 2007. San Francisco, USA: High prevalence of XDR-TB from a tertiary care hospital in India. Abstract A510. [Google Scholar]
- 13.Rodrigues C. XDR TB-Perspectives from a Referral Tertiary care Hospital in Mumbai. In: Sood OP, Sharma SK, editors. Challenges of MDR/XDR tuberculosis in India. New Delhi, India: Ranbaxy Science Foundation; 2008. Dec, pp. 39–42. Proceedings of Round Table Conference Series. (22) [Google Scholar]
- 14.Mondal R, Jain A. Extensively drug-resistant Mycobacterium tuberculosis, India. Emerg Infect Dis. 2007;13:1429–31. doi: 10.3201/eid1309.070443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraborty N, De C, Bhattacharyya S, Mukherjee A, Santra S, Banerjee D, et al. Drug susceptibility profile of Mycobacterium tuberculosis isolated from HIV infected and uninfected pulmonary tuberculosis patients in eastern India. Trans R Soc Trop Med Hyg. 2010;104:195–201. doi: 10.1016/j.trstmh.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Rajasekaran S, Chandrasekar C, Mahilmaran A, Kanakaraj K, Karthikeyan DS, Suriakumar J. HIV coinfection among multidrug resistant and extensively drug resistant tuberculosis patients - a trend. J Indian Med Assoc. 2009;107:281–2. 284-6. [PubMed] [Google Scholar]
- 17.James P, Gupta R, Christopher DJ, Thankagunam B, Veeraraghaven B. MDR- and XDR-TB among suspected drug-resistant TB patients in a tertiary care hospital in India. Clin Respir J. 2011;5:19–25. doi: 10.1111/j.1752-699X.2009.00184.x. [DOI] [PubMed] [Google Scholar]
- 18.Balaji V, Daley P, Anand AA, Sudarsanam T, Michael JS, Sahni RD, et al. Risk factors for MDR and XDR-TB in a tertiary referral hospital in India. PLoS One. 2010;5:e9527. doi: 10.1371/journal.pone.0009527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michael JS, Shalini BE, Mathews MS. Drug Resistant Tuberculosis - An Experience of Southern States of India. In: Sood OP, Sharma SK, editors. Challenges of MDR/XDR tuberculosis in India. New Delhi, India: Ranbaxy Science Foundation; 2008. pp. 35–8. Proceedings of Round Table Conference Series (22) [Google Scholar]
- 20.Paramasivan CN, Rehman F, Wares F, Sundar Mohan N, Sundar S, Devi S, et al. First- and second-line drug resistance patterns among previously treated tuberculosis patients in India. Int J Tuberc Lung Dis. 2010;14:243–6. [PubMed] [Google Scholar]
- 21.Myneedu VP, Visalakshi P, Verma AK, Behera D, Bhalla M. Prevalence of XDR TB cases- A retrospective study from a tertiary care TB hospital. Indian J Tuberc. 2011;58:54–9. [PubMed] [Google Scholar]
- 22.Laszlo A, Rahman M, Espinal M, Raviglione M. Quality assurance programme for drug susceptibility testing of Mycobacterium tuberculosis in the WHO/IUATLD Supranational Reference Laboratory Network: five rounds of proficiency testing, 1994-1998. Int J Tuberc Lung Dis. 2002;6:748–56. [PubMed] [Google Scholar]
- 23.Jesudason MV, Mukundan U, Ohri VC, Badrinath S, John TJ. An external quality assessment service in Microbiology in India: A six-year experience. Indian J Med Microbiol. 2001;19:20–5. [Google Scholar]
- 24.Mitnick CD, Shin SS, Seung KJ, Rich ML, Atwood SS, Furin JJ, et al. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med. 2008;359:563–74. doi: 10.1056/NEJMoa0800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandhi NR, Shah NS, Andrews JR, Vella V, Moll AP, Scott M, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181:80–6. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 26.James P, Christopher DJ, Balamugesh T, Gupta R. Death of a health care worker with nosocomial extensively drug-resistant tuberculosis in India. Int J Tuberc Lung Dis. 2009;13:795–6. [PubMed] [Google Scholar]
- 27.John TJ, John SM. Paradigm shift for tuberculosis control in high prevalence countries. Trop Med Int Health. 2009;14:1428–30. doi: 10.1111/j.1365-3156.2009.02392.x. [DOI] [PubMed] [Google Scholar]
- 28.John TJ, Vashishtha VM, John SM, Sudarsanam TD. Tuberculosis control must be scientifically defined and soundly designed. Indian J Med Res. 2010;132:4–8. [PubMed] [Google Scholar]
- 29.Steingart KR, Henry M, Viviene NG, Hopewell PC, Ramsay A, Cunningham J, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:570–81. doi: 10.1016/S1473-3099(06)70578-3. [DOI] [PubMed] [Google Scholar]
- 30.John TJ. Extensively drug-resistant tuberculosis in India. Indian J Med Res. 2010;131:109–10. [PubMed] [Google Scholar]
- 31.Central TB Division.TB India 2010. DOTS-Plus guidelines. Central TB Division, Directorate General of Health Services, Ministry of Health & Family Welfare, Nirman B havan, New Delhi, 2010. Revised National TB Control Programme. India. [accessed on August 2011]. Available from: http://www.tbcindia.org .


