Abstract
Background & objectives:
N-acetyltransferases 1 and 2 (NAT1 and NAT2) are important enzymes for metabolism of tobacco carcinogens. Due to polymorphisms, improper activities of these enzymes might lead to the formation of DNA adducts that may modulate risk of tobacco related oral precancer and cancer. Previously, it was shown that NAT2 polymorphisms did not modulate the risk of oral precancer and cancer. We undertook this study to check whether polymorphisms at NAT1 can modulate the risk of oral leukoplakia and cancer either alone or in combination with NAT2.
Methods:
Genotypes at four SNPs on NAT1 were determined by TaqMan method in 389 controls, 224 leukoplakia and 310 cancer patients. Genotype data were analyzed to know haplotypes and acetylation status of individuals and, then to estimate the risk of diseases. Using our previously published NAT2 data, combination of NAT1 and NAT2 acetylation genotypes of patients and controls were also analyzed to estimate the risk of diseases.
Results:
Analysis of NAT1 genotype data revealed that 1088T and 1095C alleles exist in strong linkage disequilibrium (r2=0.97, P<0.0001) and SNPs are in Hardy-Weinberg Equilibrium (P=0.1). Wild type or normal acetylating and variant or rapid acetylating alleles were two major alleles (frequencies 0.62 and 0.36, respectively) present in the control population. NAT1 rapid acetylation could not modulate the risk of leukoplakia and cancer (OR=0.9, 95% CI: 0.6-1.3; OR=1.0, 95% CI: 0.7-1.4, respectively). Analysis of combined NAT1 and NAT2 acetylating data also showed no significant enhancement of the risk of diseases.
Interpretation & conclusions:
NAT1 rapid acetylation alone as well as combination of NAT1 rapid-NAT2 slow acetylation did not modulate the risk of oral precancer and cancer in our patient population. So, NAT1/NAT2 metabolized carcinogen products may not be involved in tobacco related oral precancer and cancer. It may be interpreted that large sample size as well as combination of polymorphisms at other candidate loci may be important to estimate the risk of a complex disease like oral cancer.
Keywords: Combination of polymorphisms, leukoplakia, NAT1, NAT2, oral cancer
Annually about 270,000 cases of oral cancer are reported worldwide and of these about 80,000 of are diagnosed in India1. Annual incidence of oral leukoplakia has been reported to be 0.2-11.7 per cent in different populations of India and about 2-12 per cent of the leukoplakia cases become malignant within several years2,3. As an early sign of damage to oral mucosa, tobacco smokers and chewers often develop different precancerous lesions, such as leukoplakia, erythroplakia, submucous fibrosis, etc., and these lesions are easily accessible to diagnosis. As leukoplakia is one of the good predictors of oral cancer, knowledge of genetic risk factors for leukoplakia may provide useful strategy to control oral cancer incidence.
Tobacco carcinogens undergo different kinds of modifications by Phase I (such as cytochrome P450, CYPs etc.) and Phase II (such as GSTs, NAT1, NAT2 etc.) enzymes either for detoxification or activation depending on the specific enzymes. It has been reported earlier that polymorphisms at GSTs, CYPs, NAT2 and different DNA repair loci, either independently or in combination, could modify the risk of oral cancer and precancer in different Indian populations4–12. But, no report is available on association between polymorphism at NAT1 locus and risk of oral precancer and cancer in Indian population. NAT1 can catalyze O-acetylation (activation) and N-acetylation (detoxification) reactions with aromatic and heterocyclic amines present in tobacco. Polymorphisms at NAT1 may result in the production of NAT1 proteins with variable enzyme activity or stability13 and NAT1 is highly polymorphic with more than 25 variant alleles in different population14. Wild type or normal allele (called as NAT1*4) contains wild-type nucleotides at all polymorphic sites but the most common variant NAT1*10 allele contains two variant nucleotides (A1088 and A1095) at 1088 and 1095nps in the 3’-untranslated region of the gene. These changes in nucleotides modify mRNA stability which, in turn, increases the activity of the NAT1 enzyme. So, this allele has been assigned as rapid acetylating allele15. Other variant alleles such as NAT1*3, NAT1*11, NAT*15, NAT1*14, etc. could have different acetylating activities but implication of these variant alleles in association study is not known since frequencies of these alleles are very low in the population16. Some studies have reported both presence and absence of association between the risk of oral/head and neck cancer17–19 and NAT1 *10 haplotype/allele (i.e. rapid acetylating allele). Investigators have also reported that NAT1 rapid acetylation status could be a modulating factor for the risk of other cancers20–22.
Two recent studies could not confirm the previous observations on association between cancer and NAT1 acetylation status16,23. In an analytical study, highest levels of DNA adducts were detected in individuals who had both NAT1 rapid and NAT2 slow acetylation activity24. A study, with low sample size, also reported significant association between fast acetylators at both NAT1 and NAT2 (i.e. with NAT2*4/NAT1*10 diplotype) and risk of head and neck cancer even though NAT1 and NAT2 could not modify risk of cancer independently25.
In Indian scenario, except one report on frequencies of NAT1 alleles in a small number of south Indian people26, there is no report of polymorphism at NAT1 in other Indian populations. The objective of this study was, therefore, to find out extent of polymorphism at NAT1 in healthy controls and estimate the risk of oral leukoplakia and cancer. Using previously published7 NAT2 acetylation data from the same patients and controls, combination of NAT1 and NAT2 acetylation status of patients and controls was also analyzed to estimate risk of leukoplakia and cancer.
Material & Methods
The study was conducted in the Human Genetics Unit, Biological Sciences Division, Indian Statistical Institute, Kolkata, India.
Patients, controls and tobacco habit: Oral leukoplakia and cancer patients were recruited from the R. Ahmed Dental College and Hospital (a primary referral hospital at Kolkata, India) during 1999-2005 with the help of an oral pathologist from the same hospital. Individuals, without any lesions in oral cavity, were recruited from outpatient department as “controls”. Patients and controls were unrelated. Information on age, sex, occupation, type of tobacco habit, tobacco use frequency and duration, alcohol intake and duration were recorded. Individuals, who had family history of any type of cancer and exposed to any carcinogen or toxic chemicals in work place, were excluded from the study. For inclusion, individuals should have any types of tobacco habits, be older than 25 yr considering the diseases have been manifested due to gene-environment interaction rather than due to genetic component/s only. The cases were confirmed as oral squamous cell carcinoma (SCC) and leukoplakia by histopathology7. Overall, 310 cancer, 224 leukoplakia and 389 control individuals were included in the study. Written informed consent was obtained from each subject. Blood sample (5 ml) was drawn and stored for DNA analysis. This study protocol was approved by the Institutional Committee for Protection of Research Risk to Humans as also Ethics Committee of ISI, Kolkata.
All individuals reported tobacco habits such as smoking of cigarette and/or bidi (a native cigarette-like stick of coarse tobacco hand-rolled in a dry tembuhurni leaf) and/or chewing/dipping of tobacco in different forms7. Patients and controls reported tobacco habits of either smoking or chewing/dipping or both. Lifetime tobacco chewing/dipping exposure was measured as chewing-year (CY): taking smokeless tobacco once in a day for 1 year=1 CY27 and similarly, dose of tobacco smoking was measured as pack-years (PY)28: 1 packet per day for 1 year=1 PY (1 pack=20 cigarettes or 40 bidies), since amount of tobacco present in one cigarette (750-1000 mg) is similar to that present in two bidies (850-1050 mg)5.
Genomic DNA was isolated from blood samples7 and study of histopathology was perfomed using cancer and precancer biopsy materials.
Genotypes at NAT1: Four single nucleotide polymorphisms (SNPs) at nucleotide positions (np) 445 (G>A), 559 (C>T), 1088 (T>A) and 1095 (C>A) on exon 2 (having rs#4987076, #4986782, #1057126, #15561) were genotyped by TaqMan method according to the published procedure29.
Haplotypes and acetylation status: Frequencies of haplotypes at NAT1 in all patients and controls were estimated from genotype data by a maximum-likelihood method using the expectation maximization algorithm (http://www.broad.mit.edu/mpg/haploview). Acetylation status of each individual was determined by genotype of that individual's haplotype data14.
Genotypes at NAT2: Polymorphic data at five SNPs at nucleotide positions (np) 341 (T>C), 481 (C>T), 590 (G>A), 803 (A>G) and 857 (G>A) (having rs#1801280, #1799929, #1799930, #1208, #1799931, respectively) and acetylation status of each individual were taken from our previous study7.
Combination of NAT1 and NAT2 acetylation data: All patients and controls were divided into sub-groups depending on the NAT1 and NAT2 acetylation status of each patient and control. Each sub-group of patients and controls was also compared to check whether a particular combination of NAT1 and NAT2 acetylation status could modify risk of diseases.
Re-sequencing of PCR products: PCR products (~15% of total samples) from NAT1 and NAT2 were re-sequenced (ABI 3100 Genetic Analyzer; Applied Biosystem, USA) to confirm the genotypes determined by TaqMan method.
Detection of “9bp deletion” polymorphism at NAT1: This insertion/deletion polymorphism is spread over the sequence between np 1065-1090 and was determined by resequencing. For analyses, Phred, Phrap, Consed (http://www.phrap.com/) were used to determine the sequence of the PCR product and “9bp insertion/deletion” polymorphism.
Statistical analysis and power calculation: Age-, sex-, and tobacco dose-adjusted risk of oral cancer and leukoplakia was calculated as odds ratios (ORs) with 95% confidence intervals (CIs) by binary logistic regression analysis using SPSS statistical package (version 10.0, SPSS Inc., Chicago, USA). Chi-square test was used for comparison of categorical variables, such as genotype/allele frequencies, sexes, smokers, chewers, etc. and t-test was used to compare continuous variables such as age, CY, PY, etc. between patient and control groups.
Power of the sample sizes used in this study was calculated using the software “Power for Genetic Association Version 2”30. The expected risk estimate (relative risk=2.0) was calculated specifying the values for a number of parameters such as minor allele frequency of 0.36, disease prevalence 0.0002, statistical power 80 per cent, marker allele frequency 0.36 (assuming disease allele is marker allele), r2=1.0, case-control ratio =1.
Results
Demographic and tobacco habit information of patient and control populations are summarized elsewhere7. About 90 per cent of smokers had habits of both cigarettes and bidis, so it was not possible to analyze bidi and cigarette smokers separately. In patient and control groups, only a few (6 controls, 10 leukoplakia and 14 cancer patients i.e., <5%) had occasional alcohol-drinking habit, therefore, alcohol consumption was also not considered in the analyses. All cancer and leukoplakia patients were incident cases and none of the controls had family history of cancer. Leukoplakia mostly affected buccal mucosa and commissural area in 146 cases (65%), buccal mucosa and alveolar sulcus in 45 (20%) and other sites including lip, tongue, etc. in 33 (15%). Most of the patients suffered from ulcerative lesion in 137 (61%) followed by homogeneous in 80 (36%) and nodular in 7 (3%) types of leukoplakia. Cancer affected sites were buccal mucosa and alveolar sulcus in 161 (52%), lip in 47 (15%), tongue in 37 (12%), buccal sulcus in 34 (11%), and retro molar area in 31 (10%). Morphologically these were classified as well in 201 (65%), moderately in 53 (17%) and poorly in 56 (18%) differentiated SCC.
The power of the study was >80 per cent with the sample sizes of 389 controls, 224 leukoplakia and 310 cancer samples. Polymorphic data at SNP on NAT1 were checked for Hardy-Weinberg equilibrium31 but no deviation was observed (P=0.1 for SNP at np1095). Analysing genotype data for pair-wise linkage disequilibrium (LD), it was observed that the 1088T allele and 1095C allele were in strong positive LD (r2= 0.97, P<0.0001). Since the homozygote variant genotypes at np445 and 559 were absent in the populations, LD was not estimated at these two SNPs. Some genotypes (~15% of total samples), determined by TaqMan method were cross-checked by sequencing method to ensure that genotypes were correctly determined.
Variant homozygotes at 445 np, 559 np and “9 bp del” polymorphic sites were absent and frequencies of heterozygotes at these SNP sites were very low. So, risk of disease was not observed to be modified by these SNPs. Frequencies of major, heterozygous and variant genotypes at each of 1088 and 1095np polymorphic sites were observed to be similar among control, leukoplakia and cancer samples and, hence, risk of diseases was not modulated by these genotypes (Table I). Alleles present in each genotype have been arranged into haplotypes in the order: np 445(G/A)-np 559(G/A)-np 1088 (T/C)-np 1095 (C/A). The G445-G559-T1088-C1095 haplotype, which contains major alleles at all loci, was most common in control and patient populations followed by G445-G559-A1088-A1095 haplotype with variant alleles at np 1088 and 1095. Each haplotype corresponds to different acetylation status, such as normal or rapid or slow acetylating allele. Haplotype G445-G559-T1088-C1095, designated as *4 allele, is a reference haplotype with normal acetylation status and G445-G559-A1088-A1095 haplotype allele is variant *10 allele with rapid acetylation status14,16. Other haplotypes frequencies were very low or absent in the samples. None of the haplotypes modified the risk of leukoplakia and cancer in the population (Table II). Each individual depending on the diplotype (i.e. haplotype in the pair of chromosomes) was designated as normal or rapid or slow acetylator in all three populations. Distribution of diplotypes or acetylators was not significantly different in controls, leukoplakia and cancer samples (Table III).
Table I.
Distribution of genotypes in leukoplakia, cancer and control populations and risk of diseases

Table II.
Distributions of different NAT1 haplotypes and acetylation phenotypes in patients and controls
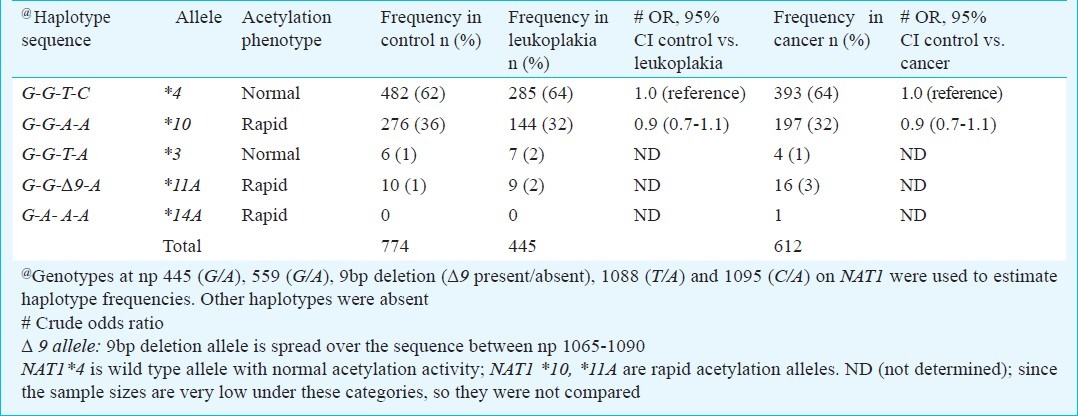
Table III.
Distribution of NAT1 diplotypes and acetylation phenotypes in leukoplakia, cancer and control populations and risk of diseases

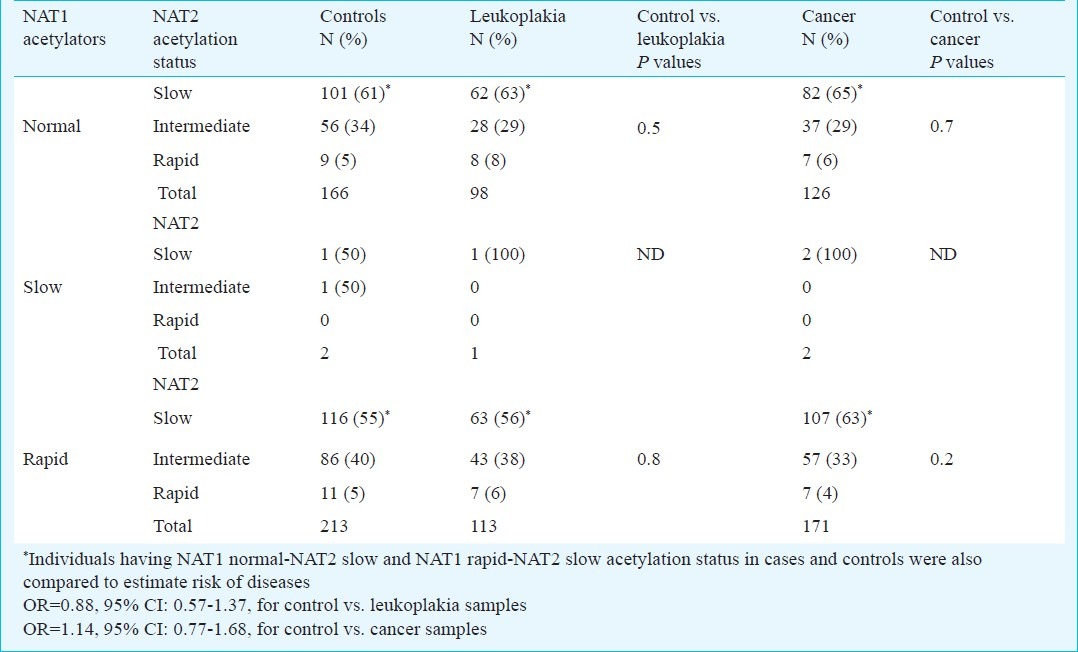
Among the patients and controls, some had only smoking habit, some of them had only tobacco chewing habit and remaining had mixed tobacco habit. Stratifying individuals on the basis of tobacco habit, it was observed that NAT1 rapid acetylators having smoking or chewing or mixed tobacco habits were not susceptible to leukoplakia or cancer in this study (Table IV). Working on these patients and controls, previously it was shown that NAT2 slow acetylation could not modulate the risk of leukoplakia and cancer7. Risk of the diseases could also be estimated using combination of NAT1 and NAT2 acetylation data of each individual in patients and controls. In this way, patients and controls were sub-grouped as having NAT2 slow, intermediate and rapid acetylation status in NAT1 normal, slow and rapid acetylators (Table V). Distribution of combined NAT1 normal-NAT2 slow, NAT1 normal-NAT2 intermediate and NAT1 normal-NAT2 rapid acetylators in cases and controls were not significantly different. Similarly, distribution of NAT1 rapid-NAT2 slow, NAT1 rapid-NAT2 intermediate and NAT1 rapid-NAT2 rapid acetylators in cases and controls were also not significantly different. Acetylators with NAT1 rapid-NAT2 slow combination were also not significantly susceptible to leukoplakia and cancer when compared with NAT1 normal-NAT2 slow combination (OR=0.88, 95% CI: 0.57-1.37 for leukoplakia; OR=1.14, 95% CI: 0.77-1.68 for cancer, Table V).
Table IV.
Distributions of NAT1 acetylators having different types of tobacco habit and risk of disease
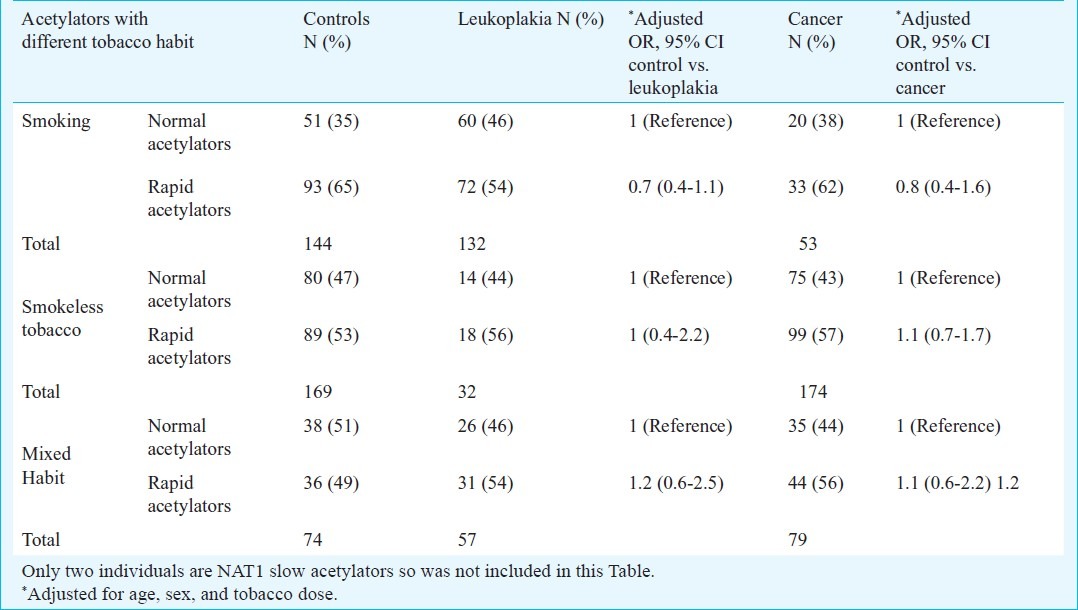
Table V.
Comparison of distribution of NAT1 acetylators with different NAT2 acetylation status in patients and controls
Discussion
Numbers of males and females, age and number of smokers and chewers were not similar in controls and leukoplakia and cancer patients (data not shown)7. So, adjustments for age, sex, and tobacco doses were done to estimate the risk of disease. In India, males use both smoking and smokeless tobacco whereas females use mostly smokeless tobacco. Although smokers and smokeless tobacco users are affected by leukoplakia and cancer but comparatively more male patients as well as smokers were present in leukoplakia population. One of the reasons might be that leukoplakia is not life threatening initially, so females (mostly housewives from low income families) did not visit hospital due to several procedural steps required in the hospital. As a result, females, who were mostly smokeless tobacco users, were less represented in leukoplakia population.
Distribution of normal (43%), rapid (56%) and slow acetylators (1%) in the control population was observed to be similar to those (39, 60 and 1%, respectively) in Caucasian population19, but rapid acetylation status was different from those in Japanese and Turkish populations18,32. Frequencies of NAT1*4 and NAT1*10 alleles in this control population (0.62 and 0.36, respectively) were different from those in a south Indian population (0.51 and 0.17, respectively) settled in Singapore26.
Similar to a study on head and neck cancer in Turkish population25, we also did not find any association between polymorphism at NAT1 and risk of oral cancer. But unlike previous studies on Japanese and Western populations18,19, rapid acetylation (i.e. NAT1*10 allele) did not modulate the risk of oral cancer in this Indian population. Although sample sizes became low, NAT1 rapid acetylation could not modify risk of disease among tobacco habit-based stratified samples such as smokers, chewers and mixed habituals. This difference in result between Indian and Western and Japanese populations might be explained by small sample sizes studied in those populations, so possibility of false positive result could not be ruled out. Other explanations for this difference in observation might be attributed to the facts that majority of the cancer patients in our study had tobacco chewing/dipping habit (data not shown)7 whereas Western and Japanese populations had mostly tobacco smoking and alcohol abuse habit.
In a study, acetylation of N-hydroxy-4-aminobiphenyl was measured for analyses of carcinogen-DNA adducts and the highest levels of DNA adducts were found in individuals who had both NAT1 rapid and NAT2 slow acetylation activity24. NAT1 is expressed in the oral cavity itself; so NAT1 rapid acetylators might have higher levels of NAT1-catalyzed O-acetylated carcinogens to produce DNA adducts. Although NAT2 is not expressed in oral tissue, the effect of NAT2 is thought be mediated by metabolism in liver or gut and part of the modified carcinogens might be excreted through saliva. In another study with low sample size, significant association was observed between combined NAT1-NAT2 fast acetylation (i.e. NAT2*4 and NAT1*10) and risk of head and neck cancer25. We also considered NAT1 and NAT2 acetylation data of each individual to analyze the risk of disease. Since, acetylation status at NAT1 and NAT2 of each individual was assigned on the basis of genotypes, each patient or control was grouped into sub-groups with different combinations of NAT1-NAT2 acetylation status. Different combinations of NAT1-NAT2 acetylation sub-groups among patients and controls were compared to estimate the risk of disease. But, no association was observed between the risk of disease and different combinations of NAT1-NAT2 acetylation genotypes. One of the possibilities might be low sample size in each sub-group after combination of NAT1-NAT2 acetylation phenotypes. Since the sample size in each NAT1-NAT2 acetylation sub-group among patients and controls became low, they were not further stratified on the basis of tobacco habits to estimate risk of disease.
In conclusion, our results showed that NAT1 and/or NAT2 phenotypes did not enhance the risk of oral cancer and leukoplakia either alone or in combination. So, potential public health impact of this result is minimal at the moment as there is insufficient evidence implicating NAT polymorphism in the aetiology of oral cancer. Patients and controls could not be recruited matching sex, age and tobacco habits due to logistic problems and this was one of the limitations of the study. Another limitation was that the same control population was used to compare the data of leukoplakia and cancer patients but the number of smokers was more in the leukoplakia patients whereas number of smokeless tobacco users was more in cancer patients compared to controls. It would have been better to recruit two matched control populations for leukoplakia and cancer patients groups. In any association study, sample sizes play an important role to dissect out a real picture, thus a study with large sample size from an ethnic population is desirable.
Acknowledgment
Authors acknowledge the assistance and contribution of Dr Nilabja Sikdar, Prof. Ranjan R. Paul (Oral pathologist, presently at Guru Nanak Dental Science and Research, Panihati, Kolkata), Debabrata Sutradhar and Badal Dey. The Department of Science and Technology, Government of India, provided partial financial support for this work. The first author (MM) received senior research fellowship from the Council of Scientific & Industrial Research (CSIR), Government of India, New Delhi.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008. GLOBOCAN 2008. Int J Cancer. 2010;127:2893–17. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Definition of leukoplakia and related lesions: an aid to studies to precancer. Oral Surg Oral Med Oral Pathol. 1978;46:517–39. [PubMed] [Google Scholar]
- 3.Gupta PC, Mehta FS, Daftary DK, Pindborg JJ, Bhonsle RB, Jalnawalla PN, et al. Incidence rates of oral cancer and natural history of oral precancerous lesions in a 10-year follow-up study of Indian villagers. Comm Dental Oral Epidemiol. 1980;8:287–33. doi: 10.1111/j.1600-0528.1980.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 4.Sikdar N, Mahmud SA, Paul RR, Roy B. Polymorphism in CYP1A1 and CYP2E1 genes and susceptibility to leukoplakia in Indian tobacco users. Cancer Lett. 2003;30:33–42. doi: 10.1016/s0304-3835(03)00156-3. [DOI] [PubMed] [Google Scholar]
- 5.Sikdar N, Paul RR, Roy B. Glutathione S-transferase M3 (A/A) genotype as a risk factor for oral cancer and leukoplakia among Indian tobacco smokers. Int J cancer. 2004;100:95–101. doi: 10.1002/ijc.11610. [DOI] [PubMed] [Google Scholar]
- 6.Majumder M, Sikdar N, Paul RR, Roy B. Increased risk of oral leukoplakia and cancer among mixed tobacco users carrying XRCC1 variant haplotypes and cancer among smokers carrying two risk genotypes: one on each of two loci, GSTM3 and XRCC1 (codon 280) Caner Epidemiol Biomark Prev. 2005;14:2106–12. doi: 10.1158/1055-9965.EPI-05-0108. [DOI] [PubMed] [Google Scholar]
- 7.Majumder M, Sikdar N, Ghosh S, Roy B. Polymorphisms at XPD and XRCC1 DNA repair loci and increased risk of oral leukoplakia and cancer among NAT2 slow acetylators. Int J Cancer. 2007;120:2148–56. doi: 10.1002/ijc.22547. [DOI] [PubMed] [Google Scholar]
- 8.Anantharaman D, Samnant TA, Sen S, Mahimkar MB. Polymorphisms in tobacco metabolism and DNA repair genes modulate oral precancer and cancer risk. Oral Oncol. 2011;47:866–72. doi: 10.1016/j.oraloncology.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Anantharaman D, Chaubal PM, Kannan S, Bhisey RA, Mahimkar MB. Susceptibility to oral cancer by genetic polymorphisms at CYP1A1, GSTM1 and GSTT1 loci among Indians: tobacco exposure as a risk modulator. Carcinogenesis. 2007;28:1455–62. doi: 10.1093/carcin/bgm038. [DOI] [PubMed] [Google Scholar]
- 10.Nair UJ, Nair J, Mathew B, Bartsch H. Glutathione S-transferase M1 and T1 null genotypes as risk factors for oral leukoplakia in ethnic Indian betel quid/tobacco chewers. Carcinogenesis. 1999;20:743–8. doi: 10.1093/carcin/20.5.743. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran S, Ramadas K, Hariharan R, Rejnish RK, Pillai MR. Single nucleotide polymorphisms of DNA repair genes XRCC1 and XPD and its molecular mapping in Indian oral cancer. Oral Oncol. 2006;42:350–62. doi: 10.1016/j.oraloncology.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal D, Gupta S, Agarwal D, Gupta OP, Agarwal M. Role of GSTM1 and GSTT1 polymorphism: susceptibility to oral submucous fibrosis in the North Indian population. Oncology. 2010;79:181–6. doi: 10.1159/000318533. [DOI] [PubMed] [Google Scholar]
- 13.Butcher NJ, Boukouvala S, Sim E, Minchin RF. Pharmacogenetics of the arylamine N-acetyltransferases. Pharmacogenomics J. 2002;2:30–42. doi: 10.1038/sj.tpj.6500053. [DOI] [PubMed] [Google Scholar]
- 14.NAT1 and NAT2 Nomenclature. Available from: http://lousville.edu/medschool/pharmacology/NAT.html .
- 15.Bell DA, Badawi AF, Lang NP, Ilett KF, Kadlubar FF, Hirvonen A. Polymorphism in N-acetyl transferase 1 (NAT1) polyadenylation signal: association of NAT*10 allele with higher N-acetylation activity in bladder and colon tissue. Cancer Res. 1995;55:5226–9. [PubMed] [Google Scholar]
- 16.Kidd LCR, Vancleave TT, Doll MA, Srivastava DS, Thaker B, Komolafe O, et al. No association between variant N-acetyltransferase genes, cigarette smoking and prostate cancer susceptibility among men of African descent. Biomark Cancer. 2011;3:1–13. doi: 10.4137/BIC.S6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jourenkova-Mironova N, Wikman H, Bouchardy C, Mitrunen K, Dayer P, Benhamou S, et al. Role of arylamine N-acetyltransferase 1 and 2 (NAT1 and NAT2) genotypes in susceptibility to oral/pharyngeal and laryngeal cancers. Pharmacogenetics. 1999;9:533–7. [PubMed] [Google Scholar]
- 18.Katoh T, Keneko S, Boissy R, Watson M, Ikenmura K, Bell DA. A pilot study testing the association between N-acetyltransferases 1 and 2 and risk of oral squamous cell carcinoma in Japanese people. Carcinogenesis. 1998;19:1803–7. doi: 10.1093/carcin/19.10.1803. [DOI] [PubMed] [Google Scholar]
- 19.Olshan A, Wiessler MC, Watson MA, Bell DA. GSTM1, GST1, GSTP1, CYP1A1 and NAT1 polymorphisms, tobacco use and the risk of head and cancers. Cancer Epidemiol Biomark Prev. 2000;9:185–91. [PubMed] [Google Scholar]
- 20.Hein DW, Doll MA, Fretland AJ, Leff MA, Webb SJ, Xiao GH, et al. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol Biomark Prev. 2000;9:29–42. [PubMed] [Google Scholar]
- 21.Agundez JA. Polymorphisms of human N-acetyltransferases and cancer risk. Curr Drug Metab. 2008;9:520–31. doi: 10.2174/138920008784892083. [DOI] [PubMed] [Google Scholar]
- 22.McKay JD, Hashibe M, Hung RJ, Wakefield J, Gaborieau V, Dabrowska NS, et al. Sequence variants of NAT1 and NAT2 and other xenometabolic genes and risk of lung and aerodigestive tract cancers in central Europe. Cancer Epidemiol Biomark Prev. 2008;17:141–7. doi: 10.1158/1055-9965.EPI-07-0553. [DOI] [PubMed] [Google Scholar]
- 23.Sharma S, Cao X, Wilkens LR, Yamamoto J, Lum-Jones A, Henderson BE, et al. Well-done meat consumption, NAT1 and NAT2 acetylator genotypes and prostate cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomark Prev. 2010;19:1866–70. doi: 10.1158/1055-9965.EPI-10-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badawi AF, Hirvonen A, Bell DA, Lang NP, Kadlubar FF. Role of aromatic amine acetyltransferases, NAT1 and NAT2, in carcinogen-DNA adducts formation in the human urinary bladder. Cancer Res. 1995;55:5230–7. [PubMed] [Google Scholar]
- 25.Demokan S, Suoglu Y, Gözeler M, Demir D, Dalay N. N-acetyltransferase 1 and 2 gene sequence variants and risk of head and neck cancer. Mol Biol Rep. 2010;37:3217–26. doi: 10.1007/s11033-009-9905-8. [DOI] [PubMed] [Google Scholar]
- 26.Zhao B, Lee EJ, Yeoh PN, Gong NH. Detection of mutations and polymorphisms of N-acetyltransferase 1 gene in Indian, Malay and Chinese populations. Pharmacogenetics. 1998;8:299–304. doi: 10.1097/00008571-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Buch SC, Notani PN, Bhisey RA. Polymorphisms at GSTM1, GSTM3 and GSTT1 gene loci and susceptibility to oral cancer in an Indian population. Carcinogenesis. 2002;44:803–7. doi: 10.1093/carcin/23.5.803. [DOI] [PubMed] [Google Scholar]
- 28.Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: Modeling total exposure and intensity. Cancer Epidemiol Biomark Prev. 2006;15:517–23. doi: 10.1158/1055-9965.EPI-05-0863. [DOI] [PubMed] [Google Scholar]
- 29.Doll MA, Hein DW. Rapid genotype method to distinguish frequent and/or functional polymorphisms in human N-acetyltransferase-1. Anal Biochem. 2002;301:328–32. doi: 10.1006/abio.2001.5520. [DOI] [PubMed] [Google Scholar]
- 30.Menashe I, Rosenberg PS, Chen BE. PGA: power calculator for case-control genetic association analyses. BMC Genet. 2008;9:36. doi: 10.1186/1471-2156-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crow JF. Eighty years ago: The beginnings of population genetics. Genetics. 1988;119:473–6. doi: 10.1093/genetics/119.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arslan S, Degerli N, Bardakci F. Distribution of N-acetyltransferase Type 1 (NAT1) genotypes and alleles in a Turkish Population. Genet Mol Biol. 2004;27:162–4. [Google Scholar]


