Abstract
Background & objectives:
Age adjusted incidence rate of lung cancer in India ranges from 7.4 to 13.1 per 100,000 among males and 3.9 to 5.8 per 100,000 among females. The factors affecting survival in lung cancer patients in India are not fully understood. The current study was undertaken to evaluate the factors affecting survival in patients diagnosed with lung cancer attending a tertiary care cancer institute in Bangalore, Karnataka, India.
Methods:
Consecutive patients with primary lung cancer attending Bangalore Institute of Oncology, a tertiary care centre at Bangalore, between 2006 and 2009 were included. Demographic, clinical, radiological data were collected retrospectively from the medical records.
Results:
A total of 170 consecutive subjects (128 males, 42 females) diagnosed to have lung cancer; 151 non-small cell lung cancer (NSCLC) and 19 small cell lung cancer (SCLC) were included. A higher proportion of never-smokers (54.1%) were observed, mostly presenting below the age of 60 yr. Most subjects were in stage IV and III at the time of diagnosis. More than 50 per cent of patients presented with late stage lung cancer even though the duration of symptoms is less than 2 months. The 30-month overall survival rates for smokers and never-smokers were 32 and 49 per cent, respectively. No significant differences were observed in 30 month survival based on age at presentation, gender and type of lung cancer. Cox proportional hazards model identified never-smokers and duration of symptoms less than 1 month as factors adversely affecting survival.
Interpretation & conclusions:
Our results showed that lung cancer in Indians involved younger subjects and associated with poorer survival as compared to other ethnic population. Studies on large sample need to be done to evaluate risk factors in lung cancer patients.
Keywords: Duration of symptoms, histopathological type, lung cancer, never smokers, small cell lung cancer, survival
Lung cancer is one of the commonest malignancies among men1 and one of the leading causes of death globally1,2. Despite improvement in diagnostics, staging and treatment modalities, the prognosis remains poor1. Age adjusted incidence rate of lung cancer in India ranges from 7.4 to 13.1 per 100,000 among males and 3.9 to 5.8 per 100,000 among females. Five year survival of 12-14 per cent has been reported for non-small cell lung cancer and 2-3 per cent for small cell lung cancer1,3,4.
Various groups have evaluated factors associated with survival of lung cancer patients. Smokers with lung cancer have been found to have higher all cause-mortality and cancer-specific mortality in some studies5–10 but not in others11,12. Smoking related co-morbidities in patients with lung cancer can impact survival and 20-40 per cent of non-metastatic lung cancer patients die without cancer progression3. Increasing age was associated with higher mortality rates in lung cancer patients in Japan4 in both men and women. Some studies have reported better prognosis in adenocarcinoma1,11 while others have found better survival in squamous cell carcinoma14. Some studies report that women have better survival rates than men8,14, while in other studies, higher survival rates were seen in men13. Poor survival has been documented with more advanced lung cancer1. Unlike in the western world, a significant proportion of lung cancer patients in India are never-smokers and it was not clear whether the survival in never-smoking lung cancer patients is different from smokers. Studies evaluating the factors associated with survival in lung cancer patients are useful as these generate data on prognostic variables, which could be used to develop models to predict treatment response and survival in newly detected lung cancer patients. These findings can also be used to conduct detailed investigations to understand the pathobiology of lung cancer in better way especially tumour biology and host responses to explain these differences in survival which could in the future direct development of novel treatment modalities. The current study was undertaken with the aim to determine 30-month survival rates in lung cancer patients and the factors that affect survival in patients with lung cancer in our population.
Material & Methods
One hundred and seventy consecutive patients with primary lung cancer attending the Bangalore Institute of Oncology, a tertiary care centre at Bangalore, Karnataka, India between 2006 and 2009 were included in the study. Demographic, clinical, radiological data were collected retrospectively from the medical records. The data on survival was collected prospectively. The demographic variables including age, gender, smoking status and area of residence were collected. Patients were classified as never-smokers when they had never smoked in the past5. Patients who were former or current smokers were classified as smokers. The clinical presentation of these subjects with various symptoms related to lung cancer and the duration of first symptom before confirmation of the diagnosis of lung cancer were recorded. The radiological evaluation included chest X-rays, CT scan, magnetic resonance imaging (MRI), ultrasonography of the abdomen, positron emission tomography (PET) and bone scans for staging of lung cancer based on the American Joint Committee on Cancer (AJCC) staging 200215. Histopathology of all the patients was confirmed by lung biopsy, fine needle aspiration cytology (FNAC) lung, bronchial washings and pleural fluid and pleural biopsy specimens16. Presence of co-morbid conditions like diabetes, hypertension, ischaemic heart disease or pulmonary tuberculosis was noted. The main outcome of the study was all-cause mortality in smokers and never-smokers. The Central Ethics Committee of Bangalore Institute of Oncology approved the study protocol.
Statistical analysis: Survival was measured from date of confirmation of diagnosis to date of death. Date of death was collected from medical records and where unavailable, telephonic contact of the patient's relatives was conducted to ascertain the present status of the subject. When contact could not be established in spite of three attempts, patients were classified as lost to follow up. The patients’ survival status as on June 1, 2009 was utilized for survival analysis. The overall survival curves and the 30 month survival rates between smokers and non-smokers, histological subgroups, age, gender, stage of lung cancer at presentation and duration of symptoms were analyzed using the Kaplan-Meier method and difference between the groups were assessed using the log rank test. The differences between smokers and never-smokers were analyzed using the χ2 test. Cox proportional hazards analysis was performed and unadjusted hazards ratio and its 95% confidence intervals were calculated. A multivariate Cox proportional hazards analysis was performed utilizing the factors significantly associated in univariate analysis.
Results
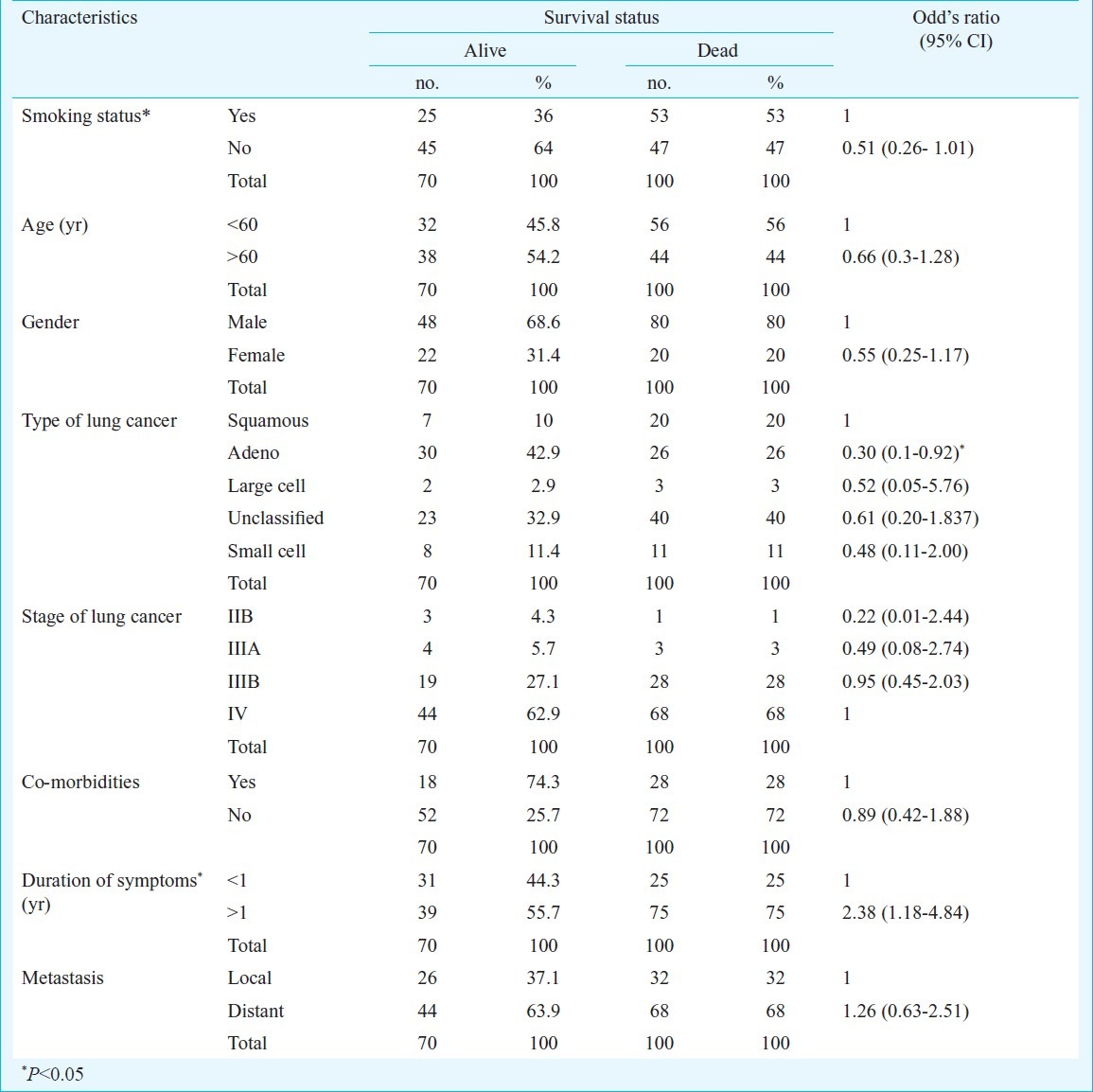
The survival status of 16 of the 170 subjects could not be ascertained as they were lost to follow up, but have been included in the survival analysis. There were 151 non-small cell lung cancer (NSCLC) and 19 small cell lung cancer (SCLC) patients in the study group. There were 128 males and 42 females. None of the females were smokers. The demographic and clinical characteristics of the subjects are presented in Table I. More than half of the patients with lung cancer were never smokers [92 (54.1%)]. A higher proportion of never-smokers [63 (68.3%)] presented before the age of 60 yr as compared to smokers [31 (40.3%)]. The mean age of presentation in non-smokers was 50.55 ± 11.53 yr and that for smokers was 56.78 ± 13.32 yr. The most common stage of lung cancer at presentation was stage IV, followed by stage IIIB, both of which accounted for [61 (96.7%)] of non-small cell lung cancer presentation at diagnosis among smokers and [81 (92%)] among never-smokers. Nearly three-fourths of small cell lung cancer patients presented in the extensive stage. Adenocarcinoma was the most common non small cell lung cancer and nearly one-third of the subjects had unclassified lung cancer. A higher proportion of never-smokers had local metastasis [58 (63%)] and smokers had distant metastasis [40 (51.3%)]. Nearly one-third of the subjects presented with symptoms of less than one month duration and three-fourth within three months. Less than one-fourth of the subjects had co-morbidities, hypertension [21 (12.5%)], diabetes [21 (12.5%)] and ischaemic heart diseases [9 (5.14%)]. Majority of the patients opted for treatment [149 (87.6%)].
Table I.
Characteristics of demographic and clinicopathologic variables with respect to survival status
Kaplan Meier survival analysis: At the time of analysis, with a follow up of 30 months, a total number of 100 deaths (58.8%) had occurred. Seventy patients (41.2%) were alive, which included 16 patients who were lost for follow up. Subjects with small cell lung cancer were excluded from the Kaplan Meier survival analysis. A subgroup analysis comparing survival between non-small cell lung cancer and small cell lung cancer was carried out separately and did not show any significant difference (data not shown). There were no significant differences in survival across age, gender, type of lung cancer and presence of distant metastasis. The survival rates at 30 months according to Kaplan Meier analysis was higher in patients above the age of 60 yr (32% in patients with age less than 60 yr, 46% in those above 60 yr) and in females (37.5% in males, 52% in females). Squamous cell carcinoma had the least survival at the end of 30 months (survival rates in patients with squamous, adenocarcinoma and others were 26, 53 and 38%, respectively) and patients with distant metastasis had lower survival (44% in patients with local metastasis vs. 39 per cent in patients with distant metastasis). The 30 month survival for stage IV lung cancer was 27 per cent and stage IIIB was 32 per cent. A significant difference in survival was observed according to smoking status and duration of symptoms suggestive of lung cancer (P<0.05). The 30-month overall survival rate for smokers and never-smokers were 32 and 49 per cent, respectively, and the 30-month survival was 34 per cent in patients with symptoms more than one month versus 55 per cent in patients who had symptom duration of less than 1 month.
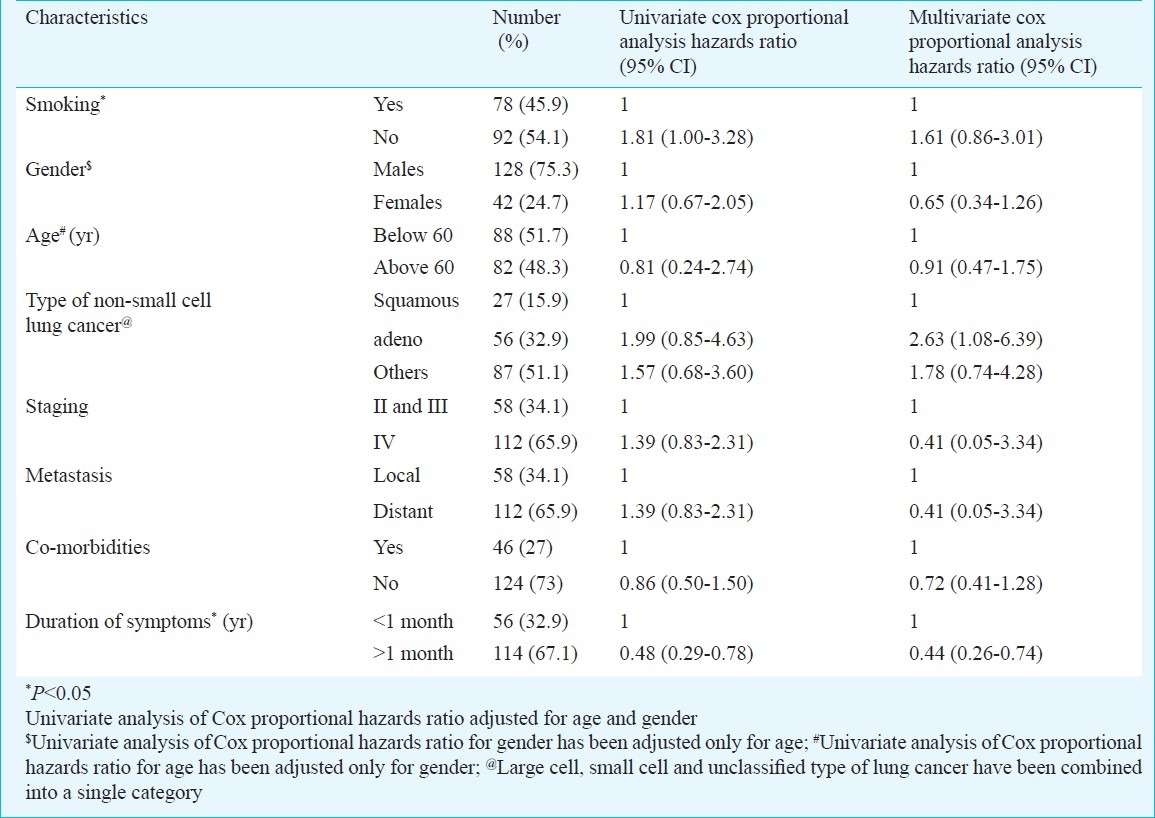
Cox-proportional hazards analysis: Univariate Cox proportional hazards ratios were calculated for different variables (Table II). The unadjusted hazards ratios were not different across age groups, gender, type of lung cancer, presence of distant metastases and staging. The hazards ratio of death increased in non-smokers and with shorter duration of symptoms (Table II). Multivariate Cox proportional hazards model for the factors utilized in univariate analysis, found that the patients with adenocarcinoma and with shorter duration of symptoms of less than one month had significantly higher hazards of death (Table II).
Table II.
Univariate cox proportional hazards analysis of factors affecting survival in subjects with lung cancer
Discussion
There is a paucity of data on lung cancer survival in the Indian population. Lung cancer survival studies have demonstrated different characteristics of lung cancer and factors affecting survival in Asians. This study showed the preponderance of never-smokers with lung cancer (>50%) as compared to smokers, earlier age at presentation in never-smokers, short duration of symptoms before presentation (>70% presented with symptoms less than 3 months), advanced stage of presentation with short duration of symptoms (>60% in stage IV) and paucity of associated co-morbidities (<25%). Adenocarcinoma was the most common lung cancer among those that could be classified. Most of the subjects presented with advanced lung cancer and there was no difference in survival according to age, gender, type of lung cancer and presence of distant metastases.
The main outcome of the study was all-cause mortality in smokers and never-smokers. As suggested by Tammenagi3 all-cause mortality was chosen over lung cancer specific mortality in the present study due to the following reasons: (i) There is no reason to think that death in lung cancer subjects occur due to cancer or competing causes in a mutually exclusive manner, (ii) There is a great likelihood of assignment bias, that is, many subjects with lung cancer have been assigned cause of death as lung cancer even when carefully followed up patients in an earlier study17 have shown no evidence of cancer progression; and (iii) Misclassification in the cause of death can be expected when clinical autopsies to confirm the cause of death are not routinely performed and such misclassifications could lead to spurious associations18.
Earlier studies have demonstrated that survival is better in never-smoker lung cancer patients as compared to smokers, both current and former smokers, smokers of either gender, at any stage, all age groups, across ethnicities and histopathological subtypes3–6. A significant difference in the 5-year overall survival rates for never-smokers (23%) versus smokers (16%) was observed and smoking was an independent risk factor for death7. Bryant6 reported better 5-year survival rates among never-smokers (64%) compared to smokers (56%). Five-year survival rates were poorer if the subjects had smoked greater than 20 pack years; 48 per cent (for 20-40 pack years) and 35 per cent (for >40 pack years). The stage-specific 5-year survival rate was greater for never-smokers compared to smokers across all stages; stage I (75% vs. 62%), stage II (53% vs. 46%) and stage III (41% vs. 36%), respectively. In advanced lung cancer, no differences in survival between smokers and never-smokers could be observed9,16. We observed that the never-smokers had poorer survival as compared to smokers in univariate Cox's proportional hazards analysis adjusted for age and gender, but not in multivariate Cox's proportional hazards analysis. A significant proportion of our subjects with lung cancer were non-smokers. Never-smokers had a 60 per cent higher hazard of dying as compared to smokers. It has been suggested earlier that lung cancer in never-smokers is a separate disease entity with a different pathobiology19,20. Risk factors other than tobacco smoking are important in these subjects such as biological, environmental, occupational and socio-economic and include cooking fumes21, hormones22 and viral infections23. More studies are needed to understand non-smoking lung cancer pathogenesis and disease behaviour in the Indian population.
The duration of symptoms at presentation and survival is a complex variable encompassing patient behaviour, clinical course, functioning of the health system and tumour biology24. Studies on lung cancer subjects assessing the symptom duration and survival, found a relationship between duration of symptoms and survival18,19,24. Lung cancer patients with a longer duration of symptoms at presentation had more advanced stage of lung cancers and consequently, poorer survival25,26. In our study longer duration of symptoms was found to be associated with a better survival both on univariate and multivariate Cox's proportional hazards analysis. Subjects with longer duration of symptoms had less than 50 per cent hazards of dying as compared to subjects with symptoms of duration less than one month at the time of diagnosis. The reason for this could include both patient and physician associated factors as well as tumour biology in the Indian population. Patients may have poor perception of their symptoms and may not relate it to lung cancer until they find it troublesome, the primary physician may treat symptomatically and thus the patients may find relief from their early symptoms. Though the duration of symptoms was obtained by a physician interview of the patients for this study, the data were obtained by chart review and a detailed questionnaire may be needed to elicit symptoms that were mild enough for the patients to ignore, but were related to lung cancer.
It has been observed that never-smokers were diagnosed with lung cancer 5 years earlier than their smoking counterparts27. Toh et al5 noted that younger patients with lung cancer were more likely to be never-smokers in Asian population, but conflicting reports originated in the western population. In Nordquist's study population, 30 per cent of never-smokers were below 55 yr compared to 36 per cent of current smokers and they have not studied the age of presentation to overall survival status7. In our study, the mean age at diagnosis in smokers were 56.78 ± 13.32 yr and in never smokers were 50.55 ± 11.53 yr confirming the earlier observations27. Never smokers with lung cancer in our study presented 6 years earlier to smokers with lung cancer.
Toh et al27 reported that age at diagnosis was an important prognostic factor affecting survival. Every 10-year increase in age resulted in approximately 30 per cent increase in hazard of dying. Tammenagi et al3 also reported an approximately 40 per cent increase in hazard for every 10-year increase in age. In our study we observed that increasing age (>60 yr) at diagnosis did not confer a higher hazard of dying due to lung cancer [Univariate HR 0.81 (95% CI, 0.24-2.74), multivariate HR 0.91 (95% CI 0.47-1.75)].
Studies evaluating gender effects on survival showed varying results. Bryant6 and Tammenagi3 could not demonstrate any significant survival benefits in women as compared to men. Earlier studies had shown that women with NSCLC had better survival rates than men8,9,28,29. We did not find any difference in survival between men and women after adjusting for age.
Increasing stage of NSCLC has been found to deleteriously affect survival3,5,6,19 with an increasing HR of dying with increasing stage as compared to stage I lung cancer. For each stage the hazard of dying was greater for smokers as compared to never-smokers12 and the greatest effect was observed in stage I and II lung cancers. Staging has been shown to be an important prognostic factor in patients with adenocarcinoma12. We did not observe any significant difference in survival in subjects with stage IV as compared to combined stage II and III lung cancer in our population, which is due to the fact that we did not have sufficient subjects in stage I and II lung cancer unlike other studies.
Co-morbidities could be important determinants of lung cancer survival. Smokers especially are at an increased risk of co-morbidities3 and other malignancies. Several studies30,31 have shown that around 20-40 per cent of patients with non-metastatic lung cancer died without any evidence of cancer progression, perhaps due to associated co morbid conditions3. Our study population had low prevalence of co-morbidities, and a non-significant lower hazards of death was observed in subjects with lung cancer without co morbidities, which is most likely due to the small sample size and the lower prevalence of co-morbidities in the study population.
Our study had several limitations. Most of the data except on present survival were collected from chart review, with attendant limitations accompanying a retrospective study. The study population consisted mostly of subjects with advanced lung cancer (stage III and stage IV) and there were not many subjects with stage I and II lung cancer and thus the observations were limited to advanced lung cancer. The comparison of smokers and never-smokers was limited to active smoking only and the details of presence of exposure, duration and degree of exposure to passive smoking and exposure to other noxious gases such as biomass and fossil fuels could not be elicited as there were no direct patient interviews. The sample size was small and may not have been powered adequately for several sub group analysis and therefore, the results need to be confirmed in a larger study.
In conclusion, our study demonstrated that the survival rate at 30 months was higher in patients >60 yr of age, in females than in males. Over 85 per cent of the subjects opted for treatment, but survival rates were lower compared to other ethnic populations. A higher proportion of unclassified (poorly differentiated) lung cancer was observed. Never-smokers presented with lung cancer earlier, lung cancer in Indians appeared to be more aggressive, involved younger subjects, was associated with poorer survival as compared to other ethnic populations and presented in advanced stages of lung cancer with a short duration of symptoms before diagnosis. There is a need for studies to evaluate the risk factors for lung cancer in never-smokers population including gene profiling to evaluate disease susceptibility.
Acknowledgment
The authors acknowledge the contribution of Dr Amruthavalli, Associate Professor, Centre for Information Science and Technology, University of Mysore, Karnataka.
References
- 1.Makitaro R, Paakko P, Huhti E, Bloigu R, Kinnula VL. Prospective population-based study on the survival of patients with lung cancer. Eur Respir J. 2002;19:1087–92. doi: 10.1183/09031936.02.00048302. [DOI] [PubMed] [Google Scholar]
- 2.Barnes DJ. The changing face of lung cancer. Chest. 2004;126:1718–21. doi: 10.1378/chest.126.6.1718. [DOI] [PubMed] [Google Scholar]
- 3.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest. 2004;125:27–37. doi: 10.1378/chest.125.1.27. [DOI] [PubMed] [Google Scholar]
- 4.Ito Y, Ohno Y, Rachet B, Coleman MP, Tsukuma H, Oshima A. Cancer survival trends in Osaka, Japan: the influence of age and stage at diagnosis. Jpn J Clin Oncol. 2007;37:452–8. doi: 10.1093/jjco/hym047. [DOI] [PubMed] [Google Scholar]
- 5.Toh CK, Gao F, Lim WT, Leong SS, Fong KW, Yap SP, et al. Never-smokers with lung cancer: epidemiologic evidence of a distinct disease entity. J Clin Oncol. 2006;24:2245–51. doi: 10.1200/JCO.2005.04.8033. [DOI] [PubMed] [Google Scholar]
- 6.Bryant A, Cerfolio RJ. Differences in epidemiology, histology, and survival between cigarette smokers and never-smokers who develop non-small cell lung cancer. Chest. 2007;132:185–92. doi: 10.1378/chest.07-0442. [DOI] [PubMed] [Google Scholar]
- 7.Nordquist LT, Simon GR, Cantor A, Alberts WM, Bepler G. Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest. 2004;126:347–51. doi: 10.1378/chest.126.2.347. [DOI] [PubMed] [Google Scholar]
- 8.Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol. 2002;13:1087–93. doi: 10.1093/annonc/mdf187. [DOI] [PubMed] [Google Scholar]
- 9.McDuffie HH, Klaassen DJ, Dosman JA. Female-male differences in patients with primary lung cancer. Cancer. 1987;59:1825–30. doi: 10.1002/1097-0142(19870515)59:10<1825::aid-cncr2820591024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Yoshino I, Kawano D, Oba T, Yamazaki K, Kometani T, Maehara Y. Smoking status as a prognostic factor in patients with stage I pulmonary adenocarcinoma. Ann Thoracic Sur. 2006;81:1189–93. doi: 10.1016/j.athoracsur.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu H, Tominaga S, Nishimura M, Urata A. Comparison of clinico-epidemiological features of lung cancer patients with and without a history of smoking. Jpn J Clin Oncol. 1984;14:595–600. [PubMed] [Google Scholar]
- 12.Linden G, Dunn JE, Jr, Hom PH, Mann M. Effect of smoking on the survival of patients with lung cancer. Cancer. 1972;30:325–8. doi: 10.1002/1097-0142(197208)30:2<325::aid-cncr2820300203>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Kirsh MM, Tashian J, Sloan H. Carcinoma of the lung in women. Ann Thoracic Sur. 1982;34:34–9. doi: 10.1016/s0003-4975(10)60849-1. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson MK, Wang J, Hoffman PC, Haraf DJ, Olak J, Masters GA, et al. Sex-associated differences in survival of patients undergoing resection for lung cancer. Ann Thoracic Sur. 2000;69:245–9. doi: 10.1016/s0003-4975(99)01078-4. discussion 9-50. [DOI] [PubMed] [Google Scholar]
- 15.Frederick L Greene, Balch CM, Flem ID. AJCC cancer staging handbook:TNM Classification of malignant tumours: Springer. 2002:469. [Google Scholar]
- 16.Prasad R, James P, Kesarwani V, Gupta R, Pant MC, Chaturvedi A, et al. Clinicopathological study of bronchogenic carcinoma. Respirology. 2004;9:557–60. doi: 10.1111/j.1440-1843.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 17.Miller AB, Yurgalevitch S, Weissfeld JL, Prostate LC. Ovarian Cancer Screening Trial Project T. Death review process in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled Clin Trials. 2000;21(6 Suppl):400S–6S. doi: 10.1016/s0197-2456(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 18.Goodman MT, Kolonel LN, Wilkens LR, Yoshizawa CN, Le Marchand L. Smoking history and survival among lung cancer patients. Cancer Causes & Control: CCC. 1990;1:155–63. doi: 10.1007/BF00053167. [DOI] [PubMed] [Google Scholar]
- 19.Gealy R, Zhang L, Siegfried JM, Luketich JD, Keohavong P. Comparison of mutations in the p53 and K-ras genes in lung carcinomas from smoking and nonsmoking women. Cancer Epidemiol Biomarkers Prev. 1999;8:297–302. [PubMed] [Google Scholar]
- 20.Hainaut P, Pfeifer GP. Patterns of p53 G-->T transversions in lung cancers reflect the primary mutagenic signature of DNA-damage by tobacco smoke. Carcinogenesis. 2001;22:367–74. doi: 10.1093/carcin/22.3.367. [DOI] [PubMed] [Google Scholar]
- 21.Metayer C, Wang Z, Kleinerman RA, Wang L, Brenner AV, Cui H, et al. Cooking oil fumes and risk of lung cancer in women in rural Gansu, China. Lung Cancer. 2002;35:111–7. doi: 10.1016/s0169-5002(01)00412-3. [DOI] [PubMed] [Google Scholar]
- 22.Taioli E, Wynder EL. Re: Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst. 1994;86:869–70. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 23.Cheng YW, Chiou HL, Sheu GT, Hsieh LL, Chen JT, Chen CY, et al. The association of human papillomavirus 16/18 infection with lung cancer among nonsmoking Taiwanese women. Cancer Res. 2001;61:2799–803. [PubMed] [Google Scholar]
- 24.Maguire A, Porta M, Malats N, Gallen M, Pinol JL, Fernandez E. Cancer survival and the duration of symptoms. An analysis of possible forms of the risk function. ISDS II Project Investigators. Eur J Cancer. 1994;30A:785–92. doi: 10.1016/0959-8049(94)90293-3. [DOI] [PubMed] [Google Scholar]
- 25.Bourke W, Milstein D, Giura R, Donghi M, Luisetti M, Rubin AH, et al. Lung cancer in young adults. Chest. 1992;102:1723–9. doi: 10.1378/chest.102.6.1723. [DOI] [PubMed] [Google Scholar]
- 26.Skarin AT, Herbst RS, Leong TL, Bailey A, Sugarbaker D. Lung cancer in patients under age 40. Lung Cancer. 2001;32:255–64. doi: 10.1016/s0169-5002(00)00233-6. [DOI] [PubMed] [Google Scholar]
- 27.Toh CK, Wong EH, Lim WT, Leong SS, Fong KW, Wee J, et al. The impact of smoking status on the behavior and survival outcome of patients with advanced non-small cell lung cancer: a retrospective analysis. Chest. 2004;126:1750–6. doi: 10.1378/chest.126.6.1750. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson MK, Skosey C, Hoffman PC, Golomb HM. Sex-associated differences in presentation and survival in patients with lung cancer. J Clin Oncol. 1990;8:1402–7. doi: 10.1200/JCO.1990.8.8.1402. [DOI] [PubMed] [Google Scholar]
- 29.Agudo A, Ahrens W, Benhamou E, Benhamou S, Boffetta P, Darby SC, et al. Lung cancer and cigarette smoking in women: a multicenter case-control study in Europe. Int J Cancer. 2000;88:820–7. doi: 10.1002/1097-0215(20001201)88:5<820::aid-ijc21>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 30.Harpole DH, Jr, Herndon JE, 2nd, Wolfe WG, Iglehart JD, Marks JR. A prognostic model of recurrence and death in stage I non-small cell lung cancer utilizing presentation, histopathology, and oncoprotein expression. Cancer Res. 1995;55:51–6. [PubMed] [Google Scholar]
- 31.Tammemagi MC, McLaughlin JR, Mullen JB, Bull SB, Johnston MR, Tsao MS, et al. A study of smoking, p53 tumor suppressor gene alterations and non-small cell lung cancer. Ann Epidemiol. 2000;10:176–85. doi: 10.1016/s1047-2797(99)00048-4. [DOI] [PubMed] [Google Scholar]


