Abstract
Fifty years ago several thousand children were born with severe limb defects after their mothers had been given thalidomide in pregnancy. This tragedy caused procedures for licensing new medicines to become much stricter. Where, nevertheless, significant side effects were found it became common to sue for damages. These consequences have caused possibly an even greater disaster damaging many more people and threatening ruin to health services everywhere. The huge increase in both time and cost in bringing medicines to market is increasing their price to unsupportable levels; and only wealthy companies are now able to do so. This requires reform as does litigation for ‘statistical’ harmful effects.
Introduction
This year is the 50th anniversary of the withdrawal of thalidomide from the market, following what was probably the greatest ever pharmaceutical disaster.
Thalidomide was patented by Grunenthal in Germany in 1954, possibly having been developed originally as an antidote to nerve gas poisoning. However, it was launched in October 1957 as a sedative, a pain killer and an anti-emetic suitable for treating morning sickness in pregnancy. The following year it was licensed in the UK and in much of the rest of the world with the exception of the USA. There, Frances Kelsey—the Inspector at the Federal Drugs Authority (FDA)—wanted to see more pre-clinical studies because not all the rats in some of the animal experiments had been adequately sedated. That the USA was almost alone in not licensing thalidomide caused the FDA later to become even more risk averse than other regulatory authorities in its licensing procedures. In the pre-clinical testing, no tests had been performed on pregnant animals to check the effects on the foetus. Such testing was not usual at that time as it was generally not believed that drugs would cross the placenta and harm the foetus.1
However, between 1957 and 1961, when the drug was withdrawn, more than 10 000 children in 46 countries were born with congenital deformities, most usually in the skeletal system, of which phocomelia, the absence of limbs, was the most common. In the UK, 2000 affected babies were born, of whom only 466 survived.
This was a great tragedy though in terms of the number of people affected, it falls far short of the lethal consequences of the withdrawal of DTT as a pesticide in 1972 which, it is estimated, has caused several million deaths from malaria.2 While thalidomide was withdrawn in 1961, it has subsequently returned to the market for quite different indications. It has been proved of considerable value in treating erythema nodosum leprosum, a form of cutaneous leprosy, and also in treating blood cancer and multiple myeloma. It is a sad reflection that there have been some further cases of phocomelia in Brazil, where there are many cases of erythema nodosum leprosum,3 demonstrating how difficult it is to make sure that women being treated do not become pregnant.
The immediate consequences of the thalidomide tragedy were that testing all drugs for teratogenicity (possible ill effects on the foetus in pregnant animals) became universal. The affected children were also quite properly paid compensation since phocomelia is extremely rare and one can therefore be confident that all the cases seen were caused by the drug.
A further consequence was that the procedures for licensing drugs became much more rigorous, much lengthier and very much more expensive. These changes in the procedures for licensing drugs were accompanied by an extraordinary reduction in the public tolerance of risk in regard to all prescribed pharmaceuticals. So much so that it became customary to believe that any prescribed drug should be absolutely safe. This is an impossible aspiration as there can be no doubt that any compound with any pharmacological effect can produce undesirable as well as desirable reactions. (Curiously, similar risk aversion does not extend to herbal medicines or bush teas whose supply is virtually uncontrolled. These alternative medicines are by no means always safe. For example, there have been deaths from Aristolochia—a kidney poison—which has been found in some herbal remedies).4
An unintended consequence of these changes was that drugs have become ruinously expensive. It can now take more than 10 years and cost more than a billion dollars to bring a new drug to market.5 This in turn means that only large companies with very deep pockets can now afford to take drugs all the way from discovery to the market. This is itself undesirable since it does not allow innovative smaller companies to undertake drug development and this has probably prevented a variety of inventive and novel candidate drugs from coming into the market. It has similarly become relatively uneconomic to develop drugs for diseases that are neither so common that a ‘blockbuster’ drug can be developed or on the other hand so rare that the special ‘orphan drug’ regulations apply.6 Diseases that fall between these have been relatively neglected. To give an example from my own field of interest—the complement system—there has long been an interest among the specialists in this field to develop therapeutics aimed at modifying complement function. However, this has always proved difficult because the target diseases were all rather uncommon. However, in the last few years, it has become recognized that an extremely common disease, age-related macular degeneration, is largely due to genetic predispositions related to the complement system and this has transformed the attitude towards developing therapeutics in this field.
A final consequence stemming from the thalidomide affair is a substantial increase in litigation against drug companies whenever any harmful side effects occur. This has added further to drug costs and has led to drugs being withdrawn when there is no adequate reason for so doing. These problems of this ‘litigation culture’ will be discussed further below.
The cartoon (Figure 1) exemplifies the Law of Unintended Consequences better than any number of descriptions. It should be hung in the office of every decision maker since the Law of Unintended Consequences is ubiquitous. It was defined by R.K. Merton7 who stated that ‘the Law of Unintended Consequences often cited but rarely defined is that actions of people and especially of government always have effects that are unanticipated or unintended’. He identified five sources of unanticipated consequences: (i) ignorance and (ii) error, which are almost always involved; (iii) the imperious immediacy of interest (of which a good example may be the current changes being proposed in the National Health Service where the immediacy of interest has blinded the government to the harmful consequences which have been pointed out to them repeatedly); (iv) basic values—a good example here is the EU Physical Agents Directive.8 This draft directive would have led to all medical magnetic resonance imaging becoming impossible since the workers would have been subjected to more static radiation than the directive allowed. When this was pointed out to the relevant Directorate at the Commission, the response was that ‘all workers deserve equal protection’. This is an entirely fatuous response since, if implemented, it would lead immediately to the abolition of the fire service, closely followed by the police, medicine and nursing, deep sea fishing, coal mining … ; (5) the final source was ‘self-defeating prediction’. A possible example here is provided by the targets for waiting times which the Government felt would lead to patients being treated more rapidly. In reality the effect was rather the reverse since if, for example, there are penalties if an outpatient is not seen within a given time, the immediate effect is to reduce the number of patients who are booked into every clinic.
Figure 1.
The Drowned Prince. Reproduced with permission from the artist.
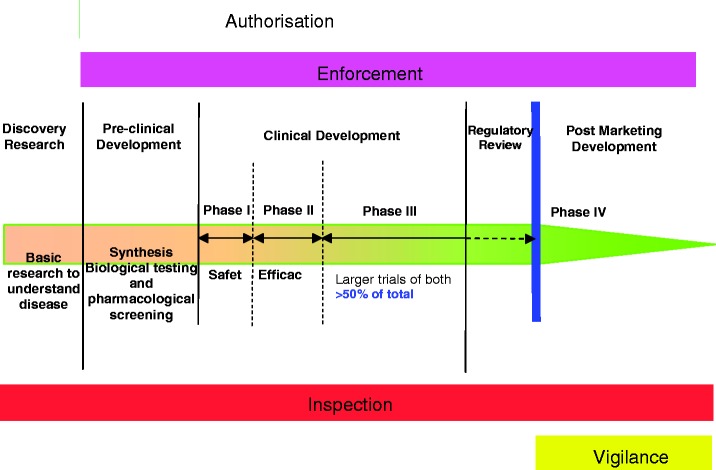
The drug development process
This process applies to all prescribed drugs, albeit not to alternative and herbal medicines (Figure 2). (The European Commission has tried to make the regulatory authorities provide an analogous process for homeopathic medicines9 which since they essentially contain only water has proved to be a somewhat difficult exercise). The regulatory process involves preclinical studies ensuring the purity and consistency of the drug formulations; studies of drug metabolism and clearance; and animal studies of safety and efficacy. Clinical studies follow to assess safety (Phase 1) and efficacy (Phase 2). There is no dispute that all such studies are necessary to protect the public against dangerous and sometimes fraudulent drugs.
Figure 2.
Drug development process. By courtesy of Sir Kent Woods at MRHA.
As can be seen in Figure 2, these necessary studies are essentially completed by the end of Phase 2 of clinical development. What follows, Phase 3, are much more extensive human studies done on patients comparing the test drug with either placebo or best available treatment. Their aim is to provide more information about efficacy and to identify less common side effects. In reality, Phase 2 studies may detect side effects to about one in a hundred and Phase 3 to about one in a thousand. Side effects significantly rarer than this will be discovered only after release. Phase 3 trials are very expensive, comprising more than half the total cost of drug development. They are very lengthy and may take several years; and they produce immense amounts of data which need to be analysed, and this further slows down the approval system. This degree of evaluation before marketing is unique for pharmaceuticals. With all other consumer items, it takes place after they are on the market. After drugs are released, further development continues. Most studies to discover new targets, and to assess exactly how best to use the drug, are already done by controlled trials carried out after licensing. Figure 2 shows all the stages of drug development in which the Medicines and Healthcare products Regulatory Agency (MHRA) is involved and these go from the very earliest to well after licensing. I suggest that Phase 3 trials are not really necessary and probably do not lead to an increase in QALYS (quality adjusted life years) when the increased safety from identifying relatively rare side effects is likely to be outweighed by the harm caused by a good drug being made available years later and at vastly increased expense than it would have been at the end of Phase 2. Furthermore, no clinical trial group is ever fully representative of the population to which the drug is finally given. One reason for this is that being prepared to take part in a clinical trial is itself a confounding factor. Furthermore, in real life there are people who comply badly with treatment; who are taking other medicaments; or have other diseases, all of which are all usually excluded from clinical trials. For these reasons, efficient post-marketing surveillance is probably better for assessing the value of a drug than any trial.
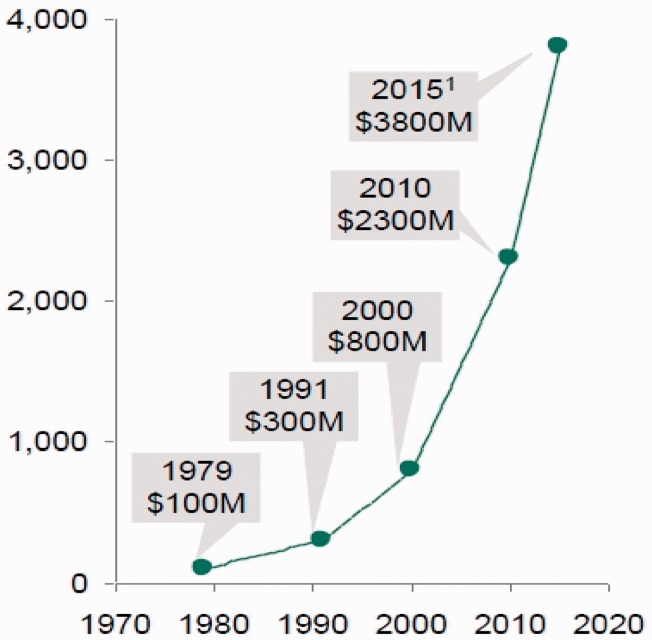
The cost per molecule coming to market has increased in recent years in an alarming fashion and shows no sign of slowing down (Figure 3).
Figure 3.
Cost per molecule (including cost of failure). The Boston Consulting Group 2011.5
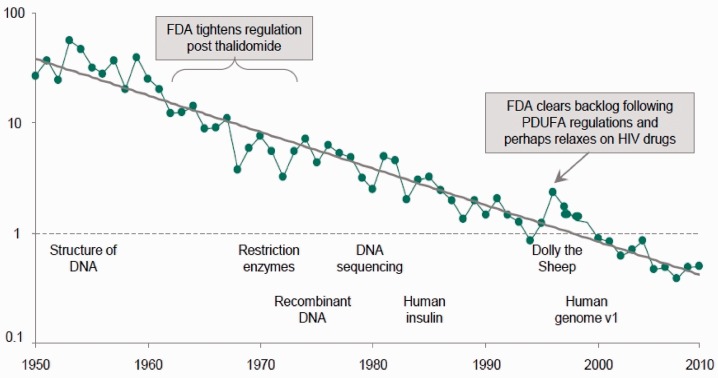
This has been accompanied by a sharp reduction in the number of new medical entities (NMEs) coming to market per million dollars research and development spend (Figure 4).
Figure 4.
NMEs per $B R&D spent (inflation adjusted). Reproduced and modified with permission from Bernstein Research paper which forms the basis of reference 10.
There has been a fall of ∼100-fold over the last 60 years in drugs coming to market per billion dollars research and development spend. The Bernstein Research Group,10 whose data this is, suggests causes for this. The first, the ‘cautious regulator problem’, is what concerns us here. This they define as the ‘cumulative ratchet effect of the regulators’ low risk tolerance where each sin by the industry or genuine drug misfortune tightens the ratchet and few events ever loosen it’. It may be added that the risk aversion of the FDA in the USA is driven largely by fear of Congress. The members of the FDA are said to live in a continuous state of worried anticipation of being telephoned by congressmen. In the UK it is likely that it is fear of the tabloid press that similarly motivates some of the actions of the MHRA. The effect on the cost of drugs has certainly been dramatic. In one study of new anti-cancer drugs at launch, it was estimated that the current monthly cost of treatment at 2007 prices was about $7000 in 2005–2008, compared to a figure of about $500 in 1985–1989 with a steady increase in between.11 It is quite clear that this range of increase is insupportable even in countries with very high GDP, let alone those who are poorer. There is a roughly linear relationship between health expenditure and GDP and even the richest countries do not spend more than about $6000 per year per head of population, itself a colossal sum.
The remedies
It is about 10 years since I have been campaigning on this issue. The first public manifestation was an article in the New Statesman of 23 July 2001 by Michael Hanlon with the provocative name ‘How the law keeps us ill’. There he made all the points that have been made above including some on litigation which will follow. Five years ago, the Cooksey report12—‘A review of UK health research funding’ - was published. The final chapter of this report was on the subject of ‘A new drug development pathway‘ and in this chapter Sir David Cooksey recommends changes to the regimen of drug regulation which have some similarity to what is proposed here. Recommendation 18 of the Executive Summary reads: ‘the review believes that if the UK is to succeed in achieving its health and economic objectives, the government must consider ways of bringing drugs that address UK health priorities to market faster but without compromising patient safety. It is increasingly clear that the current way of developing drugs in the private sector is unsustainable in the long term’. He goes on to recommend the earlier conditional licensing of new drugs and the use of the NHS national programs to ensure more rapid assessment of emerging side effects and efficacy over longer periods. This part of the Cooksey Report was totally ignored by the government and no action has been taken to implement any of his recommendations. I remember being invited by Sir Alasdair Breckenridge to take part in a Medicines and Healthcare Regulatory Authority meeting on the Cooksey Report because, he told me, I was the only person he had met who agreed with Cooksey. In the last 5 years, however, this has changed and these ideas may now have reached the point when their time has come. More recently proposals have even been made in the USA to amend the FDA processes. Kay Hagan, a US senator has proposed that changes should be made to derive fast-track evaluations of medicines for conditions with no approved cures.
I would propose that, where Phase 2 trials show favourable risk-benefit, remedies should be made available to those patients who agree to waive their litigation rights and who further agree to participate fully in follow-up surveillance. Such a system would, of course, need to be trialled. Introducing such changes in the ‘big bang’ manner so beloved of politicians is certainly unwise. Interested patients would be given a brochure containing the results up to the end of Phase 2 expressed in understandable language and explaining the risks and uncertainties. Those that then wish to proceed would sign a legally binding indemnity against any possible ill effects.
Unfortunately, this is currently not possible because it conflicts with the strict liability provisions of the Consumer Protection Act of 1987 and the European Directive on Product Liability. This is a problem that would need to be sorted out. It would also be necessary to persuade the patient population at large that no remedy is ever entirely risk free and the myth that they ought to be should finally be abandoned. The great advantage of having a system of this kind running in parallel with the conventional drug licensing is that it would allow the real cost per QALY that is saved by Phase 3 to be measured. It is likely that these figures would be negative and that there are real advantages to patient survival and wellbeing (quite apart from the economic benefits) of having more rapid drug licensing of drugs that have done well in early development.
The legal situation
Historically, a pre-requisite for imposing liability for damages on a drug company (as on any manufacturer) was the ability to prove fault. Where, as usual, there was no direct contractual link between the manufacturer and the consumer, liability would have been in the tort of negligence, according to the principles set out by the House of Lords in the famous case of Donoghue v Stevenson.13 To succeed in such a claim, it would have been necessary for the patient to show that his or her injuries were the result of the manufacturer’s failure to comply with the behavioural standards of what was (and is) referred to as the standard of the ‘reasonable man’: meaning in this case, a reasonable manufacturer in the position of the defendant. The law helped the consumer to establish fault by allowing negligence to be established by circumstantial rather than direct evidence—a rule which lawyers sometimes expressed (and still express) in Latin as ‘the doctrine of res ipsa loquitur’. Where something went wrong which, on the face of it, could not have gone wrong without someone being negligent, a prima facie case would be established and it would be up to the defendant to rebut it. As Lord Wright said in one of the leading cases, Grant v Australian Knitting Mills, ‘[the claimant] is not required to lay his finger on the exact person in all the chain who was responsible or to specify what he did wrong …’.14 In theory, strict liability might exist under the law of contract in a case where a direct contractual link between manufacturer and consumer existed. In such a case the manufacturer might be strictly liable for breach of warranty of fitness for purpose under the Sale of Goods Acts. But as the patient rarely buys his medicines directly from the manufacturer, normally a manufacturer would only be liable to a patient who claimed to have suffered ill-effects from his medicine in the law of tort, which in those days meant the tort of negligence.
When describing this body of legal rules the past tense has been used, although as every lawyer knows, or ought to know, it still exists, enabling drug companies that were demonstrably negligent to be sued on that account. But as will be explained shortly, negligence liability at common law has long been outflanked by a statutory form of strict liability created by the Consumer Protection Act 1987.
Another feature of the rules as they formerly existed at common law was that if a patient agreed to take a medicine that he knew had not been fully tested, and on the basis that he voluntarily assumed the risk, that would preclude any later claim by the patient if some harm later materialized. This was because of another legal rule that lawyers customarily express in Latin: volenti non fit injuria—no legal injury is done to a person who consents to it. Unlike the common law rules establishing liability described in the previous paragraph, which still exist (albeit in the background), this rule restricting liability has now been seriously qualified. First, in 1977 section two of the Unfair Contract Terms Act was enacted. This invalidates, within the context of ‘business liability’, any contractual term or notice to the extent that it would ‘exclude or restrict [a defendant’s] liability for death or personal injury resulting from negligence’; and for good measure, subsection three goes on to provide that ‘Where a contract term or notice purports to exclude or restrict liability for negligence a person’s agreement to or awareness of it is not of itself to be taken as indicating his voluntary acceptance of any risk’. Continuing in this spirit and going further, section seven of the Consumer Protection Act 1987 then expressly invalidated any agreement or undertaking by which the consumer of a product might be said to agree to bear the risk of its causing him damage for which the manufacturer would otherwise be liable as a result of the strict liability imposed on manufacturers by Part I of this Act. According to this section:
The liability of a person by virtue of this Part to a person who has suffered damage caused wholly or partly be a defence in a product, or to a dependant or relative of such a person, shall not be limited or excluded by any contract term, by any notice or by any other provision.
The Consumer Protection Act 1987 was enacted to implement the European Community Directive 85/374/EC, usually known as the Product Liability Directive (The Act was notable in that it was the first occasion that the UK government implemented an EC Directive by means of an Act of Parliament, rather by an Order under the European Communities Act of 1972. To a person from outside the legal scene, this seems an unfortunate precedent!). The impetus behind this Directive, as is well known, was the thalidomide tragedy, and the difficulties which the previous law had allegedly presented to the deformed children in establishing the conditions necessary to engage the tortious liability the manufacturers.
Section 2 of the 1987 Act imposes on the ‘producer’ of a product (and on certain other people too) a new form of tortious liability where ‘damage is caused wholly or partly by a defect in a product’. The meaning of ‘defect’ is then explained in Section 3, which provides that ‘there is a defect in a product for the purposes of this Part if the safety of the product is not such as persons generally are entitled to expect’.
When this provision was first enacted, doubts existed as to quite how strict this new liability would be. These doubts turned upon the meaning of the phrase ‘persons generally are entitled to expect’. Just what is the level of safety in respect of products that persons generally are ‘entitled’ to expect? In reality, no product can be made absolutely safe, however much care the manufacturer is prepared to take. So can it be said that public is ‘entitled’ to expect products to be safer than it is possible for manufacturers with the exercise of reasonable care and skill to make them? When the courts were called upon to answer this question, they did so with a resounding ‘yes’.
The case in this line of decisions which is of particular relevance to medical law is A and Others versus the National Blood Authority and Others.15 There was a test case, the litigation being brought by 114 claimants who had been infected with hepatitis C virus by blood transfusion or blood products. The claimants did not assert negligence on the part of the National Blood Authority but sued on the basis that the defendants were subject to strict or objective liability by virtue of the Consumer Products Act. In this claim they were successful, even though at the time in question the risk of contamination, though known, was unavoidable.
Mr Justice Burton’s judgment in the case is very long and very erudite. But at the risk of drastic over-simplification, I think the key points that emerge from it as far as the present discussion is concerned are the following. First, people are not entitled to expect absolute safety in all circumstances. But secondly, there can still be liability even when the level of safety they do expect is physically impossible. And thirdly, risk-benefit calculations do not count and it is irrelevant that nothing could have been done to make the product safer. From my perspective, which is that of a research scientist and a citizen whose natural reflex is to feel that in principle nobody should be held liable (whether civilly or criminally) unless they are in some way to blame, the first of these three propositions makes good sense; the second is neither sensible nor just; and the third is completely mad, in as much as assessment of risk-benefit is entirely central to all decision making in medicine. Risk-benefit analysis is also important because it allows consideration of public as well as individual benefit. (Vaccination is a good example here.)
Though widely regarded as a ‘good thing’ because it provides automatic compensation for the consumer who suffers illness or injury, it seems to me that, when viewed from the other end of the telescope, the strict liability that is now imposed on manufacturers of drugs is in fact distinctly bad. First, as a matter of morality it seems (at least to me) to be unfair to impose liability on those who are not to blame. To this, as I am aware, the orthodox answer is ‘the law of tort is not concerned with blame, but with loss distribution; so it is right to make the manufacturer compensate for the loss, because he can afford to pay compensation out of the profits that he makes’. But that leads to the second objection, which is this. If the immediate consequences of the law are good in that those who suffer injury or illness receive compensation, in the case of medicines (if no other types of product) the broader social consequences are bad because—as is the thesis of this article—it helps to create a situation in which useful medicines are less readily available.
I believe that the common law of negligence, by which this area was previously governed, was inherently superior to the strict liability that since 1987 has supplanted it. It is desirable, of course, that those who suffer unexpected consequences from new medicines should be compensated. But if this is to be done, the proper way to do it is, surely, is not by extending the civil liability of the drug companies but to set up a general compensation scheme that is funded by the State from general taxation, as was done over 30 years ago, indeed, in the case of vaccine damage, by the Vaccine Damage Payments Act 1979. This Act was passed in consequence of concern about the fact that, in rare cases, undesirable side effects can result from vaccination against whooping-cough. The argument was that vaccination against whooping-cough and a number of other serious diseases greatly reduces the incidence of these diseases and hence is highly beneficial for society in general. And in the light of that, it is fair that society in general should compensate the small number of individuals who suffer unfortunate side effects as a result of it. (The background to the Act is explained at some length by Richard Jones in his commentary to the Act in Current Law Statutes. Information on the scheme can be found online at http://www.direct.gov.uk/en/MoneyTaxAndBenefits/BenefitsTaxCreditsAndOtherSupport/Disabledpeople/DG_10018714).
The litigation culture
Where companies or people cause harm by negligence, fraud, deceit or other malfeasance, they should expect to be called to account. However, where patients suffer harm from a very rare side effect, or where the harm is only statistically related to the drug use and direct causality cannot be established, the growing practice of litigation against the drug companies has no obvious justification and has certainly done great harm to health care. The fear of litigation is, in reality, what drives a great deal of the regulation of medicines. The regulators claim that their regulation is there to achieve favourable risk-benefit and cost-benefit ratios—indeed that is what National Institute for Health and Clinical Excellence (NICE) was set up to do—but to some extent this is certainly a pious fiction and it is the fear of litigation that drives a lot of the activity. There is little doubt that the public, especially those who comment on this subject on blogs, enthusiastically support suing drug companies. It is also clear that they fail to appreciate that it is they the consumers who really pay the compensation in higher drug prices. There is also an element of great unfairness in this as it seeks to compensate only those where fault can be shown; whereas others may suffer similarly where no fault can be shown and get no compensation at all.16 It would obviously be more desirable to have a system where proper medical and social care is available to those who need it, independent of whether or not their problem is somebody else’s fault.
There are two revealing examples of recent class actions involving drugs. The first concerns AstraZeneca and the drug Seroquel, where substantial sums were recently paid in settlement of claims, the merit of which has never been decided in any court.17 On 18 March 2010, a jury in a New Jersey court ruled in favour of AstraZeneca by rejecting a plaintiff’s claims that Seroquel caused his alleged injuries. This was the first product liability case to go to trial. Nine previous cases were dismissed by both federal and state court judges, and approx 2600 additional cases have been abandoned by the plaintiffs’ attorneys. On 9 August 2010, Astra Zeneca agreed with attorneys representing approximately 17 500 Seroquel product liability claimants in the USA to pay approximately $198 million. Terms of settlement were not revealed.
If there were any association between taking Seroquel and diabetes and obesity it would be only statistical and in no individual case could one be sure that the diabetes and obesity suffered by one of the patients with psychosis who take Seroquel was actually due to the drug.
The second example is that of GlaxoSmithKline and the drug Rosiglitazone (Avandia). Rosiglitazone is a PPARγ activator which is used to improve insulin sensitivity in Type 2 Diabetes and has been claimed to increase cardiovascular (CVS) events. Compared with Pioglitazone (Actos), Rosiglitazone was associated with an increased risk of the composite of acute myocardial infarction, stroke, heart failure, or all-cause mortality in patients ≥65 years. Odds Ratio for this composite was 1.18 (1.12–1.23). This corresponds to 1.68 (1.27–2.08) excess events per 100 person-years of treatment,18 i.e. Pioglitazone is a slightly safer drug with regard to CVS events. However, there are some doubts about an association with bladder cancer.
In February 2011 GlaxoSmithKline paid $250 million to settle 5500 suits, an average of $46 000 per plaintiff—the same amount paid in the 10 000 suit settlement in July 2010 but $40 000 per plaintiff less than a previous payment of $60 million for 700 lawsuits—a total of $770 million. This is all recovered in the cost of medicines. It is the consumer and not the drug company who pays. Presumably this persuades the companies to settle these actions, which could be seen, in my view, as the equivalent of paying off hostage takers.
These class actions, which come largely from the USA, are frequently advertised and Figure 5 shows an advertisement from a website related to both Rosiglitazone (Avandia) and Pioglitazone (Actos), where a group of lawyers is actively pursuing people promising that they would include them in such actions without any fees or expenses unless recovery is obtained. There are serious moral objections to this sort of advertising and of course these lawyers do not act entirely out of altruism but also for their own profit.
Figure 5.
Advertising class actions—a public harm.
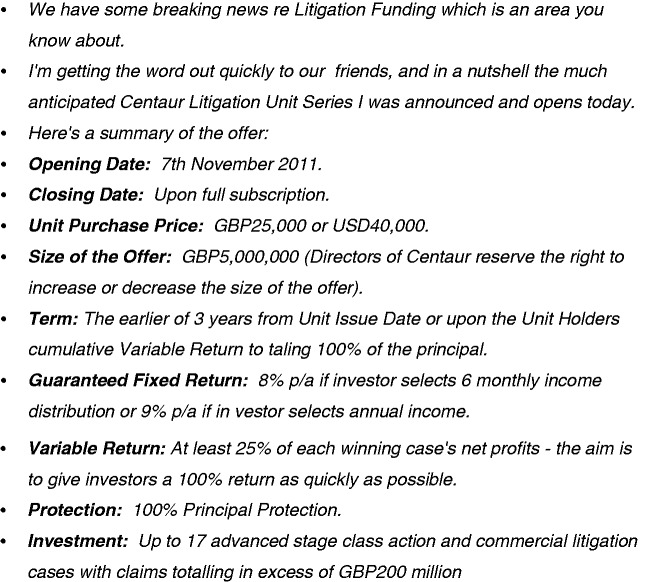
Figure 6 reproduces an email which I was sent unsolicited just before I gave this lecture and is a UK example. This is from an investor group who are wishing to launch a litigation fund in order to raise money for such class actions and promising their investors a substantial level of return. This strikes me as being ethically equivalent to, or even worse than, investing in tobacco shares.
Figure 6.
Litigation funding guarantees significant returns.
There are examples in the UK where class actions have been abandoned because they seemed unlikely to succeed. One is a class action against Sanofi Pasteur, on the grounds that sodium valproate, a valuable anti-epileptic drug, was claimed to produce birth defects. This was abandoned by the Legal Services Commission, after they had spent £3 million on it, because they were advised that there was not sufficient prospect of success. The plaintiffs’ lawyer commented that they were ‘forced to abandon’ the action not because the battle in court was lost but because continuing would be too great a financial risk for the claimants. He was also concerned about legal aid funding for group actions to ensure proper access to justice for individuals who suffer serious drug-induced injuries. It is curious that he clearly feels that it is the public duty to support such actions whether they succeed or not. Sodium valproate is a valuable drug and if patients need it to treat their convulsions they should take adequate precautions against pregnancy.
However, the litigation culture does not only cost money. It can also cost lives. Perhaps the most tragic example of this is the example of the Wyeth rotavirus vaccine. Wyeth introduced a living oral vaccine against rotavirus in 1998. Rotavirus is the commonest viral cause of diarrhoea, particularly in children and particularly in sub-Saharan Africa it is a major cause of death in early childhood. The vaccine was highly effective but it was found in trials to be associated with an increased incidence of intussusception, a complication where a piece of bowel folds itself into the next piece of bowel, probably because lymphoid follicles in the gut become swollen as a result of the vaccination. Intussusception is an uncommon spontaneous event in children and is not usually lethal. The vaccine was estimated to give rise to one extra case per 10 000 children vaccinated. For this reason the Advisory Committee on Immunisation Practices withdrew its recommendation for the use of the vaccine and Wyeth decided to withdraw it altogether in the USA. They then also withdrew it from sale anywhere in 1999, even though the risk:benefit ratio in Africa was totally different from that in the USA. In the West rotaviral diarrhoea can be unpleasant and cause admission to hospital, but rarely kills children. In Africa it is a major cause of death and is responsible for about forty percent of the deaths from diarrhoea. No new vaccines against rotavirus were introduced until 2006 when Merck introduced one, and in 2008 when GlaxoSmithKline introduced another. These vaccines give less intussusception, about one extra case per 50 000 children vaccinated, and they remain in use. However, between 1999 and 2006 approximately three million children died of rotavirus infection in Africa. While not all of these would have been prevented by vaccination, a very substantial number would and this is a real tragedy.
At the root of this particular calamity is another important legal problem which is that the law recognizes a distinction between harm done by doing ‘something’ as compared with harm caused by doing ‘nothing’. In the case of litigation for vaccine damage, if a child suffers a side effect from a vaccine, compensation can be claimed from the vaccine manufacturers, but if many children die of a disease from which they could have been protected by vaccination—but were not—there is no liability. This distinction plays a large part in the controversies about active or passive euthanasia. Ethically the distinction is highly contentious with philosophers arguing plausibly and passionately on both sides. However, in the particular context of a doctor’s duty of care to patients there is a very strong case for denying any distinction and most doctors would agree to this. When a doctor is faced with having to make a decision, doing nothing is just one choice among many and is not categorically different from anything else. Unfortunately, however, it has become embedded into the legal system in the terms of a couplet from A. H. Clough’s (1819–61) deeply ironic rendering of the Ten Commandments ‘The Latest Decalogue’.19 ‘Thou shalt not kill but needs not strive officiously to keep alive’ was quoted by the late Lord Donaldson of Leamington in a letter to The Times in 2004 when he wrote ‘what became of the age old medical commandment “thou shalt not kill but needs not strive officiously to keep alive”’. He was wrong on both counts. It is not a medical commandment at all and it is no older than the Victorian age. It is clearly meant ironically as the following two couplets clearly show ‘thou shalt not steal, an empty feat when it’s so lucrative to cheat, thou shalt not covet but tradition approves all forms of competition’. It is almost certain that Arthur Clough would be mortified to see his admonition used in a sense exactly to the opposite of its intended meaning and it is unfortunate that Lord Donaldson is not alone in his unhappy misinterpretation of Clough’s couplet.
So what is to be done?
Abolish strict liability in this area and replace it with liability based on negligence;
Revise the definition of negligence so that, in deciding whether it was negligent or not to seek to develop a new drug, account is taken of the consequences of doing nothing as well as the consequences of trying to do something.
Change the law on waivers so that any patient who is prepared to try a new medicine, together with the risk that it may have unknown side effects, is at liberty to do so;
Abolish, at least in this area, no win no fee arrangements.
Much of this litigation culture was imported from the USA where there is no National Health Service; where illness can be a financial disaster; and where drugs are advertised directly to patients—all of which can affect motives for litigation. Here, where there is a National Health Service which should be able to provide necessary care for all, including those who have suffered drug side effects, the situation is quite different and so should be our attitude to litigation.
Acknowledgements
This article is an edited version of the Ver Heyden de Lancey Lecture, delivered on 18 November 2011 in Cambridge. The author is greatly indebted particularly to Professor John Spencer and also to Dr. David Howarth for their help with the legal aspects of this article.
Conflict of interest: None declared.
References
- 1.Dally A. Thalidomide: was the tragedy preventable? The Lancet. 1998;351:1197–9. doi: 10.1016/S0140-6736(97)09038-7. [DOI] [PubMed] [Google Scholar]
- 2.Williams W. Wrongful ban on DDT costs lives. Eco Imperialism. 2004 [ http://www.eco-imperialism.com] Accessed October 2011. [Google Scholar]
- 3.Paumgartten FJ, Chahoud I. Thalidomide embryopathy cases in Brazil after 1965. Reprod Toxicol. 2006;22:1–2. doi: 10.1016/j.reprotox.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Cosyns JP. Aristolochic acid and ‘Chinese herbs nephropathy’: a review of the evidence to date. Drug Saf. 2003;26:33–48. doi: 10.2165/00002018-200326010-00004. [DOI] [PubMed] [Google Scholar]
- 5.The Boston Consulting Group. Life Sciences R&D: Changing the Innovation Equation in India. Cambridge, MA: US-India BioPharma & Healthcare Summit; 2011. [ http://www.bcg.com/expertise_impact/biopharma_summit.aspx] Accessed October 2011. [Google Scholar]
- 6. Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on orphan medicinal products. Official Journal of the European Communities 22 January 2000.
- 7.Merton RK. The unanticipated consequences of purposive social action. Am Sociol Rev. 1936;1:894–904. [Google Scholar]
- 8. Directive 2006/25/EC of the European Parliament and of the Council of 5 Apr 2006 on the minimum health and safety requirements regarding the exposure of workers to risks arising from physical agents (artificial optical radiation) (19th individual Directive within the meaning of Article 16(1) of Directive 89/391/EEC). Official Journal of the European Union 27 April 2006.
- 9.Rose L. British health care regulation moves away from science. Biologist. 2007;54:3–5. [Google Scholar]
- 10.Scannell J, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Disc. 2012;11:191–200. doi: 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- 11.Bach PB. Limits on medicare’s ability to control rising spending on cancer drugs. New Eng J Med. 2009;360:626–33. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 12.Cooksey D. A Review of UK Health Research Funding. London: HMSO; 2006. [ http://www.hm-treasury.gov.uk] Accessed October 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [1932] Appeal Cases 562.
- 14. [1936] Appeal Cases 85, at page 100.
- 15. [2001] 3 All England Law Reports 289; [2001] Lloyd's Reports Medical 187; (2001) 60 Butterworths Medico-legal Reports 1.
- 16.Cane P. Atiyah’s Accidents, Compensation and the Law. 7th. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- 17.Jack A. AstraZeneca faces $520m Seroquel fine. Financial Times. 2010:28th April, Page 16. [Google Scholar]
- 18.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with Rosiglitazone or Pioglitazone. JAMA. 2010;304:411–8. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 19.Clough AH. The Latest Decalogue. In: Mulhauser FL, editor. The Poems of Arthur Hugh Clough. 2nd. London: Oxford University Press; 1974. [Google Scholar]