Abstract
Maternal smoking during pregnancy is associated with low birth weight. Common variation at rs1051730 is robustly associated with smoking quantity and was recently shown to influence smoking cessation during pregnancy, but its influence on birth weight is not clear. We aimed to investigate the association between this variant and birth weight of term, singleton offspring in a well-powered meta-analysis. We stratified 26 241 European origin study participants by smoking status (women who smoked during pregnancy versus women who did not smoke during pregnancy) and, in each stratum, analysed the association between maternal rs1051730 genotype and offspring birth weight. There was evidence of interaction between genotype and smoking (P = 0.007). In women who smoked during pregnancy, each additional smoking-related T-allele was associated with a 20 g [95% confidence interval (95% CI): 4–36 g] lower birth weight (P = 0.014). However, in women who did not smoke during pregnancy, the effect size estimate was 5 g per T-allele (95% CI: −4 to 14 g; P = 0.268). To conclude, smoking status during pregnancy modifies the association between maternal rs1051730 genotype and offspring birth weight. This strengthens the evidence that smoking during pregnancy is causally related to lower offspring birth weight and suggests that population interventions that effectively reduce smoking in pregnant women would result in a reduced prevalence of low birth weight.
INTRODUCTION
Low birth weight and preterm birth are important risk factors for perinatal morbidity and mortality (1). Furthermore, lower birth weight is associated with an increased risk of chronic diseases in adulthood, including cardiovascular disease, high blood pressure, coronary heart disease, type 2 diabetes and adult mortality (2–5). Maternal smoking during pregnancy is strongly associated with low birth weight (6,7). Babies whose mothers smoked during pregnancy are on average 150 to 200 g lighter at birth than babies with non-smoking mothers (6,8). The 2006 legislation in Scotland, which banned smoking in public areas, has been associated with a reduced number of preterm births and small for gestational age babies (9).
Genetic variation at the CHRNA5–CHRNA3–CHRNB4 locus on chromosome 15q25 is robustly associated with the quantity of smoking in those who smoke (10–14). Each additional copy of the risk allele (T) of the single-nucleotide polymorphism (SNP), rs1051730, is associated with an increase in smoking quantity of approximately one cigarette per day (12). The variant is not associated with smoking initiation, since it is equally prevalent in smokers and never-smokers (12). The rs1051730 SNP is also strongly associated with a reduced ability of women to quit smoking during pregnancy, and with a higher number of cigarettes per day in pregnant women who smoke (15,16).
Studies of women from the UK (15) and the Netherlands (17) have independently investigated the association between the 15q25 variant and birth weight. Both studies observed reductions in birth weight with each additional copy of the maternal risk allele in women who smoked during pregnancy [−28 g (95% confidence interval—95% CI: −59 to 2 g) per T-allele in n = 1829 UK women; −38 g (95% CI: −89 to 13 g) per T-allele in n = 610 Dutch women]. However, statistical power was limited by the available sample sizes, and neither association achieved P < 0.05. The association between the maternal rs1051730 variant and birth weight has not been investigated in a well-powered sample of mothers and offspring, and furthermore, any potential associations with fetal genotype have not been examined.
We therefore performed a meta-analysis of 26 241 women from 14 studies to assess the evidence for association between maternal rs1051730 and birth weight, stratified by maternal smoking status in pregnancy. We hypothesized that, due to its strong association with smoking quantity, there would be an association between the maternal risk allele and lower offspring birth weight in women who smoked during pregnancy, but no association with birth weight in the non-smokers. Such an interaction would be interpreted within a Mendelian randomization context as providing evidence of the causal nature of an association between maternal smoking and offspring birth weight (18).
The 15q25 variant is strongly associated with lung cancer in smokers. Several analyses have shown that the association with self-reported number of cigarettes per day is insufficient to explain the lung cancer association (12,19,20). These results initially raised the possibility that the 15q25 variant might increase vulnerability to the harmful effects of tobacco smoke in those who are exposed, independently of smoking behaviour. If this were the case, we might expect to see an association between fetal rs1051730 genotype and birth weight, which is independent of maternal genotype, in the offspring of mothers who smoked. However, a recent study showed a stronger association with objectively measured cotinine levels than with self-reported number of cigarettes per day. This suggested that the locus works predominantly by influencing tobacco exposure in smokers, and that although cigarettes reported per day have the advantage of enabling the necessarily large sample sizes for genetic association studies, they fail to capture smoking exposure fully (21). Based on this, and other recent evidence (22), we would not expect to observe an association between fetal rs1051730 genotype and birth weight that is independent of maternal genotype. We examined evidence of association between fetal rs1051730 genotype and birth weight in 25 090 offspring from 11 studies. In a subset of 12 489 mother–offspring pairs from eight studies, we were able to test the association between fetal genotype and birth weight that was independent of maternal genotype.
RESULTS
The basic characteristics of study participants are presented in Table 1.
Table 1.
Basic characteristics of study participants
| Study | Sample group | Mothers, na (% of male offspring) | Age in years, mean (SD) | Mean birth weight of offspring in grams (SD) | Median gestational age at delivery in weeks (IQR) | N available for fetal genotype analysis |
|---|---|---|---|---|---|---|
| ALSPAC | All pregnancies | 6323 (50.8) | 28.4 (4.7) | 3474 (477) | 40 (39–41) | 6875 |
| Non-smoking pregnancies | 4687 (50.2) | 29.0 (4.5) | 3516 (469) | 40 (39–41) | 5226 | |
| Smoking in the first trimester only | 313 (51.8) | 26.9 (5.1) | 3535 (507) | 40 (39–41) | 336 | |
| Smoking continued after the first trimester | 1049 (53.2) | 26.7 (5.0) | 3308 (461) | 40 (39–41) | 1035 | |
| Total smoking pregnancies | 1636 (52.6) | 26.6 (5.0) | 3356 (480) | 40 (39–41) | 1649 | |
| BWHHS | All pregnancies | 2211 (48.3) | NA | 3297 (542) | NA | NA |
| Non-smoking pregnancies | 1702 (49.0) | NA | 3293 (543) | NA | NA | |
| Smoking in the first trimester only | NA | NA | NA | NA | NA | |
| Smoking continued after the first trimester | NA | NA | NA | NA | NA | |
| Total smoking pregnancies | 509 (46.0) | NA | 3312 (537) | NA | NA | |
| DNBC-GOYA | All pregnancies | 1804 (52.0) | 29.2 (4.2) | 3643 (495) | 40 (39–41) | NA |
| Non-smoking pregnancies | 1338 (52.2) | 29.4 (4.1) | 3683 (491) | 40 (39–41) | NA | |
| Smoking in the first trimester only | 154 (51.9) | 28.0 (4.5) | 3695 (504) | 40 (39–41) | NA | |
| Smoking continued after the first trimester | 312 (51.0) | 28.6 (4.6) | 3447 (459) | 40 (39–41) | NA | |
| Total smoking pregnancies | 466 (51.6) | 28.4 (4.6) | 3434 (454) | 40 (39–41) | NA | |
| DNBC-PTB | All pregnancies | 991 (52.6) | 29.8 (4.0) | 3701 (465) | 40 (40–41) | 991 |
| Non-smoking pregnancies | 731 (53.4) | 30.1 (3.8) | 3760 (449) | 40 (40–41) | 731 | |
| Smoking in the first trimester only | 60 (53.3) | 28.7 (4.0) | 3634 (516) | 41 (40–41) | 60 | |
| Smoking continued after the first trimester | 178 (50.6) | 29.3 (4.6) | 3516 (452) | 40 (40–41) | 178 | |
| Total smoking pregnancies | 260 (53.4) | 29.1 (4.5) | 3535 (470) | 40 (40–41) | 260 | |
| EFSOCH | All pregnancies | 808 (51.7) | 30.5 (5.1) | 3512 (474) | 40 (39–41) | 712 |
| Non-smoking pregnancies | 650 (52.3) | 31.2 (4.7) | 3545 (467) | 40 (39–41) | 566 | |
| Smoking in the first trimester only | 48 (45.8) | 28.4 (5.7) | 3598 (378) | 41 (39–41) | 42 | |
| Smoking continued after the first trimester | 91 (50.5) | 27.6 (5.7) | 3280 (509) | 40 (39–41) | 87 | |
| Total smoking pregnancies | 158 (49.4) | 27.8 (5.7) | 3375 (479) | 40 (39–41) | 146 | |
| Generation R | All pregnancies | 3384 (49.5) | 31.2 (4.5) | 3529 (498) | 40 (39–41) | 2258 |
| Non-smoking pregnancies | 2512 (49.0) | 31.6 (4.1) | 3567 (495) | 40 (39–41) | 1731 | |
| Smoking in the first trimester only | 303 (49.2) | 31.0 (4.3) | 3539 (488) | 40 (39–41) | 196 | |
| Smoking continued after the first trimester | 569 (51.5) | 29.8 (5.6) | 3353 (479) | 40 (39–41) | 331 | |
| Total smoking pregnancies | 872 (50.7) | 30.2 (5.2) | 3418 (490) | 40 (39–41) | 527 | |
| HAPO | All pregnancies | 3661 (51.3) | 30.5 (5.4) | 3524 (463) | 40 (39–41) | 2872 |
| Non-smoking pregnancies | 3113 (51.2) | 30.8 (5.3) | 3560 (453) | 40 (39–41) | 2480 | |
| Smoking in the first trimester only | NA | NA | NA | NA | NA | |
| Smoking continued after the first trimester | NA | NA | NA | NA | NA | |
| Total smoking pregnancies | 548 (51.6) | 28.3 (5.9) | 3321 (466) | 40 (39–41) | 392 | |
| MIDSPAN | All pregnancies | 700 (49.5)b | 27.3 (4.8) | 3458 (485) | 40 (40–40) | NA |
| Non-smoking pregnancies | 408 (49.3)b | 27.8 (4.7) | 3533 (459) | 40 (40–40) | NA | |
| Smoking in the first trimester only | NA | NA | NA | NA | NA | |
| Smoking continued after the first trimester | NA | NA | NA | NA | NA | |
| Total smoking pregnancies | 292 (49.7)b | 26.5 (4.8) | 3341 (501) | 40 (40–40) | NA | |
| MoBa | All pregnancies | 763 (46.0) | 28.8 (3.4) | 3682 (419) | 40 (39–41) | 521 |
| Non-smoking pregnancies | 660 (47.1) | 29.0 (3.3) | 3689 (416) | 40 (39–41) | 482 | |
| Smoking in the first trimester only | NA | NA | NA | NA | NA | |
| Smoking continued after the first trimester | NA | NA | NA | NA | NA | |
| Total smoking pregnancies | 103 (38.8) | 27.5 (4.0) | 3642 (415) | 40 (39–41) | 39 | |
| NCCGP | All pregnancies | 567 (46.7) | 28.7 (5.7) | 3445 (496) | 40 (39–41) | 544 |
| Non-smoking pregnancies | 425 (44.7) | 29.6 (5.1) | 3493 (501) | 40 (39–41) | 406 | |
| Smoking in the first trimester only | NA | NA | NA | NA | NA | |
| Smoking continued after the first trimester | NA | NA | NA | NA | NA | |
| Total smoking pregnancies | 142 (52.8) | 25.9 (6.4) | 3300 (451) | 40 (39–40) | 138 | |
| NFBC1966c | All pregnancies | 2068 (49.6) | 26.3 (3.8) | 3522 (469) | 40 (38–42) | 4721 |
| Non-smoking pregnancies | 1748 (49.3) | 26.4 (3.8) | 3545 (464) | 40 (38–42) | 3873 | |
| Smoking in the first trimester only | 110 (49.1) | 23.5 (3.2) | 3499 (422) | 40 (39–41) | NA | |
| Smoking continued after the first trimester | 210 (51.9) | 27.1 (3.4) | 3347 (495) | 40 (39–41) | NA | |
| Total smoking pregnancies | 320 (50.9) | 25.8 (3.8) | 3399 (476) | 40 (39–41) | 848 | |
| Raine | All pregnancies | 1206 (51.2) | 28.9 (5.7) | 3459 (472) | 39 (38–40) | 1266 |
| Non-smoking pregnancies | 904 (53.3) | 29.5 (5.6) | 3516 (471) | 39 (38–40) | 942 | |
| Smoking in the first trimester only | 45 (48.9) | 25.6 (5.8) | 3438 (369) | 39 (38–40) | 47 | |
| Smoking continued after the first trimester | 215 (46.0) | 27.6 (5.8) | 3248 (434) | 39 (38–40) | 231 | |
| Total smoking pregnancies | 302 (45.0) | 27.1 (5.7) | 3288 (431) | 39 (38–40) | 324 | |
| 1958BC_T1DGCc | All pregnancies | 836 (49.9) | 25.8 (5.4) | 3379 (469) | 40 (40–41) | 1985 |
| Non-smoking pregnancies | 551 (50.3) | 26.9 (5.5) | 3438 (460) | 41 (40–41) | 1190 | |
| Smoking in the first trimester only | 82 (50.0) | 24.4 (4.1) | 3458 (421) | 40 (40–42) | 650 | |
| Smoking continued after the first trimester | 203 (48.8) | 23.3 (4.6) | 3188 (461) | 41 (40–41) | 145 | |
| Total smoking pregnancies | 285 (49.1) | 23.6 (4.5) | 3266 (465) | 41 (40–41) | 795 | |
| 1958BC_WTCCC2c | All pregnancies | 919 (49.6) | 26.0 (5.3) | 3328 (477) | 40 (40–41) | 2345 |
| Non-smoking pregnancies | 582 (50.7) | 27.1 (5.2) | 3372 (483) | 40 (40–41) | 1431 | |
| Smoking in the first trimester only | 225 (50.2) | 23.7 (4.8) | 3189 (461) | 40 (40–41) | 746 | |
| Smoking continued after the first trimester | 112 (42.9) | 24.7 (4.7) | 3380 (421) | 40 (40–42) | 168 | |
| Total smoking pregnancies | 337 (47.8) | 24.0 (4.8) | 3252 (456) | 40 (40–41) | 914 |
IQR, inter-quartile range; NA, not available.
aN includes mothers with genotype of rs1051730, smoking status and birth weight, sex and gestational age of offspring available.
bIn the MIDSPAN study, analysis allowed for multiple offspring per mother: n = 1479 pregnancies (896 non-smoking; 583 smoking).
cThe fetal rs1051730 genotype analyses of the NFBC1966, 1958BC_T1DGC and 1958BC_WTCCC2 studies relate to births of the mothers themselves, in addition to other participants in these birth cohorts (with maternal smoking data obtained from their own mothers). For all other studies, the fetal genotype analyses were performed on the offspring of the women included in the analyses of maternal rs1051730 genotype.
Association between maternal rs1051730 genotype and offspring birth weight
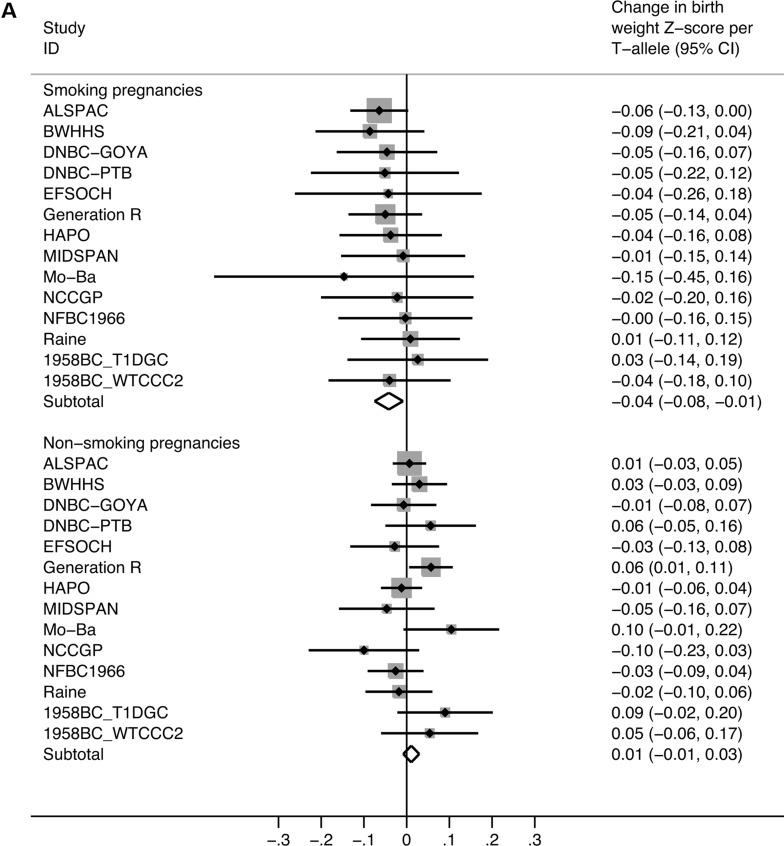
In women who smoked during pregnancy, each additional T-allele, which is associated with greater smoking quantity, was associated with a 0.04 standard deviation (SD) (95% CI: 0.01–0.08 SD) lower birth weight (P = 0.014). This equates, approximately, to a reduction of 20 g (95% CI: 4–36 g) per T-allele. However, in the never-smokers, we estimated only a 0.01 SD change in birth weight per maternal T-allele (95% CI: −0.01 to 0.03 SD) [or 5 g (95% CI: −4 to 14 g); P = 0.260; Table 2; Fig. 1A; see Supplementary Material, Table S2 for individual study results]. There was little detectable heterogeneity within the non-smoking and smoking strata [I2 = 21.5% (95% CI: 0–68), P = 0.220 for non-smokers; I2 = 0% (95% CI: 0–55), P = 0.997 for pregnancy smokers]. However, there was evidence of heterogeneity between the strata (P = 0.007), indicating an interaction between genotype and smoking status in their association with birth weight.
Table 2.
Results of meta-analyses of the association between maternal rs1051730 genotype and birth weight, stratified by maternal smoking status
| Sample group | Analysis details | Total n of participants | Change in offspring birth weight Z-score per maternal T-allele (95% CI) | Association P-value | Heterogeneity P-value (Cochran's Q-test) | % Inconsistency (I2) estimate (95% CI) |
|---|---|---|---|---|---|---|
| Non-smoking pregnancies | Basic analysisa of all available studies | 20 011 | 0.011 (−0.008 to 0.030) | 0.260 | 0.220 | 21.5 (0, 68) |
| Offspring rs1051730 genotype included as an additional covariable in all available studies | 9869 | 0.001 (−0.030 to 0.031) | 0.974 | 0.384 | 6 (0, 70) | |
| Basic analysisa excluding BWHHS, MIDSPAN and NCCGP studies | 17 476 | 0.013 (−0.007 to 0.034) | 0.190 | 0.264 | 18.9 (0, 59) | |
| Smoking in the first trimester only | Basic analysisa of all available studies | 1227 | 0.043 (−0.032 to 0.118) | 0.259 | 0.270 | 19.4 (0, 61) |
| Smoking continued after the first trimester | Basic analysisa of all available studies | 3052 | −0.052 (−0.098 to −0.006) | 0.028 | 0.837 | 0.0 (0, 65) |
| Total smoking pregnancies | Basic analysisa of all available studies | 6230 | −0.042 (−0.075 to −0.008) | 0.014 | 0.997 | 0.0 (0, 55) |
| Offspring rs1051730 genotype included as an additional covariable in all available studies | 2620 | −0.060 (−0.117 to −0.002) | 0.042 | 0.876 | 0.0 (0, 68) | |
| Basic analysisa excluding BWHHS, MIDSPAN and NCCGP studies | 5287 | −0.041 (−0.077 to −0.005) | 0.026 | 0.990 | 0.0 (0, 60) |
aBasic analysis: linear regression of offspring birth weight Z-score against maternal rs1051730 genotype (coded as 0, 1 or 2 T-alleles), with offspring sex and gestational age as covariables.
Figure 1.
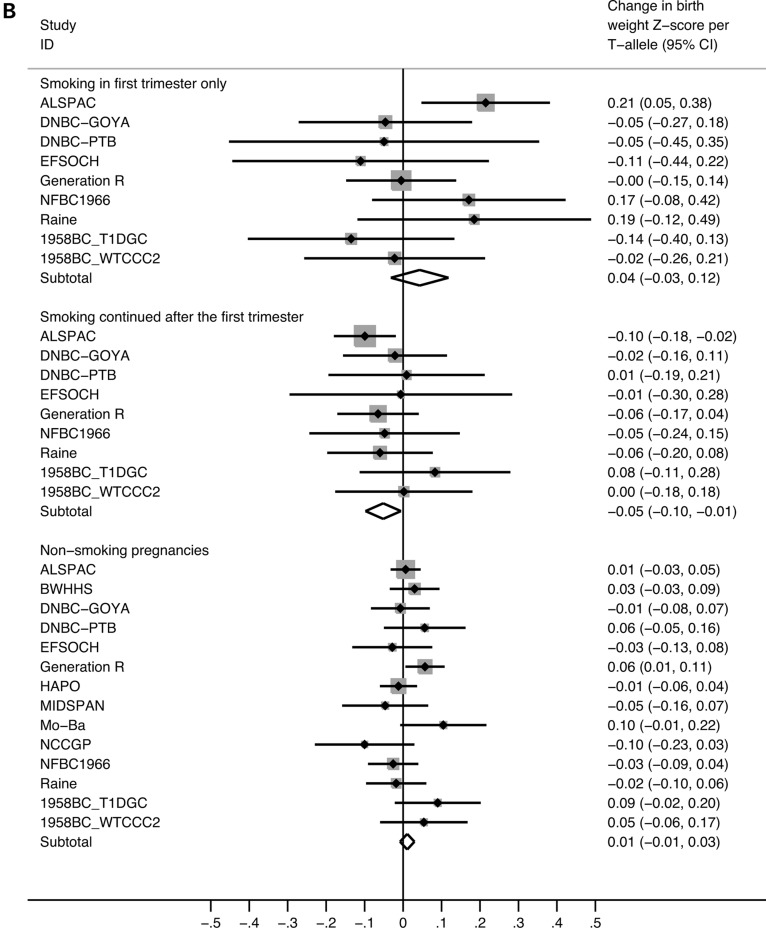
Meta-analysis plots of the association between maternal rs1051730 genotype and offspring birth weight, stratified by maternal smoking status. (A) Pregnancies of non-smoking versus smoking mothers. There was evidence of heterogeneity between the strata (P = 0.007). In the smoking pregnancies, the effect size equates to a 20 g (95% CI: 4–36 g) lower birth weight per T-allele. In the non-smoking pregnancies, there was no evidence of association [birth weight difference per T-allele: 5 g (95% CI: −4 to 14 g)]. (B) Pregnancies of non-smoking mothers versus mothers who smoked only in the first trimester versus mothers who continued to smoke after the first trimester. There was evidence of heterogeneity between the first trimester smoking and continued smoking strata (P = 0.035). There was no evidence of association in the first trimester smoking pregnancies [birth weight difference per T-allele: 21 g (95% CI: −15 to 57 g)]. However, in pregnancies of continued smokers, there was a 24 g (95% CI: 3–45 g) lower birth weight per T-allele.
The effect size estimates observed for both smoking and non-smoking pregnancies were similar (i) when adjusting for fetal rs1051730 genotype in available samples, and (ii) in a sensitivity analysis excluding the studies with less certain smoking exposure data [BWHHS (The British Women's Heart and Health Survey), MIDSPAN and NCCGP (North Cumbria Community Genetics Project); Table 2].
For 4279 women who smoked during pregnancy, data were available on whether or not they continued to smoke after the first trimester. There was evidence of heterogeneity between the effect size estimates in the first trimester smokers and continued smokers (P = 0.035; Fig. 1B). In women who reported smoking only during the first trimester, the estimated change in birth weight per T-allele was a 0.04 SD increase [95% CI: −0.03 to 0.12 SD; (P = 0.259); equivalent to an increase of 21 g (95% CI: −15 to 57 g)] per T-allele. In contrast, we observed an association between genotype and a 0.05 SD lower birth weight per T-allele (95% CI: 0.01–0.10 SD; P = 0.028), in women who smoked beyond the first trimester. This is equivalent to a reduction of 24 g (95% CI: 3–45 g) per T-allele.
Association between fetal rs1051730 genotype and offspring birth weight
In a meta-analysis of the 25 090 offspring with fetal rs1051730 genotype available, we observed no evidence of association with birth weight in the offspring whose mothers did not smoke in pregnancy [0.00 SD (95% CI: −0.01 to 0.02 SD; P = 0.665; equivalent to 0 g (95% CI: −5 to 9 g)]. There was moderate heterogeneity in the non-smoking group [I2 = 43.8% (95% CI: 0–72), P = 0.058]. In the offspring whose mothers did smoke during pregnancy, the estimated change in birth weight per T-allele was −0.03 SD [(95% CI: −0.06 to 0.01 SD; P = 0.108; equivalent to −14 g (95% CI: −29 to 5 g); Supplementary Material, Figure S1]. Low heterogeneity was observed for smokers [I2 = 0.0% (95% CI: 0–60), P = 0.618]. Weak evidence of heterogeneity was observed between the two groups (P = 0.105). On adjustment for maternal rs1051730 genotype, the effect size estimates were 0.01 SD (95% CI: −0.02 to 0.04 SD; P = 0.634) [equivalent to 5 g increase in birth weight (95% CI: −9 to 19 g)] and −0.02 SD (−0.08 to 0.04 SD; P = 0.538) [equivalent to a 9 g reduction in birth weight (95% CI: −38 to 19 g)] in the offspring of non-smoking and smoking mothers, respectively. Low heterogeneity was observed for both groups [non-smokers: I2 = 0.0% (95% CI: 0–68), P = 0.900; smokers: I2 = 12.0% (95% CI: 0–71), P = 0.333]. No evidence of heterogeneity was observed between the two groups (P = 0.444).
DISCUSSION
In a meta-analysis of 26 241 women from 14 studies, we have found strong evidence of a gene × environment interaction between maternal smoking behaviour and genotype of the 15q25 variant, rs1051730, in relation to offspring birth weight (P = 0.007). In women who smoked during pregnancy, there was evidence that each additional copy of the T-allele, which is associated with higher smoking quantity, was associated with a 20 g (95% CI: 4–36 g) reduction in offspring birth weight. In non-smoking mothers, the estimated change in birth weight was 5 g (95% CI: −4 to 14 g) per T-allele. Although the result is as expected, given prior knowledge of the role of the SNP in smoking behaviour and the observational link between smoking and low birth weight, the result provides an important proof-of-principle: when clearly defined, genetic and behavioural risk factors can interact to influence a phenotype.
Our results strengthen the evidence that maternal cigarette smoking during pregnancy is causally associated with lower offspring birth weight. Conventional epidemiological studies are at risk of confounding by lifestyle or socioeconomic factors. Genetic variants should not be related to the confounding factors that distort associations in conventional observational epidemiological studies (23). We have shown previously that the rs1051730 variant is not associated with several confounding factors of smoking behaviour (e.g. age, education level and occupation) (15). Hence, we can be confident that the association we have observed between maternal rs1051370 genotype and offspring birth weight, together with the null association in never-smokers, reflects a causal relationship between smoking during pregnancy and birth weight when considered within a Mendelian randomization framework (18). These data add to the evidence from previous epidemiological studies and clinical trials (6,9,24–30).
Each additional risk allele of rs1051730 is associated with approximately one extra cigarette per day reported by smokers (12,21). Our effect size estimates of a 20 g (95% CI: 4–36 g) lower birth weight per allele in smokers and a 24 g (95% CI: 3–45 g) lower birth weight per allele in women who smoked beyond the first trimester represent unconfounded estimates of this exposure on birth weight. These estimates are consistent with evidence from a prospective study of maternal smoking during pregnancy which reported a 27 g lower birth weight per cigarette per day smoked in the third trimester (31). As a consequence of the relatively small effect sizes, genetic variation at the 15q25 locus would be expected to explain only a very small proportion of the association between low birth weight and adverse health outcomes in the offspring.
Subdivision of the pregnancy-smoker group showed evidence that the association between maternal genotype and birth weight may differ depending on whether women smoked during the first trimester only [21 g (95% CI: −15 to 57 g) increase in birth weight per T-allele] or continued to smoke beyond the first trimester [24 g (95% CI: 3–45 g) lower birth weight per T-allele]. Although power was limited, particularly in the women who smoked only in the first trimester, there was evidence of heterogeneity between the strata (P = 0.035). This suggests, in line with previous evidence (31), that if women quit smoking during the first trimester of pregnancy, the adverse effects of smoking on birth weight are reduced. This result should, however, be treated with some caution. We stratified the data on whether or not women gave up smoking, which may be influenced both by rs1051730 genotype (15) and by smoking quantity, which itself influences birth weight. Stratification on smoking cessation could therefore potentially induce distorted relationships between genotype and birth weight. Women who continue smoking are likely to be heavier smokers than those who do not, and this might apply especially to the T-allele carriers. Further studies are necessary to ascertain the extent of this problem, for example, using cotinine levels as a more reliable indicator of smoking quantity.
Studies of the association between the 15q25 variant and lung cancer have reported a stronger association with disease than can be accounted for by the quantity of cigarettes per day (12,19,20). Possible explanations for this include the likely scenario that the quantity of cigarettes per day fails to capture smoking exposure fully, but also have raised the possibility that the 15q25 variant might increase vulnerability to the harmful effects of tobacco smoke independently of smoking behaviour. To test whether the effects of maternal smoking exposure could be modified by the fetal genotype of rs1051730, we additionally examined evidence of association between fetal rs1051730 genotype and birth weight in 25 090 offspring from 11 studies. Maternal and fetal genotypes are correlated (r ≈ 0.5), so if there is no true association between fetal genotype and birth weight, we would still expect to observe an effect size of approximately half the magnitude of the association between maternal genotype and birth weight in offspring of smoking pregnancies. Our observed effect size estimate of −14 g (95% CI: −29 to 5 g) is consistent with this expectation. On adjustment for maternal genotype in 12 489 mother–offspring pairs, the estimate of the association between fetal genotype and birth weight that is independent of maternal genotype was −9 g (95% CI: −38 to 19 g). Together our results suggest that any influence of the rs1051730 variant on birth weight is mediated by maternal smoking behaviour. This supports the hypothesis that the CHRNA5–CHRNA3–CHRNB4 locus works wholly through modulating smoking behaviour and not by an alternative mechanism.
We acknowledge some limitations to our study. First, in BWHHS, MIDSPAN and NCCGP, we made some assumptions regarding smoking status in pregnancy due to incomplete data. This may have resulted in a misclassification of pregnancy smokers versus non-smokers. However, a sensitivity analysis excluding these studies produced very similar effect size estimates of the associations within each stratum. Second, the individual studies in the meta-analysis used different collection and classification methods for the smoking data. We found little evidence of heterogeneity between studies within each stratum. Third, smoking status was self-reported, and previous studies using biochemical markers suggest that women may not admit to smoking during pregnancy (32). Any such misreporting is likely to result in an underestimate, rather than an overestimate of the difference between smokers and non-smokers. In addition, a recent meta-analysis, which included the BWHHS and MIDSPAN studies, has shown strong agreement between associations of the rs1051730 variant and both self-reported cigarettes per day and cotinine levels (21). Fourth, our statistical evidence for association with birth weight in the pregnancy smokers (P = 0.014), and for the genotype × smoking status interaction (P = 0.007), is modest in relation to levels now considered necessary for robust genetic association studies (33). However, the evidence for association between rs1051730 and smoking quantity is extremely robust, giving high prior odds of association with birth weight, and the strength of evidence we have observed is as expected, given the available power and effect size estimates.
In conclusion, the maternal rs1051730 variant is associated with lower birth weight when mothers smoke during pregnancy, but not in non-smoking mothers. In mothers who smoked beyond the first trimester of pregnancy, there was an association between the variant and lower birth weight, but not in mothers who smoked only in the first trimester, although this observation requires further careful evaluation. Our study strengthens the evidence that smoking during pregnancy is causally related to lower birth weight.
MATERIALS AND METHODS
Study participants
We analysed data on a total of 26 241 pregnant women and 25 090 offspring from 14 study samples. Participants were included in our analyses if they were of European descent. Only singleton births of gestational age ≥37 weeks were analysed. None of the contributing studies had data available on cotinine levels measured during pregnancy, so we used self-reported smoking status. All participants gave informed consent and ethical approval was obtained from the relevant local review committees.
The Avon Longitudinal Study of Parents and Children
This is a prospective study which recruited pregnant women from Bristol, UK, with expected delivery dates between April 1991 and December 1992 (34,35). Ethical approval was obtained from the ALSPAC (Avon Longitudinal Study of Parents and Children) Law and Ethics Committee in addition to the local National Health Service (NHS) Research Ethics Committee. Details of smoking behaviour, collected from questionnaires administered in the 18th and 32nd gestational weeks, have been described previously (15). Birth weight and gestational age were obtained from obstetric records. In the current analyses, we included 6323 women and 6875 children with rs1051730 genotype, birth weight and smoking data available.
The British Women's Heart and Health Survey
The BWHHS study randomly selected and recruited 4286 women aged 60–79 years, from 23 British towns, between 1999 and 2001 (36). At baseline, the women were interviewed, examined, completed questionnaires and had detailed reviews of their medical records. Smoking history (never, former and current, including details of age started and quit) was self-reported at baseline, either at the research nurse interview or in the mailed questionnaires (with very high levels of agreement between both sources when data were available from both). The study had no direct information about pregnancy smoking status. However, the women were born between 1920 and 1940 and predominantly gave birth to their offspring between 1940 and 1970. This period was prior to public health warnings about the adverse effects of smoking during pregnancy. We therefore assumed that women who started smoking before the age of 21 and who quit after the age of 45 continued to smoke during pregnancy. The birth weight, sex and gestational age of the first-born child were collected via questionnaires. Gestational age was recorded as ‘early’, ‘on-time’ or ‘late’ and therefore exclusion of women who gave birth earlier than 37 weeks was not possible. A total of 2211 women were included in the analysis after the exclusion of participants with non-European ethnicity or who had missing rs1051730 genotype, smoking or birth weight data. Fetal genotype was not available.
Danish National Birth Cohort—Genetics of Extreme Overweight in Young Adults and Preterm Birth Controls studies
The Danish National Birth Cohort (DNBC) is a collection of data on 92 274 pregnant women recruited between 1996 and 2002, from their first antenatal visit to their general practitioner. Women were interviewed twice during pregnancy (16 and 30 weeks gestation). Smoking data were collected during the first pregnancy interview, where women were asked about smoking during early pregnancy and about current smoking status at the time of the interview. Birth weight and gestational age were obtained from registry data, based on obstetric records.
The DNBC contributed data from two substudies. A total of 1804 women from a randomly drawn sample of the DNBC mothers were genotyped as part of the Genetics of Extreme Overweight in Young Adults (GOYA) study (37,38) and included in our meta-analysis. The Preterm Birth Controls (PTB) substudy of the DNBC also contributed 991 women to our meta-analysis, who had infants delivered at 39 or 40 weeks of gestation (39). Fetal genotype was available for the DNBC-PTB study (n = 991), but for not the DNBC-GOYA study. A total of 19 women, who met our inclusion criteria, were recruited to both the DNBC-GOYA and DNBC-PTB studies. These women were excluded from the DNBC-GOYA sample to ensure that they were not counted twice in the meta-analyses.
The Exeter Family Study of Childhood Health (EFSOCH)
This is a prospective study of children born between 2000 and 2004, and their parents, from a geographically defined region of Exeter, UK (40). A questionnaire was administered during the 28th gestational week, asking about lifetime, pre-pregnancy, first trimester and current smoking quantity. Birth weight was measured by trained study personnel, and gestational age was collected from obstetric records. We included 808 women and 712 offspring with rs1051730 genotype, birth weight and maternal smoking data available.
Generation R
The Generation R Study is a population-based prospective cohort study from fetal life until young adulthood in a multi-ethnic urban population in Rotterdam, the Netherlands (41). Information about maternal smoking was obtained by self-administered questionnaires in the first, second and third trimesters. Smoking at enrolment was assessed in the first questionnaire by asking each woman whether she smoked during pregnancy (categories: no smoking, first trimester only smoking, continued smoking). This questionnaire was sent to all women, regardless of the gestational age at enrolment. To assess smoking habits in the second and third trimesters, women were asked whether they smoked in the past 2 months (categories: no, yes) in the second and third questionnaires. Birth weight and gestational age data were obtained from medical records and hospital registries. In the current analysis, 3384 women of European descent with rs1051730 genotype, birth weight and maternal smoking data available were included. Fetal genotype data were also available for 2258 offspring meeting our inclusion criteria.
Hyperglycemia and Adverse Pregnancy Outcome study
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study was designed to investigate the role of maternal hyperglycaemia on pregnancy outcome (42). Birth weight measurements by trained study personnel, and ascertainment of gestational age data, have been described in detail previously (43). The study participants attended their local study centre for a visit at 24–32 weeks of gestation. Prior to, or during, this visit, they were asked how many cigarettes they had smoked, on average, per day during this pregnancy. For the purposes of the current study, the answers of the respondents were classified as non-smokers or smokers. The HAPO study contributed 3661 women and 2872 offspring of European descent to our current analysis. These participants were recruited from nine study centres: Bellflower, CA, USA (n = 62); Chicago, IL, USA (n = 250); Providence, RI, USA (n = 35); Cleveland, OH, USA (n = 204); Toronto, Canada (n = 326); Belfast, UK (n = 754); Manchester, UK (n = 1024); Brisbane, Australia (n = 689); Newcastle, Australia (n = 317).
MIDSPAN family study
There are four MIDSPAN population cohorts in Scotland (44). The three original studies took place between 1964 and 1976. In 1996, the next generation was studied, when 2338 (1298 female and 1040 male) offspring aged 30–59 years of couples from the Renfrew/Paisley Study were recruited into the Family Study. The birth weight and gestational age for 1825 births from 828 women in the Family Study that had reported at least one pregnancy prior to screening were recorded by questionnaires. Smoking during pregnancy was derived from data detailing the age at which smoking commenced, smoking ceased and age at birth. It was assumed that smokers who smoked while pregnant did not temporarily reduce or stop smoking during pregnancy. Where this assumption is wrong, non-exposed pregnancies will be miscategorized as exposed, and the effect on birth weight underestimated. Anecdotal evidence suggests women became much more likely to cut down or stop over the decades from the 50s to the 90s, so miscategorization due to unreported pauses in smoking around pregnancy will become more frequent in later decades and the negative effect of smoking on birth weight will appear to lessen over the decades. To test this, births were categorized by year of birth into 50s–60s (number of mothers smoking while pregnant = 146 out of 295), 70s–80s (number of mothers smoking while pregnant = 267 out of 645) and 90s (number of mothers smoking while pregnant = 170 out of 539), and tested for an interaction between this variable and smoking while pregnant (P = 0.69). There was no evidence for a reduction over time in the effect of smoking on birth weight, so no strong evidence of miscategorization of pregnancies as exposed. A sensitivity analysis, using a conservative version of smoking during pregnancy (excluding women who started or stopped smoking within 2.5 years of giving birth), gave very similar results. After removal of 210 births (59 mothers) with missing or inconsistent data regarding pregnancies or maternal smoking behaviour, twin births and births with gestational age below 37 weeks, and a further 136 births (69 mothers) due to missing maternal rs1051730 genotype, the current analyses included 700 mothers and 1479 births. Fetal genotype was not available.
The Norwegian Mother and Child Cohort Study
The Norwegian Mother and Child Cohort Study (MoBa) study is a nation-wide pregnancy cohort that from 1999 to 2008 included more than 107 000 pregnancies. It is maintained at the Norwegian Institute of Public Health. Pregnant women were invited to participate through a postal invitation after they signed up for routine ultrasound examination in their local hospital. The participation rate was 43% (45). The women were asked to provide biological samples and to answer questionnaires during pregnancy and after birth up to the age of 7 for the child. The first questionnaire, answered in gestational weeks 15–17, covered health, chemical and physical factors in the environment, lifestyles and socioeconomic and demographic factors, and the second questionnaire was a food frequency questionnaire answered prospectively in regard to spontaneous preterm delivery outcome. The records of the participating women in the Medical Birth Registry of Norway (MBRN) were included in the data set (45). This study uses version 4 of the data files made available for research in 2008. The study has been approved by the Regional Committee for Ethics in Medical Research and the Data Inspectorate in Norway. A total of 763 women were included in our analyses, with genotype data available for 521 offspring.
North Cumbria Community Genetics Project
The NCCGP is a community-based DNA-banking project, consisting of DNA and tissue samples from approximately 3000 mothers and approximately 7000 offspring born between 1996 and 2003 (46). Maternal smoking status during pregnancy was determined by a self-report questionnaire completed within the first 12 weeks of pregnancy. Specifically, pregnant mothers were asked whether they were currently smoking and if they had smoked at all during the pregnancy, responses from which were utilized to classify participants as non-smokers or smokers. Reported details regarding lifetime smoking habits were limited, hence it was not always possible to distinguish ever-smokers from never-smokers prior to this pregnancy. Birth weight and gestational age were obtained from hospital records. A total of 567 women were included in our analyses, with rs1051730 genotype data available for 544 offspring.
Northern Finland 1966 Birth Cohort (NFBC1966)
This study includes individuals born in the two northernmost provinces of Finland to mothers with expected dates of delivery in 1966 (47). The participants of the NFBC 1966 cohorts were used in both the fetal and maternal analyses. A total of 2068 women included within the birth cohort, with rs1051730 genotype, gave birth and were included in our ‘Maternal genotype’ meta-analysis. Maternal smoking, birth weight and gestational age data were collected from birth registry of their first-born offspring who met our inclusion criteria. For the analyses of fetal rs1051730 genotype, we were able to include 4721 participants from the birth cohort (including the 2068 women) with medical record data on their own birth weights and ‘smoking in pregnancy’ data collected from their mothers via a questionnaire.
The Raine study
The Western Australian Pregnancy Cohort (Raine) study is a prospective pregnancy cohort that recruited 2900 pregnant women between May 1989 and November 1991. Self-reported maternal smoking status was collected from questionnaires at 18 and 34 weeks of gestation. Birth weight was measured immediately after birth by midwives, and gestational age was ascertained based on the date of the last menstrual period (48). The current analysis included 1206 women and 1266 offspring who met our inclusion criteria.
The 1958 British Birth Cohort (1958BC-T1DGC and 1958BC-WTCCC2)
The 1958BC is a national cohort of UK subjects born during the same week in March 1958 (49). For the analysis of smoking status and maternal genotype, information is from the cohort members (genotype and smoking during pregnancy) and their first born offspring (birth weight and gestational age). Maternal smoking status prior to and during pregnancy and information on birth weight and gestational age for the first-born offspring were obtained by a structured questionnaire. For the analysis of smoking status and fetal genotype, information is from cohort members (genotype, birth weight and gestational age) and their mothers (smoking in pregnancy). ‘Smoking in pregnancy’ data came from maternal report after the birth, and birth weight and gestational age of the cohort members were obtained from their medical records. Genetic information was obtained through two subsamples from case–control studies that had used the 1958BC as a source for population controls: 3000 samples were randomly selected as part of the Wellcome Trust Case Control Consortium [WTCCC2, (50)] and 2592 distinct samples were selected as part of the Type 1 Diabetes Genetics Consortium [T1DGC (51)]. In our meta-analyses of maternal genotype data, we included 836 and 919 women from the T1DGC and WTCCC2 subsamples, respectively. In our meta-analyses of fetal genotype data, the respective totals were 1985 and 2345 offspring.
Genotyping and quality control
The rs1051730 polymorphism was genotyped in all available samples. The genotyping method and quality control summaries are presented in Supplementary Material, Table S1.
Statistical methods
Analyses in individual studies
Within each study, we stratified by maternal smoking status. For our main analyses, women were classified as smokers or non-smokers during pregnancy. For our second analyses, in the eight studies with available information, pregnancy smokers were further divided into those who smoked only in the first trimester and those who continued to smoke after the first trimester. We excluded multiple births, births before 37 weeks of gestation and individuals of non-European descent from all analyses. Birth weight (BW) was standardized to Z-scores within each included sample [(BW value − mean BW)/SD of BW]. To test for an association between birth weight and maternal rs1051730 genotype within each stratum, we performed linear regression of birth weight Z-score against genotype (coded as 0, 1 or 2 T-alleles; additive genetic model), with offspring sex and gestational age as covariables. In the HAPO study, the analysis was additionally adjusted for study centre. In the MIDSPAN study, which included information on multiple offspring per mother, the data are multilevel, with birth weights being clustered within mothers, who are nested within families. The analysis model was fitted using the lme function in the nlme package in R (www.r-project.org), with random intercepts for families and mothers within families.
Fetal rs1051730 genotype was available for analysis in 11 studies (Table 1). The association between fetal genotype and birth weight Z-score was analysed by linear regression within each stratum, within each study, with the same covariables and exclusions as used in the analyses of maternal genotype.
Maternal and fetal genotypes are correlated (r ≈ 0.5). To examine the associations with birth weight of maternal and fetal genotypes independently of one another, we used mother–offspring pairs in eight studies (n = 12 489; ALSPAC, EFSOCH, DNBC-PTB, Generation R, HAPO, MoBa, NCCGP, Raine). We checked for Mendelian errors, and any pairs demonstrating inconsistencies were excluded from our analysis. Within each of these studies, we performed a linear regression analysis of birth weight Z-score against maternal genotype, fetal genotype, sex and gestational age in both the pregnancy smoking and non-smoking strata.
Meta-analyses
We combined the regression coefficients and standard errors from the individual study analyses by performing inverse variance meta-analyses with fixed effects in each of the smoking or non-smoking strata (defined above). We additionally performed a sensitivity analysis, excluding the three studies in which assumptions were made about pregnancy smoking exposures due to incomplete data (BWHHS, MIDSPAN and NCCGP). All meta-analyses were performed using the METAN module, developed for STATA (52), and a P-value <0.05 was considered to represent evidence of association. We estimated the percentage of total variation among study estimates that is due to between-study heterogeneity using the I2 statistic (53) and derived 95% CIs for this statistic using the user-written Stata command, ‘heterogi’. Conventionally, I2 values of 25, 50 and 75% represent low, moderate and high heterogeneity, respectively. Additionally, we used Cochran's Q-test to evaluate the statistical evidence for between-study heterogeneity, both within and between the strata defined by smoking status. The test of heterogeneity between strata served as a test of interaction between genotype and smoking status.
Estimation of approximate overall effect sizes in grams
To convert the overall results from birth weight Z-scores into grams, we multiplied the effect size and 95% CI values by a representative value of the SD of birth weight. We took the median study SD of birth weight within each stratum as the representative value (479 g for pregnancy smokers; 472 g for non-smokers).
Power calculation
Our sample of 6230 women who smoked during pregnancy and 20 011 pregnancy non-smokers gave us, respectively, 88% and >99% power to detect an effect on birth weight of 28 g (15) per maternal rs1051730 risk allele (frequency 38%) at P < 0.05 (two-sided, assuming a mean birth weight of 3500 g and an SD of 480 g). The equivalent power estimates in the sample of 6032 offspring with fetal genotype available from smoking pregnancies and 19 058 offspring from non-smoking pregnancies were 88% and >99%, respectively.
SUPPLEMENTARY MATERIAL
FUNDING
Researchers were funded by the European Centre for the Environment and Human Health (part of the Peninsula College of Medicine and Dentistry, which is a joint entity of the University of Exeter, the University of Plymouth and the NHS in the southwest) and is supported by investment from the European Regional Development Fund (ERDF) and the European Social Fund (ESF) Convergence Programme for Cornwall and the Isles of Scilly (J.T.); The Dutch Kidney Foundation (C08.2251) (H.R.T.); the Netherlands Organization for Health Research and Development (ZonMw 90700303, 916.10159) (V.W.V.J.); the Higher Education Funding Council for England (C.L.R.); National Institute for Health Research (A.T.H. and C.P.); the University of British Columbia, Clinician Investigator Program (J.W.Y.N.); UK Medical Research Council and University of Bristol (D.A.L. and G.D.S.); Wellcome Trust [WT 084762MA (L.P.), 085541/Z/08/Z (R.M.F.) and 085515 (M.-J.A.B.)]. Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust.
Supplementary Material
ACKNOWLEDGEMENTS
We are extremely grateful to the participants and families who contributed to all of the studies and the teams of investigators involved in each one. These include interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
ALSPAC: This publication is the work of the authors, and Rachel Freathy, Debbie Lawlor and George Davey Smith will serve as guarantors for the contents of this paper. The Avon Longitudinal Study of Parents and Children (ALSPAC) has core support provided by the UK Medical Research Council, the Wellcome Trust (grant ref: 092731) and the University of Bristol.
BWHHS: The British Women's Heart and Health Study (BWHHS) is funded by the Department of Health (England) Policy Research Programme and the British Heart Foundation.
DNBC: The DNBC was established with major funding from the Danish National Research Foundation. Additional support for the DNBC has been obtained from the Danish Pharmacists' Fund, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Augustinus Foundation and the Health Fund of the Danish Health Insurance Societies.
DNBC-GOYA: The DNBC-GOYA study was supported by the Wellcome Trust (grant WT084762).
DNBC-PTB: The GWAS data for the DNBC study of preterm birth was done within the Gene, Environment Association Studies (GENEVA) consortium, with funding provided through the US National Institutes of Health (NIH) Genes, Environment and Health Initiative (GEI; U01HG004423). Assistance with genotype cleaning and general study coordination for the preterm birth project were provided by the GENEVA Coordinating Center (U01HG004446). Genotyping was performed at the Johns Hopkins University Center for Inherited Disease Research, with support from the NIH GEI (U01HG004438).
EFSOCH: The Exeter Family Study of Childhood Health (EFSOCH) was supported by South West NHS Research and Development, Exeter NHS Research and Development, the Darlington Trust and the Peninsula National Institute of Health Research (NIHR) Clinical Research Facility at the University of Exeter. The opinions given in this paper do not necessarily represent those of NIHR, the NHS or the Department of Health.
Generation R: The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR-MDC), Rotterdam. We gratefully acknowledge the contribution of children and parents, general practitioners, hospitals, midwives and pharmacies in Rotterdam. The generation and management of GWAS genotype data for the Generation R Study were done at the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC, the Netherlands. We would like to thank Karol Estrada, Dr Tobias A. Knoch, Anis Abuseiris, Luc V. de Zeeuw and Rob de Graaf for their help in creating GRIMP, BigGRID, MediGRID and Services@MediGRID/D-Grid (funded by the German Bundesministerium fuer Forschung und Technology; grants 01 AK 803 A-H, 01 IG 07015 G) for access to their grid computing resources. We thank Mila Jhamai, Manoushka Ganesh, Pascal Arp, Marijn Verkerk, Lizbeth Herrera and Marjolein Peters for their help in creating, managing and QC of the GWAS database. Also, we thank Karol Estrada and Carolina Medina-Gomez for their support in the creation and analysis of imputed data. The Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development (ZonMw 21000074).
HAPO: This study was funded by grants R01-HD34242 and R01-HD34243 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Diabetes, Digestive, and Kidney Diseases, U01 HG004415 from the National Human Genome Research Institute, M01-RR00048 and M01-RR00080 by the National Center for Research Resources and by the American Diabetes Association. Genotyping was funded by Diabetes UK grant RD08/0003692.
MoBa: The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no NO-ES-75558), NIH/NINDS (grant no. 1 UO1 NS 047537-01) and the Norwegian Research Council/FUGE (grant no. 151918/S10 and no 183220/S10), Norwegian Research Council, Oslo, Norway (FUGE 183220/S10). Swedish government grants to researchers in public health service (ALF) (ALFGBG-136431), Sahlgrenska University Hospital, Sahlgrenska Academy, Gothenburg, Sweden, Swedish Medical Society, Stockholm, Sweden (2008-21198) and Jane and Dan Olsson Research Foundation, Gothenburg, Sweden.
MIDSPAN: We thank Dr Mark Upton, who led the Midspan Offspring Study, and the members of the research team who collected the data used in this analysis. The offspring study in MIDSPAN was supported by grants from the Wellcome Trust and the NHS Cardiovascular Research and Development Programme.
NCCGP: We thank all of the participants of the North Cumbria Community Genetics Project and previous members of the study team, in particular Professor Sir John Burn and Mrs Pat Jonas. Funding for genotyping and sample analysis of NCCGP samples has been provided from a number of sources including the National Institute of Health Research.
NFBC1966: We thank Professor Paula Rantakallio (launch of NFBC1966 and initial data collection), Ms Sarianna Vaara (data collection), Ms Tuula Ylitalo (administration), Mr Markku Koiranen (data management), Ms Outi Tornwall and Ms Minttu Jussila (DNA biobanking). This work was supported by the Academy of Finland (project grants 104781, 120315, 129418 and Center of Excellence in Complex Disease Genetics and SALVE), University Hospital Oulu, Biocenter, University of Oulu, Finland (75617), the European Commission (EURO-BLCS, Framework 5 award QLG1-CT-2000-01643), NHLBI (5R01HL087679-02) through the STAMPEED programme (1RL1MH083268-01), NIH/NIMH (5R01MH63706:02), ENGAGE project and grant agreement (HEALTH-F4-2007-201413) and the Medical Research Council, UK (G0500539, G0600705, PrevMetSyn/SALVE).
Raine: We gratefully acknowledge the Raine Study participants and their families and the Raine Study Team for cohort co-ordination and data collection. We also thank the NHMRC for their long-term contribution to funding the study and the Telethon Institute for Child Health Research for long-term support of the study. Core management for the Raine study is funded by The University of Western Australia (UWA), Telethon Institute for Child Health Research, Raine Medical Research Foundation, UWA Faculty of Medicine, Dentistry and Health Sciences, Curtin University and the Women and Infants Research Foundation. Collection, extraction and genotyping of maternal and child DNA in Raine was funded by the Australian National Health and Medical Research Council and the Women and Infants Research Foundation. Antenatal data collection was supported by the Raine Medical Research Foundation.
1958BC_T1DGC and 1958BC_WTCCC2: Dr Sue Ring and Dr Wendy McArdle (University of Bristol) and Mr Jon Johnson (Centre for Longitudinal Studies, Institute of Education, London) are thanked for help with data linkage. The study was supported by the Medical Research Council (MRC G0601653 and SALVE/PrevMedsyn). Collection of DNA in the 1958 Birth Cohort was funded by the MRC grant G0000934 and the Wellcome Trust grant 068545/Z/02. This research used resources provided by the Type 1 Diabetes Genetics Consortium, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases, National Human Genome Research Institute, National Institute of Child Health and Human Development and Juvenile Diabetes Research Foundation International (JDRF) and supported by U01 DK062418. This study makes use of data generated by the Wellcome Trust Case-Control Consortium II (083948). The Medical Research Council provides funds for the MRC Centre of Epidemiology for Child Health.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Battaglia F.C., Lubchenco L.O. A practical classification of newborn infants by weight and gestational age. J. Pediatr. 1967;71:159–163. doi: 10.1016/s0022-3476(67)80066-0. [DOI] [PubMed] [Google Scholar]
- 2.Barker D.J., Hales C.N., Fall C.H., Osmond C., Phipps K., Clark P.M. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 3.Jarvelin M.R., Sovio U., King V., Lauren L., Xu B., McCarthy M.I., Hartikainen A.L., Laitinen J., Zitting P., Rantakallio P., et al. Early life factors and blood pressure at age 31 years in the 1966 northern Finland birth cohort. Hypertension. 2004;44:838–846. doi: 10.1161/01.HYP.0000148304.33869.ee. [DOI] [PubMed] [Google Scholar]
- 4.Andersen L.G., Angquist L., Eriksson J.G., Forsen T., Gamborg M., Osmond C., Baker J.L., Sørensen T.I.A. Birth weight, childhood body mass index and risk of coronary heart disease in adults: combined historical cohort studies. PLoS One. 2010;5:e14126. doi: 10.1371/journal.pone.0014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risnes K.R., Vatten L.J., Baker J.L., Jameson K., Sovio U., Kajantie E., Osler M., Morley R., Jokela M., Painter R.C., et al. Birthweight and mortality in adulthood: a systematic review and meta-analysis. Int. J. Epidemiol. 2011;40:647–661. doi: 10.1093/ije/dyq267. [DOI] [PubMed] [Google Scholar]
- 6.Jaddoe V.W., Troe E.J., Hofman A., Mackenbach J.P., Moll H.A., Steegers E.A., Witteman J.C. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: the Generation R Study. Paediatr. Perinat. Epidemiol. 2008;22:162–171. doi: 10.1111/j.1365-3016.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 7.Rantakallio P. The effect of maternal smoking on birth weight and the subsequent health of the child. Early Hum. Dev. 1978;2:371–382. doi: 10.1016/0378-3782(78)90064-6. [DOI] [PubMed] [Google Scholar]
- 8.Jarvelin M.R., Elliott P., Kleinschmidt I., Martuzzi M., Grundy C., Hartikainen A.L., Rantakallio P. Ecological and individual predictors of birthweight in a northern Finland birth cohort 1986. Paediatr. Perinat. Epidemiol. 1997;11:298–312. doi: 10.1111/j.1365-3016.1997.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 9.Mackay D.F., Nelson S.M., Haw S.J., Pell J.P. Impact of Scotland's smoke-free legislation on pregnancy complications: retrospective cohort study. PLoS Med. 2012;9:e1001175. doi: 10.1371/journal.pmed.1001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berrettini W., Yuan X., Tozzi F., Song K., Francks C., Chilcoat H., Waterworth D., Muglia P., Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol. Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat. Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorgeirsson T.E., Geller F., Sulem P., Rafnar T., Wiste A., Magnusson K.P., Manolescu A., Thorleifsson G., Stefansson H., Ingason A., et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorgeirsson T.E., Gudbjartsson D.F., Surakka I., Vink J.M., Amin N., Geller F., Sulem P., Rafnar T., Esko T., Walter S., et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducci F., Kaakinen M., Pouta A., Hartikainen A.L., Veijola J., Isohanni M., Charoen P., Coin L., Hoggart C., Ekelund J., et al. TTC12-ANKK1-DRD2 and CHRNA5-CHRNA3-CHRNB4 influence different pathways leading to smoking behavior from adolescence to mid-adulthood. Biol. Psychiatry. 2011;69:650–660. doi: 10.1016/j.biopsych.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freathy R.M., Ring S.M., Shields B., Galobardes B., Knight B., Weedon M.N., Smith G.D., Frayling T.M., Hattersley A.T. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum. Mol. Genet. 2009;18:2922–2927. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorgeirsson T.E., Stefansson K. Commentary: gene–environment interactions and smoking-related cancers. Int. J. Epidemiol. 2010;39:577–579. doi: 10.1093/ije/dyp385. [DOI] [PubMed] [Google Scholar]
- 17.Leermakers E.T.M., Taal H.R., Bakker R., Steegers E.A.P., Hofman A., Jaddoe V.W.V. A common genetic variant at 15q25 modifies the associations of maternal smoking during pregnancy with fetal growth: the Generation R Study. PLoS One. 2012;7:e34584. doi: 10.1371/journal.pone.0034584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith G.D. Use of genetic markers and gene-diet interactions for interrogating population-level causal influences of diet on health. Genes Nutr. 2011;6:27–43. doi: 10.1007/s12263-010-0181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung R.J., McKay J.D., Gaborieau V., Boffetta P., Hashibe M., Zaridze D., Mukeria A., Szeszenia-Dabrowska N., Lissowska J., Rudnai P., et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 20.Lips E.H., Gaborieau V., McKay J.D., Chabrier A., Hung R.J., Boffetta P., Hashibe M., Zaridze D., Szeszenia-Dabrowska N., Lissowska J., et al. Association between a 15q25 gene variant, smoking quantity and tobacco-related cancers among 17 000 individuals. Int. J. Epidemiol. 2010;39:563–577. doi: 10.1093/ije/dyp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munafo M.R., Timofeeva M.N., Morris R.W., Prieto-Merino D., Sattar N., Brennan P., Johnstone E.C., Relton C., Johnson P.C.D., Walther D., et al. Association between genetic variants on chromosome 15q25 locus and objective measures of tobacco exposure. J. Natl Cancer Inst. 2012;104:1–9. doi: 10.1093/jnci/djs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Broderick P., Matakidou A., Eisen T., Houlston R.S. Chromosome 15q25 (CHRNA3-CHRNA5) variation impacts indirectly on lung cancer risk. PLoS One. 2011;6:e19085. doi: 10.1371/journal.pone.0019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith G.D., Lawlor D.A., Harbord R., Timpson N., Day I., Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med. 2007;4:e352. doi: 10.1371/journal.pmed.0040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumley J., Oliver S.S., Chamberlain C., Oakley L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst. Rev. 2004:CD001055. doi: 10.1002/14651858.CD001055.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans W.N., Ringel J.S. Can higher cigarette taxes improve birth outcomes? J. Public Econ. 1999;72:135–154. [Google Scholar]
- 26.Kramer M.S. Determinants of low birth weight: methodological assessment and meta-analysis. Bull. World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer M.S., Seguin L., Lydon J., Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr. Perinat. Epidemiol. 2000;14:194–210. doi: 10.1046/j.1365-3016.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- 28.Butler N.R., Alberman E. Perinatal Problems. The Second Report of the British Perinatal Mortality Survey. Edinburgh, London: E & S Livingstone Ltd; 1969. [Google Scholar]
- 29.Hamilton B.H. Estimating treatment effects in randomized clinical trials with non-compliance: the impact of maternal smoking on birthweight. Health Econ. 2001;10:399–410. doi: 10.1002/hec.629. [DOI] [PubMed] [Google Scholar]
- 30.Smith G.D. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin. Pharmacol. Toxicol. 2008;102:245–256. doi: 10.1111/j.1742-7843.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein I.M., Mongeon J.A., Badger G.J., Solomon L., Heil S.H., Higgins S.T. Maternal smoking and its association with birth weight. Obstet. Gynecol. 2005;106:986–991. doi: 10.1097/01.AOG.0000182580.78402.d2. [DOI] [PubMed] [Google Scholar]
- 32.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob. Res. 2004;6:S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 33.Wacholder S., Chanock S., Garcia-Closas M., El Ghormli L., Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J. Natl Cancer Inst. 2004;96:434–442. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyd A., Golding J., Macleod J., Lawlor D.A., Fraser A., Henderson J., Molloy L., Ness A., Ring S., Davey Smith G. Cohort profile: The ‘Children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2012 doi: 10.1093/ije/dys064. PMID: 22507743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraser A., Macdonald-Wallis C., Tilling K., Boyd A., Golding J., Davey Smith G., Henderson J., Macleod J., Molloy L., Ness A., et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int. J. Epidemiol. 2012 doi: 10.1093/ije/dys066. PMID: 22507742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawlor D.A., Bedford C., Taylor M., Ebrahim S. Geographical variation in cardiovascular disease, risk factors, and their control in older women: British Women's Heart and Health Study. J. Epidemiol. Community Health. 2003;57:134–140. doi: 10.1136/jech.57.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nohr E.A., Timpson N.J., Andersen C.S., Smith G.D., Olsen J., Sørensen T.I.A. Severe obesity in young women and reproductive health: the Danish National Birth Cohort. PLoS One. 2009;4:e8444. doi: 10.1371/journal.pone.0008444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paternoster L., Evans D.M., Nohr E.A., Holst C., Gaborieau V., Brennan P., Prior Gjesing A., Grarup N., Witte D.R., Jørgensen T., et al. Genome-Wide Population-Based Association Study of Extremely Overweight Young Adults – The GOYA Study. PLoS One. 2011;6:e24303. doi: 10.1371/journal.pone.0024303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feenstra B., Geller F., Krogh C., Hollegaard M.V., Gortz S., Boyd H.A., Murray J.C., Hougaard D.M., Melbye M. Common variants near MBNL1 and NKX2-5 are associated with infantile hypertrophic pyloric stenosis. Nat. Genet. 2012;44:334–337. doi: 10.1038/ng.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knight B., Shields B.M., Hattersley A.T. The Exeter Family Study of Childhood Health (EFSOCH): study protocol and methodology. Paediatr. Perinat. Epidemiol. 2006;20:172–179. doi: 10.1111/j.1365-3016.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 41.Jaddoe V.W., van Duijn C.M., van der Heijden A.J., Mackenbach J.P., Moll H.A., Steegers E.A., Tiemeier H., Uitterlinden A.G., Verhulst F.C., Hofman A. The Generation R Study: design and cohort update 2010. Eur. J. Epidemiol. 2010;25:823–841. doi: 10.1007/s10654-010-9516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Contreras M., Sacks D.A., Bowling F.G., Cowley D.M., Liley H., McIntyre H.D., Tudehope D.I. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int. J. Gynaecol. Obstet. 2002;78:69–77. doi: 10.1016/s0020-7292(02)00092-9. [DOI] [PubMed] [Google Scholar]
- 43.The HAPO Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes. 2009;58:453–459. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hart C.L., MacKinnon P.L., Watt G.C., Upton M.N., McConnachie A., Hole D.J., Smith G.D., Gillis C.R., Hawthorne V.M. The Midspan studies. Int. J. Epidemiol. 2005;34:28–34. doi: 10.1093/ije/dyh348. [DOI] [PubMed] [Google Scholar]
- 45.Magnus P., Irgens L.M., Haug K., Nystad W., Skjaerven R., Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int. J. Epidemiol. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 46.Chase D.S., Tawn E.J., Parker L., Jonas P., Parker C.O., Burn J. The North Cumbria Community Genetics Project. J. Med. Genet. 1998;35:413–416. doi: 10.1136/jmg.35.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rantakallio P. The longitudinal study of the northern Finland birth cohort of 1966. Paediatr. Perinat. Epidemiol. 1988;2:59–88. doi: 10.1111/j.1365-3016.1988.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 48.Newnham J.P., Evans S.F., Michael C.A., Stanley F.J., Landau L.I. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet. 1993;342:887–891. doi: 10.1016/0140-6736(93)91944-h. [DOI] [PubMed] [Google Scholar]
- 49.Power C., Elliott J. Cohort profile: 1958 British birth cohort (National Child Development Study) Int. J. Epidemiol. 2006;35:34–41. doi: 10.1093/ije/dyi183. [DOI] [PubMed] [Google Scholar]
- 50.WTCCC. Genome-wide association study of 14 000 cases of seven common diseases and 3000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrett J.C., Clayton D.G., Concannon P., Akolkar B., Cooper J.D., Erlich H.A., Julier C., Morahan G., Nerup J., Nierras C., et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris R., Bradburn M., Deeks J., Harbord R., Altman D., Steichen T., Sterne J. METAN: Stata Module for Fixed and Random Effects Meta-analysis. Boston College Department of Economics; 2006. [Google Scholar]
- 53.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.