Abstract
Visceral leishmaniasis was first reported in Bhutan in 2006. We conducted studies of the parasite, possible vectors and reservoirs, and leishmanin skin test and risk factor surveys in three villages. Nineteen cases were reported from seven districts. Parasite typing yielded two novel microsatellite sequences, both related to Indian L. donovani. In one case village, 40 (18.5%) of 216 participants had positive leishmanin skin test results, compared with 3 (4.2%) of 72 in the other case village and 0 of 108 in the control village. Positive results were strongly associated with the village and increasing age. None of the tested dogs were infected. Eighteen sand flies were collected, 13 Phlebotomus species and 5 Sergentomyia species; polymerase chain reaction for leishmanial DNA was negative. This assessment suggests that endemic visceral leishmaniasis transmission has occurred in diverse locations in Bhutan. Surveillance, case investigations, and further parasite, vector, and reservoir studies are needed. The potential protective impact of bed nets should be evaluated.
Introduction
Seventy percent of the world's burden of visceral leishmaniasis (VL) occurs in the Indian subcontinent, comprising an estimated 162,000 to 314,000 annual cases.1 Recognized endemic VL transmission is concentrated in the Indian states of Bihar, West Bengal, Jharkand, and Uttar Pradesh, western and central districts of Bangladesh, especially Mymensingh, and in the southeastern Terai region of Nepal along the border with Bihar. However, historically the disease affected a larger area of the subcontinent. Assam, in northeastern India, suffered a devastating epidemic in the late 19th century of a disease then called “Assam fever” and later identified as VL.2 Early in the 20th century, transmission was documented to occur as far south as Madras, where the etiological agent Leishmania donovani was first described.3 Clearly, the conditions that allow VL transmission are widespread in the Indian subcontinent. In recent years, sporadic cases of VL have been reported in the Himalayan foothills in the states of Himachal Pradesh, Jammu, and Kashmir, and once more in Assam.4,5
In 2006, several cases of suspected VL were reported in the Himalayan nation of Bhutan, which shares borders with the Indian states of West Bengal and Assam. An investigation carried out in July 2007 detailed nine suspected cases; two were confirmed by the finding of typical amastigotes on smears of bone marrow aspirate and six were documented to have responded to antimonial treatment.6 The presence of known vector sand flies was also confirmed. This investigation suggested the presence of locally acquired VL cases in Bhutan for the first time. More cases were reported in 2009 and 2010, prompting the current investigation. To elucidate the nature of transmission and assess the increasing number of reported cases, the World Health Organization (WHO) undertook an assessment of VL in Bhutan in March 2011. The results of the investigation are presented in this publication.
Materials and Methods
Rapid epidemiological assessment.
The rapid assessment included review of VL cases registered by the Ministry of Health of Bhutan, visits to hospitals where VL cases had been diagnosed, and interviews with hospital authorities and treating physicians. Diagnostic bone marrow slides were examined when available. We considered a case of VL to be confirmed if the diagnosis was based on visualization of parasites on bone marrow smear or on the positive rK39 rapid test and recorded clinical resolution with antileishmanial therapy. We considered a case as probable VL if the case was reported as VL in the Ministry of Health surveillance data but the confirmatory details were lacking. Study communities were selected based on the presence of confirmed VL cases and the feasibility of traveling to the site within the time allotted for the investigation. In each site, residents were asked to gather at a central location on two occasions 48 hours apart. The investigation was explained to community leaders by a team member fluent in the local language and their formal written consent was obtained before initiating the individual consent process. Subsequently, written informed consent was obtained from each participant; parents or guardians were asked to consent for children. Demographic and epidemiological data were collected using structured household level and individual level questionnaires. All consenting participants 2 years of age or older were asked to participate in a leishmanin skin test (LST) survey. The LSTs were applied and read by health care providers with experience in intradermal test application following standard methods: 0.1 mL of Leishmania major antigen (produced under conditions of good manufacturing practices by Pasteur Institute, Tehran, Iran) was injected intradermally on the volar surface of the forearm; 48–72 hours later, induration was measured in two perpendicular directions using the “ballpoint pen” method.7 Following the international consensus definition, the LST was considered positive if the mean of the two measurements was 5 mm or greater.8,9 The Institutional Review Board of the Centers for Disease Control and Prevention reviewed the protocol and determined that the activity met the criteria for an outbreak investigation, and as such was not classified as research and did not require formal Institutional Review Board approval.
Human samples.
The diagnostic slides consisting of Giemsa-stained bone marrow smears were collected from several hospitals with reported cases of VL. They were comprehensively examined with an optical microscope at low power for amastigote forms, which were confirmed at 1,000× magnification. Each slide was then submerged for 1 hour in xylene to remove any trace of immersion oil. Slides were allowed to dry and the area of the bone marrow smear was covered with NET10 buffer (10 mM NaCl, 10 mM EDTA, 10 mM Tris HCl, pH 8.0). The material was removed by scraping, collected in a 1.5 mL eppendorf tube, and the NET10 volume filled to 200 μL for DNA extraction and further polymerase chain reaction (PCR) analysis.
Canine samples.
To assess whether the dog plays a reservoir role, in this focus a serological and parasitological investigation was carried out in a convenience sample of domestic dogs from Karuna House canine shelter, located in the outskirts of Trashigang, the district from which one of the human cases was derived. All dogs underwent clinical examination by a local veterinarian supervised by team members with experience in canine leishmaniasis (CanL). The following signs, compatible with CanL, were sought: skin lesions, lymph node enlargement, onychogryphosis, weight loss, and alopecia. For serological and PCR analysis, peripheral blood samples were collected in EDTA-containing tubes. A single popliteal lymph node aspirate was collected in a tube containing 200 μL NET10 plus 5 μg gentamicin. Informed consent was obtained from the canine shelter responsible before clinical examination and sampling of the dogs.
Serological diagnosis.
Two different procedures were used to detect anti-Leishmania antibodies in dog samples: 1) rK39 immunocromatographic test (rK39-ICT) on peripheral blood and plasma samples (25 μL), according to the procedure described by the manufacturer (Kalazar Detect Rapid Test, InBIOS International, Seattle, WA); and 2) immunofluorescence antibody test (IFAT) on plasma (1 μL) following a standard method, using 10 μL of 2 × 107 Leishmania infantum promastigotes/mL in 1× phosphate buffered saline per well as antigen (reference strain MHOM/FR/78/LEM-75). The IFAT threshold titer for positivity was 1/160.10
Collection of sand flies.
Sand flies were collected using Centers for Disease Control and Prevention (CDC) miniature light traps in four different locations in Mongar and Trashigang. The traps were placed overnight inside houses and storehouses for cereals and also at peridomestic locations such as chicken and cow shelters. The sand flies collected were preserved according to the capture site in eppendorf tubes containing silica-gel matrix until identification and further molecular analyses. Before morphological identification the last abdominal segments of females were cleared in Marc André solution; the whole body of males, head and last abdominal segment of females were mounted in Canada balsam. Identification was based on the morphology of the male genitalia and female head and spermatechae; the keys described by Lewis were followed.11,12 The remaining carcasses of the females were ground separately in 50 μL NET10 buffer and preserved for further DNA extraction and PCR analyses.
Molecular analyses.
A classical DNA extraction procedure with phenol-chloroform and ethanol precipitation was applied to all of the specimen types, which consisted of 1) scrapings from human bone marrow aspirate slides, 2) 200 μL peripheral blood from dogs, 3) 200 μL lymph node aspirate from a single dog, and 4) the ground carcasses of female sand flies. The obtained DNA was eluted in 50 μL sterile distilled water in the case of smears and sand flies and in 100 μL in the case of peripheral blood and lymph node aspirates. Five μL of DNA were used for each PCR assay.
Leishmanial DNA detection in canine samples, human bone marrow smears, and female sand flies was attempted by three PCR methods targeting different regions of the Leishmania genome: ITS1 and ITS2 using the primer pairs LITSR (5′-CTGGATCATTTTCCGATG-3′)/L5.8S (5′- TGATACCACTTATCGCACTT-3′) and L5.8SR (5′-AAGTGCGATAAGTGGTA-3′)/LITSV (5′-ACACTCAGGTCTGTAAAC-3′) as described by Kuhls and others13 and hsp70 gene using the primer pair HSP70sen (5′-GACGGTGCCTGCCTACTTCAA-3′)/HSP70ant (5′- CCGCCCATGCTCTGGTACATC-3′), as described by Fraga and others.14
The 18SrRNA gene from female sand flies was amplified using the primer pair Lu.18S 1S (5′-TGCCAGTAGTTATATGCTTG-3′)/Lu.18S AR (5′-CACCTACGGAAACCTTGTTAC-3′) as described elsewhere.15 The PCR products were run on 1.5% agarose gels stained with ethidium bromide and visualized under UV light.
DNA sequencing.
Direct sequencing of the Leishmania positive ITS1, ITS2, and hsp70 PCR products, and sand fly 18SrRNA gene was performed with the corresponding forward and reverse primers. Internal primers for sequencing were also used for hsp70, as described elsewhere.14 For the sand fly 18SrRNA gene, internal primers for sequencing were designed for this study based on conserved sequences of Phlebotomus and Lutzomyia species (Table 1). Before DNA sequencing the PCR-positive products were excised from agarose gels and purified using the QIAquick Gel Extraction Kit (QIAGEN, Qiagen Iberia SL, Spain). The Big-Dye Terminator Cycle Sequencing Ready Reaction Kit V3.1 and the automated ABI PRISM 377 DNA sequencer (Applied Biosystems, Foster City, CA) were used. Sequences obtained were analyzed and edited using the software BioEdit Sequence Alignment Editor, version 7.0.9.0.16
Table 1.
Internal primers used for the sand fly 18SrRNA gene sequencing
| Primer | Sequence (5′→ 3′) | Nucleotide position* |
|---|---|---|
| 18S-RKK | GCGTATATTAAAGTTGTTGCGG | 627–648 |
| 18S-RM | AGTAAGTGTGAGTCATAAGCTTGC | 1871–1894 |
| 18S-PDR | TCTCCGGAATCGAACCCTGATTCC | 416–393 |
| 18S-JD | GAGGTCCTATTCCATTATTCC | 999–979 |
The annealing position is given relative to GenBank accession no. AJ244359 (Phlebotomus argentipes).
DNA sequence analyses.
The hsp70 sequences were compared with L. infantum (GenBank accession nos.: FN395031-33) and L. donovani (GenBank accession nos.: FN395027-29) sequences reported by Fraga and others.14 ITS1 and ITS2 sequences were also compared with sequences retrieved from the GenBank, which are representative of the different ITS types described by Kuhls and others and the ITS1 and ITS2 sequences from a Leishmania donovani strain (MHOM/IN/1983/CHANDIGARH) isolated in Himachal Pradesh, India, not included in Kuhls'study.13 For comparison these sequences were aligned with BioEdit Sequence Alignment Editor using the ClustalW multiple alignment algorithm and were manually adjusted. Details of the sequences used for this comparison are given in Table 2.
Table 2.
Characteristics of the Leishmania strains and Bhutanese clinical isolates used for ITS sequence comparison and phylogenetic analysis
| Taxa | WHO-code | Origin | Pathology | ITS sequence type* | GenBank accession no. |
|---|---|---|---|---|---|
| L. infantum | MHOM/TN/80/IPT1 | Tunisia | VL | A | AJ000289 |
| L. donovani | MHOM/CN/00/Wangjie1 | China | VL | C | AJ000294 |
| MCAN/SD/00/LEM3946 | Sudan | CanVL | D | AJ634356 | |
| MHOM/ET/72/GEBRE1 | Ethiopia | VL | E | AJ634367 | |
| MHOM/ET/67/HU3 | Ethiopia | VL | F | AJ634373 | |
| MHOM/KE/85/NLB323 | Kenya | VL | G | AJ000297 | |
| MHOM/IN/00/DEVI | India | VL | H | AJ634376 | |
| MHOM/IN/1983/CHANDIGARH | India | VL | n.a. | AM901449 | |
| Patient 18 (BHUTAN-Trashigang)† | Bhutan | VL | n.a. | JQ730001 | |
| Patient 19 (BHUTAN-Samtse)† | Bhutan | VL | n.a. | JQ730002 |
ITS sequences type according to Kuhls and others.13 Type A has been described on Mediterranean, Chinese, and American strains, type E and F in Ethiopian and Sudanese strains, and type G in Keyan and Indian strains; n.a.: not assigned, different to those described by Kuhls and others.
Clinical samples, not culture isolates, thus WHO-code is not provided for this two samples.
Phylogenetic analysis.
Phylogenetic analysis based on the nucleotide sequences of the Leishmania ITS1 and ITS2 was performed based on maximum parsimony using the PHYLIP software package (PHYLogeny Inference Package), version 3.69.17 To test the robustness of the internal branches generated, we performed bootstrap analysis using 1,000 replications.
Results
A total of 8 confirmed and 11 probable VL cases were reported to the Ministry of Health from 2005 through March 2011 (Table 3). The cases were widely dispersed: nine cases were reported from Mongar, four from Trashiyangste, two from Samtse, and one each from Lhuenste, Tsirang, Trashigang, and Zhemgang districts (Figure 1). Interviews were conducted with four patients who had been treated for VL since 2009 (cases 14, 16, 17, and 18 in Table 3). Patient 14 was a 50-year-old man from Tsirang who had no history of travel for the previous 15 years. He was treated with 28 days of sodium stibogluconate (SSG) with good clinical response. Patient 18 was a 4-year-old boy from Trashigang who had visited Phuentsoling in southern Bhutan bordering Assam district, India, in November 2008 for 4 days. This patient had incomplete response to SSG after 35 injections and was cured with amphotericin B deoxycholate. Patients 14 and 18 had the diagnosis confirmed by the finding of leishmanial amastigotes on stained specimens of bone marrow aspirate before treatment. Patient 16 had negative results by microscopy of bone marrow smear and received the diagnosis of VL based on clinical features and the positive rK39 rapid test; he completed 28 days of SSG in early March 2011 with a resolution of symptoms. Although his home was in Mongar, he became ill while working as a porter in Trongsa from May to September 2010. Patient 17, a 28-year-old female from Mongar, was diagnosed based on clinical illness and a positive result by the rK39 rapid test and treated with 28 days of SSG; she was asymptomatic at the time of the interview but had positive results by LST during the current investigation 3 months after completion of VL treatment. One patient with positive results by the rK39 rapid test and suspected post-kala-azar dermal leishmaniasis (with no history of antecedent kala-azar) was examined in Kalapong village, Mongar. The patient had positive results by the rK39 rapid test, but both the clinical examination, and pathology suggested the more likely diagnosis was discoid lupus. The rK39 result was considered to represent a false positive caused by cross-reacting antibodies, which have been reported for this test and other serological assays for leishmaniasis in specimens from patients with rheumatological conditions.18,19
Table 3.
Visceral leishmaniasis patients reported since 2005 in Bhutan
| Patient | Year | Age | Sex | Gewog | Dzongkhag | Diagnosis | Basis of diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | 2005 | 27 | F | Trashiyangste | Probable | rK39 rapid test | |
| 2 | 2006 | 35 | M | Khoma | Lhuentse | Confirmed | Bone marrow |
| 3 | 2006 | 18 | M | Khengkhar | Mongar | Confirmed | Bone marrow |
| 4 | 2006 | 22 | M | Gyelposhing | Mongar | Probable | rK39 rapid test |
| 5 | 2006 | 34 | F | Khengkhar | Mongar | Probable | Not reported |
| 6 | 2006 | 8 | M | Nangkhor | Mongar | Probable | Not reported |
| 7 | 2009 | 12 | M | Khengkhar | Mongar | Probable | Not reported |
| 8 | 2009 | 21 | M | Sangama/Jurmey | Mongar | Probable | Not reported |
| 9 | 2009 | 11 | F | Ramjar | Trashiyangste | Confirmed | Bone marrow* |
| 10 | 2010 | 28 | M | Ramjar | Trashiyangste | Probable | Not reported |
| 11 | 2010 | F | Toetsho | Trashiyangste | Probable | Not reported | |
| 12 | 2010 | 50 | M | Jurmey | Mongar | Probable | Not reported |
| 13 | 2010 | 28 | M | Dorokha | Samtse | Probable | Not reported |
| 14 | 2010 | 50 | M | Sunkosh | Tsirang | Confirmed | Bone marrow* |
| 15 | 2010 | 60 | M | Nangkhor | Zhemgang | Probable | Not reported |
| 16 | 2010 | 16 | M | Jurmey† | Mongar | Confirmed | rK39 rapid test |
| 17 | 2010 | 28 | F | Thrinangbi | Mongar | Confirmed | rK39 rapid test |
| 18 | 2010 | 4 | M | Ozorong | Trashigang | Confirmed | Bone marrow* |
| 19 | 2011 | Changburi | Samtse | Confirmed | Bone marrow* |
Bone marrow slide specimens included in molecular analysis.
Worked as a porter in Trongsa Dzongkhag from May to September 2010.
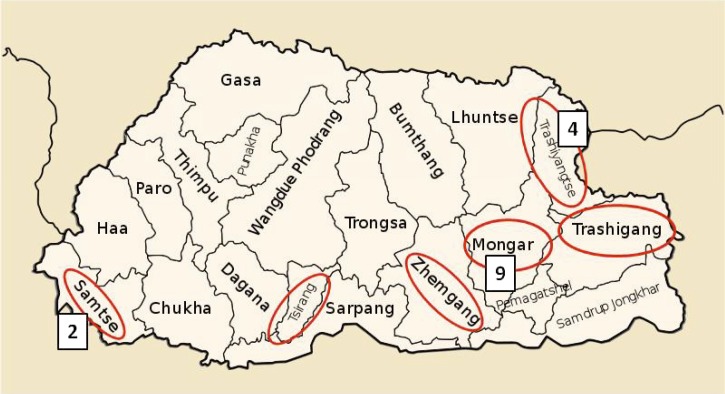
Figure 1.
Map of Bhutan with dzongkhags (districts) reporting cases of visceral leishmaniasis from 2005 to March 2011 circled in red. Nine cases were reported in Mongar, four in Trashiyangste, and two in Samtse; the other three dzongkhags each reported one case.
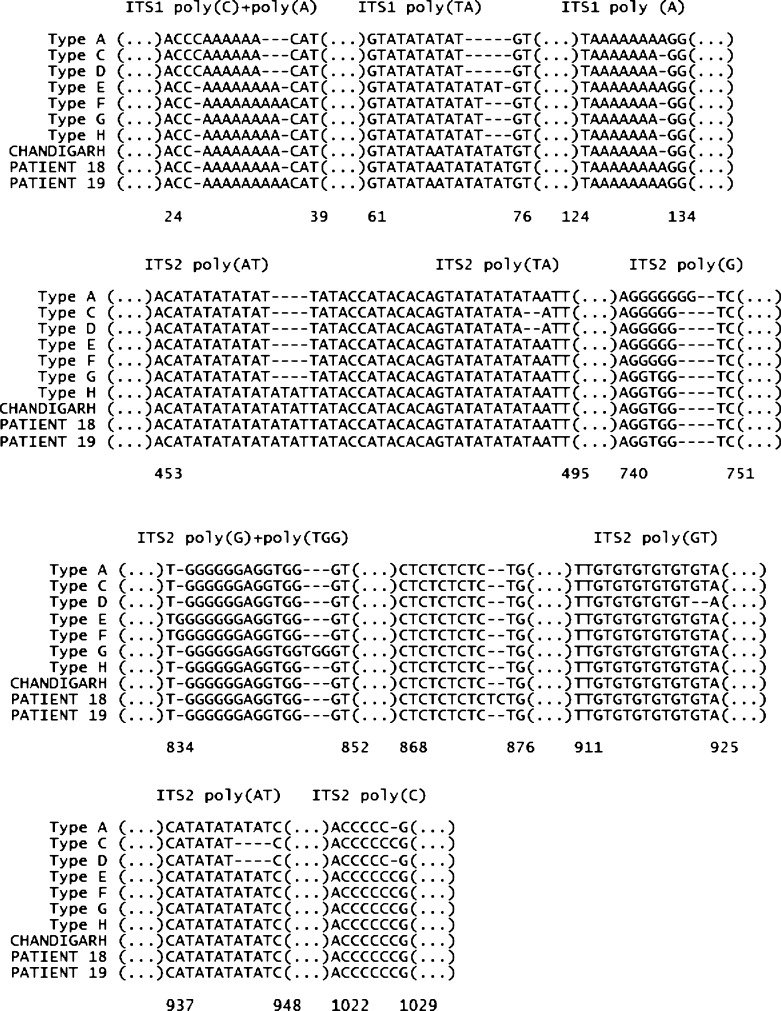
Five bone marrow slides from four patients were available for microscopic examination and molecular testing (Table 4). Amastigotes were visualized on all five slides, and four yielded positive results by at least one molecular assay. Sparse parasites were visualized in the post-treatment specimen from patient 18, and this specimen was negative by all molecular assays. Two specimens (patients 18 and 19) yielded a positive result by hsp70 PCR; their sequences, submitted to GenBank under the accession JQ729999 and JQ730000, respectively, were 100% consistent to L. donovani. All pretreatment specimens returned a positive result by ITS1 PCR, whereas only two had positive results by ITS2 PCR. Two genetic groups could be distinguished based on the polymorphic microsatellite regions included in the ITS1 sequence; specimens from patients 9, 14, and 18 presented identical sequences, whereas a distinct sequence was found in the specimen from patient 19 (Figure 2). Neither of the ITS types identified for Bhutanese clinical isolates in this analysis have been previously described; the same applies for the CHANDIGARH isolate. These specimens represent three ITS types different from those (A–H) described previously in the L. donovani complex.13
Table 4.
Results of molecular assays performed on material recovered from bone marrow smears from four visceral leishmaniasis patients, Bhutan, 2011
| Patient | hsp70 | ITS1 | ITS2 |
|---|---|---|---|
| 9 | − | + | − |
| 14 | − | + | − |
| 18 | + | + | + |
| 18* | − | − | − |
| 19 | + | + | + |
Second bone marrow specimen collected after 35-day course of sodium stibogluconate that resulted in incomplete clinical response.
Figure 2.
Partial alignment of the ITS sequence types found for the Bhutanese clinical isolates and the L. donovani complex strains representative of the different ITS types according to Kuhls and others.13 The 12 polymorphic microsatellites found previously are represented; an additional polymorphic site specific to the ITS2 sequence of patient 18 is shown (positions 868–876).
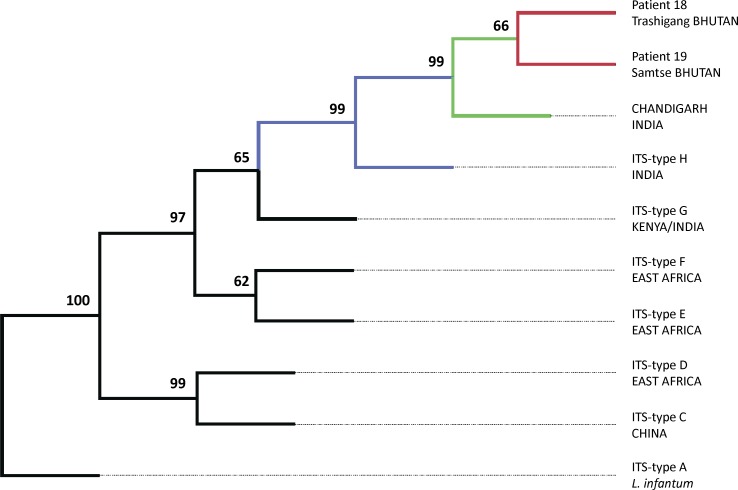
Complete sequence of both ITS1 and ITS2 was obtained only for samples from patients 18 and 19; these findings were included in the phylogenetic analysis together with sequences from other L. donovani complex strains shown in Table 2. The phylogenetic analysis revealed that Bhutanese isolates included in this study, as well as CHANDIGARH strain, are closely related to Indian isolates belonging to the ITS type H (99% bootstrap value); details are shown in Figure 3.
Figure 3.
Phylogenetic relationships of Bhutanese L. donovani clinical isolates within the L. donovani complex. The most parsimonious tree found by heuristic search is presented; it was inferred by parsimony analysis of the nucleotide sequences of ITS1 and ITS2. The numbers above the branches indicate the percentages with which a given branch is supported in 1,000 bootstrap replications. The ITS types are those referred by Kuhls and others.13.
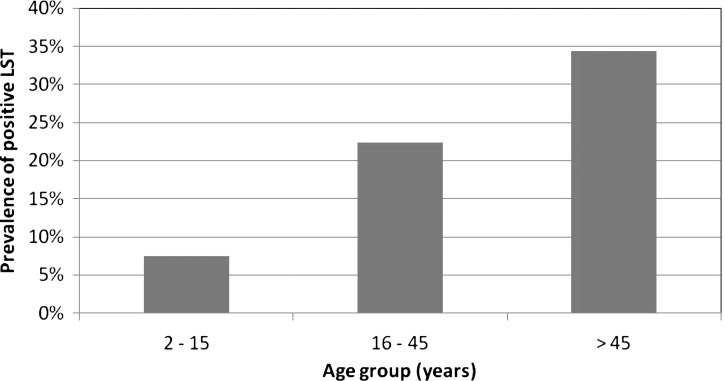
Two communities, Ozorong (Trashigang, altitude 2,000 m) and Sunkosh (Tsirang, altitude 1,300 m), were chosen for the LST survey based on the presence of VL patients 18 and 14, respectively. A third community, Sunkosh Bazar (Tsirang), located close to one of the study communities, was also included in the survey to provide a comparison group. The two study sites were located in distinct ecological zones, Ozorong in higher altitude hilly pine forests and Sunkosh in a lower altitude humid area near a large river. Demographic data were collected and a LST was applied for a total of 426 individuals, 236 from Ozorong, 76 from Sunkosh, and 114 from Sunkosh Bazar (Table 5). No other VL patients were identified in any of the study communities. Of the 426 individuals who had LST placed, 396 (93.0%) returned 48 hours later to have the test evaluated. Complete data (household level, individual level and LST results) were available for 349 individuals. Among those with LST results available, household level data were missing for 6 (1.5%) and individual data for 41 (10.4%) participants. Data for children were sparse from Sunkosh village because of the greater distance from the village to the gathering place used; adults and older children were more likely to participate than young children. By contrast, the gathering place in Ozorong was within the village, facilitating participation of all age groups. The prevalence of positive results by LST was strikingly different by community: in Ozorong, 40 (18.5%) of 216 participants had positive results, compared with 3 (4.2%) of 72 in Sunkosh and 0 of 108 in Sunkosh Bazar. Ozorong and Sunkosh differed significantly with regard to several other factors, including bed net ownership and usage, presence of dogs, goats and cattle, and the materials used for walls and floors of houses. The most important determinant of positive LST results was the village of residence, with an adjusted odds ratio of 19.4 for Ozorong compared with the other two communities (Table 6). Participants 16–45 years of age were nearly three times and those older than 45 years of age six times as likely to have positive LST results compared with children 15 years of age or younger. The pattern of rising prevalence with age was particularly marked in Ozorong (Figure 4; χ2 for trend 12.83, P < 0.001). Because there were also consistent differences between Ozorong and the other two communities in terms of bed net ownership and use, livestock and housing materials constituting epidemiological confounding, we were unable to disentangle the effects of these factors from the risk associated with the village of residence.
Table 5.
Characteristics of the study communities, rapid investigation of visceral leishmaniasis, Bhutan, 2011
| Ozorong Village (N = 236) | Sunkosh Village (N = 76) | Sunkosh Bazar (N = 114) | All N (%) | |
|---|---|---|---|---|
| Age (years) | ||||
| ≤ 15 | 83 (35) | 6 (8) | 22 (19) | 111 (26) |
| 16–45 | 117 (50) | 50 (66) | 55 (48) | 222 (52) |
| ≥ 46 | 36 (15) | 20 (26) | 37 (33) | 93 (22) |
| Female | 142 (60) | 36 (47) | 49 (43) | 227 (53) |
| Leishmanin skin test positive | 40 (19) | 3 (4) | 0 (0) | 43 (11) |
| Head of household is a farmer | 190 (83) | 74 (97) | 22 (19) | 286 (68) |
| HOH has no formal education | 177 (77) | 53 (70) | 54 (47) | 284 (68) |
| Household owns | ||||
| Dog(s) | 36 (16) | 60 (79) | 12 (11) | 108 (26) |
| Goat(s) | 8 (3) | 58 (76) | 13 (11) | 79 (19) |
| Cattle | 176 (77) | 66 (87) | 29 (25) | 271 (65) |
| Radio | 152 (66) | 31 (42) | 48 (42) | 231 (55) |
| Latrine | 225 (98) | 74 (97) | 114 (100) | 413 (98) |
| Land | 182 (79) | 76 (100) | 29 (25) | 287 (69) |
| Bed net | 88 (38) | 76 (100) | 102 (89) | 266 (63) |
| Wall material | ||||
| Bamboo or wood | 11 (5) | 0 | 11 (10) | 22 (5) |
| Mud and stone | 215 (93) | 31 (41) | 15 (13) | 261 (62) |
| Cement or stone with wood trim | 4 (2) | 45 (59) | 88 (77) | 137 (33) |
| Floor material | ||||
| Earth | 0 | 73 (99) | 10 (9) | 83 (20) |
| Wood | 200 (91) | 1 (1) | 3 (3) | 204 (50) |
| Cement (with or without wood) | 20 (9) | 0 | 101 (89) | 121 (30) |
| House has tin roof | 215 (93) | 58 (76) | 112 (98) | 388 (92) |
| Ever sleeps outside | 3 (2) | 3 (4) | 9 (9) | 15 (4) |
| Always sleeps under bed net | ||||
| Spring | 0 | 42 (59) | 15 (14) | 57 (15) |
| Summer | 35 (17) | 72 (100) | 91 (87) | 198 (51) |
| Fall | 0 | 41 (58) | 38 (36) | 79 (20) |
| Winter | 0 | 25 (35) | 10 (10) | 35 (9) |
| Travel outside village > 6 months | 11 (6) | 7 (10) | 13 (13) | 31 (9) |
Table 6.
Associations between individual and household characteristics and positive results by leishmanin skin test (LST) in unadjusted analyses and multivariable logistic regression models adjusted for community and age group, Bhutan, 2010
| LST− (N = 353) | LST+ (N = 43) | Unadjusted | Adjusted* | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |||
| Community of residence | ||||||
| Sunkosh Village or Bazar | 177 (50) | 3 (7) | Referent | Referent | ||
| Ozorong | 176 (50) | 40 (93) | 13.4 (4.1, 44.1) | < 0.0001 | 19.4 (5.7, 65.4) | < 0.0001 |
| Age (years) | ||||||
| ≤ 15 | 101 (29) | 7 (16) | Referent | Referent | ||
| 16–45 | 178 (50) | 23 (53) | 1.9 (0.8, 4.5) | 0.17 | 2.9 (1.2, 7.1) | 0.02 |
| ≥ 46 | 74 (21) | 13 (30) | 2.5 (0.9, 6.7) | 0.06 | 6.0 (2.1, 16.9) | 0.0007 |
| Male | 166 (47) | 19 (44) | 0.9 (0.5, 1.7) | 0.72 | 1.5 (0.74, 3.0) | 0.26 |
| Household head is a farmer | 227 (65) | 35 (85) | 3.1 (1.3, 7.7) | 0.012 | 1.6 (0.61, 4.1) | 0.34 |
| HoH has no formal education | 234 (67) | 28 (68) | 1.1 (0.5, 2.1) | 0.88 | 0.5 (0.22, 1.1) | 0.08 |
| Household owns | ||||||
| Dog(s) | 87 (25) | 12 (29) | 1.2 (0.6, 2.5) | 0.55 | Invalid model | |
| Goat(s) | 71 (20) | 6 (15) | 0.68 (0.3, 1.7) | 0.4 | Invalid model | |
| Cattle | 212 (61) | 34 (83) | 3.1 (1.4, 7.3) | 0.008 | 1.9 (0.8, 4.5) | 0.17 |
| Radio | 186 (53) | 24 (59) | 1.2 (0.6, 2.4) | 0.54 | 0.72 (0.4, 1.5) | 0.37 |
| Latrine | 344 (98) | 40 (98) | 0.6 (0.07, 5.1) | 0.62 | 1.5 (0.2, 14.1) | 0.73 |
| Land | 225 (64) | 36 (88) | 4.0 (1.5, 10.4) | 0.005 | 2.7 (0.99, 7.4) | 0.06 |
| Bed net | 232 (66) | 22 (54) | 0.6 (0.3, 1.1) | 0.1 | 1.8 (0.8, 3.6) | 0.14 |
| House has mud walls | 201 (58) | 38 (93) | 9.3 (2.8, 30.8) | 0.0002 | 2.3 (0.5, 10.2) | 0.28 |
| House has tin roof | 320 (91) | 39 (95) | 1.8 (0.4, 7.7) | 0.45 | 1.1 (0.2, 5.0) | 0.95 |
| House has wooden floor | 152 (44) | 30 (79) | 4.6 (2.1, 10.4) | 0.0002 | 0.6 (0.2, 1.7) | 0.3 |
| Ever sleeps outside | 14 (4) | 1 (3) | 0.6 (0.1, 4.6) | 0.61 | 1.3 (0.1, 11.7) | 0.84 |
| Always sleeps under bed net | ||||||
| Spring | 52 (16) | 3 (7) | 0.4 (0.1, 1.4) | 0.17 | Invalid model | |
| Summer | 188 (56) | 8 (20) | 0.2 (0.1, 0.4) | < 0.0001 | 0.8 (0.3, 2.3) | 0.73 |
| Fall | 74 (22) | 3 (7) | 0.3 (0.1, 0.9) | 0.04 | Invalid model | |
| Winter | 31 (9) | 3 (7) | 0.8 (0.2, 2.7) | 0.7 | Invalid model | |
| Travel outside village > 6 months | 28 (9) | 3 (8) | 0.9 (0.2, 3.0) | 0.8 | 1.2 (0.3, 4.9) | 0.77 |
Adjusted for age group and community (Ozorong versus Sunkosh village/bazar).
Figure 4.
Prevalence of positive results by the leishmanin skin test (LST) by age group in Ozorong village only, Bhutan, 2011.
Twenty-five dogs were included in the study. Most of the dogs (17 of 25) were between 1 and 3 years of age, five < 1 year of age and three > 3 years of age. With the exception of lymph node enlargement (20 of 25) the presence of other clinical signs was scarce: skin lesions (4 of 25), onychogryphosis (1 of 25), weight loss (1 of 25), and alopecia (2 of 25). Only five dogs presented two or more clinical signs. The rK39-ICT in blood and plasma, IFAT in plasma, and PCR in blood yielded negative results for all dogs. Lymph node aspirate was collected only from one dog, and it also returned a negative result by PCR.
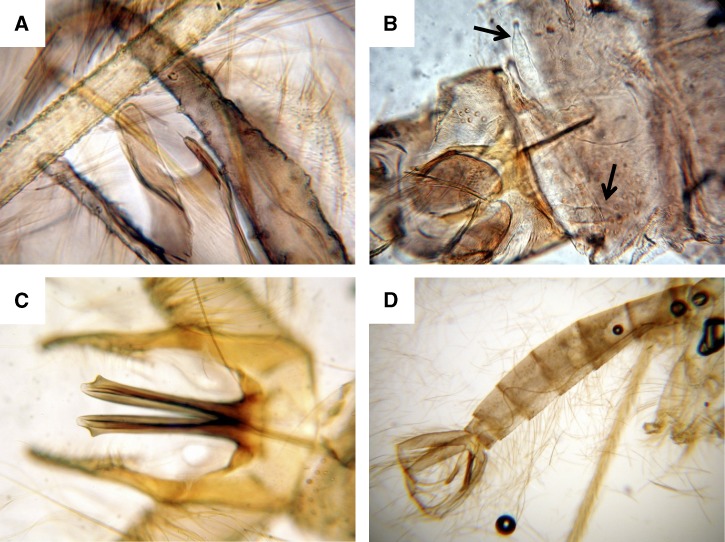
A total of 18 sand flies were collected from the four different trapping locations; 14 flies were trapped in Mongar district and 4 in Trashigang. Thirteen were Phlebotomus species (8 males and 5 females), and 5 were Sergentomyia species (2 males and 3 females) (Table 7). Because the sand flies were not prepared and mounted just after collection and were preserved in dry (silica-gel matrix), not all morphological characters were readily distinguishable, details are presented in Figure 5. Leishmanial DNA detection was negative in all female specimens. The 18SrRNA gene DNA sequence was obtained for 7 out of 8 females and submitted to the GenBank under the accession numbers indicated in Table 7.
Table 7.
Details of sand fly captures using Centers for Disease Control and Prevention (CDC) miniature light traps
| Station | Village or location (district) | Altitude (meters)* | Date | Specimens | Morphological identification | 18SrRNA gene GenBank accession no. |
|---|---|---|---|---|---|---|
| 1 | Kalapang (Mongar) | 900 | March 12–13, 2011 | Male 1 | Phlebotomus (Adlerius) longiductus | Not done |
| Male 2 | P.(A.) longiductus | Not done | ||||
| Male 3 | P. (A.) longiductus | Not done | ||||
| Male 4 | P. (Euphlebotomus) kiangsuensis | Not done | ||||
| Female 1 | P. (A.) longiductus | Not done | ||||
| Female 2 | P. (A.) longiductus | JQ790521 | ||||
| Female 3 | P. (E.) kiangsuensis | JQ790522 | ||||
| 2 | Thrinangbi (Mongar) | 1,160 | March 13–14, 2011 | Male 1 | P. (E.) kiangsuensis | Not done |
| Male 2 | P. (E.) kiangsuensis | Not done | ||||
| Male 3 | P. (E.) kiangsuensis | Not done | ||||
| Male 4 | P. (E.) kiangsuensis | Not done | ||||
| Male 5 | Sergentomyia (Parrotomyia) barraudi | Not done | ||||
| Female 1 | P. (E.) kiangsuensis | JQ790523 | ||||
| Female 2 | S. (P.) barraudi | JQ790516 | ||||
| Female 3 | S. (P.) barraudi | JQ790517 | ||||
| 3 | Karuna House (Trashigang) | 936 | March 14–15, 2011 | Female 1 | P. (Larroussius) major | JQ790519 |
| Female 2 | S.(P.) barraudi | JQ790518 | ||||
| 4 | Ozorong (Trashigang) | 2,000 | March 16–17, 2011 | Male 1 | S. (P.) barraudi | Not done |
| Female 1 | P. (L.) major | JQ790520 |
Altitude in meters above sea level.
Figure 5.
Morphological characters used for the identification of the captured sand flies. (A) P. kiangsuensis (♂) paramere. (B) P. longiductus (♀), black arrows indicate the spermathecae. (C) P. longiductus (♂) aedeagus. (D) P. longiductus (♂) abdomen, sperm-pump, and long genital filaments can be observed.
Discussion
This rapid assessment suggests that endemic VL transmission has occurred in diverse locations in Bhutan. The Leishmania species found in the specimens studied was L. donovani, including at least two distinct genotypes. Results from DNA sequencing and the phylogenetic analysis indicate that these parasites are closely related to Indian L. donovani isolates, especially ITS type H. Studies using multilocus microsatellite typing show that L. donovani isolates from the Indian subcontinent are more genetically homogeneous than those from other regions.20 Nevertheless, some degree of genetic variability has been observed using multilocus microsatellite typing and other genetic markers among isolates from the Indian subcontinent.21–23
The molecular data from this preliminary study is not sufficient to identify the origin of the parasites. One of the microscopically confirmed bone marrow smears was negative for all the molecular tests used, but this is not surprising as this post-treatment specimen had a very low parasite load. Two additional smears were also negative for hsp70 PCR and ITS2, although positive for ITS1 PCR. This finding may reflect the higher sensitivity of the ITS1 primers, which amplify a shorter target (320 bp for ITS1 versus 740 bp for ITS2) with higher copy numbers than hsp70 (∼1300 bp). Although Giemsa-stained smears are considered a good sample for PCR analysis, not all microscopically confirmed slides will return a positive result.24,25 Whether the ITS genetic profiles found in the Bhutanese isolates are specific to Bhutan or not remains unclear. However, these isolates form a cluster supported by a bootstrap value of 99% with CHANDIGARH strain, which was isolated far from Bhutan and in year 1983. A more conservative interpretation would be that they reflect part of the variability of parasites from the Indian subcontinent.
The presence of P. argentipes has previously been documented in Bhutanese communities above 2,000 m altitude.6 In the current assessment, we found a much higher prevalence of positive LST results in higher altitude Ozorong than in the lower region of Sunkosh. We initially suspected the Ozorong patient might have become infected when he traveled to the area bordering India and thus introduced the parasite into his village, but the LST age prevalence pattern was inconsistent with a single recent introduction. The pattern of rising prevalence with age is characteristic of long-standing circulation of the parasite in the community.26–29 The paucity of overt kala-azar cases in this community is unusual for anthroponotic transmission based on epidemiological studies in India and Bangladesh.30–32 The explanation is not clear, but could include the possibility of transmission from asymptomatically infected humans, or an unidentified animal reservoir. None of the dogs studied in the Trashigang canine shelter showed any marker of Leishmania infection, but the sample size was too small to yield a definitive answer regarding a potential canine reservoir role.
The low numbers of sand fly captures during this study was not unexpected, given that they were carried out in March, which is probably early in the sand fly season. We have identified five Phlebotomus longiductus specimens, a suspected vector of visceral and cutaneous leishmaniasis in Satluj river valley in Himachal Pradesh, India, but also of visceral leishmaniasis in China.33–36 Additionally, two specimens of Phlebotomus major and six of Phlebotomus kiangsuensis were found, these being proven or possible vectors of different forms of leishmaniasis in the Old World, particularly in China and India.33–35,37,38 All five Sergentomyia specimens were identified as S. barraudi, a species also described in Sichuan and Hubei provinces, China.38,39 Interestingly, we found no P. argentipes specimens, which were collected in July in the previous study,6 perhaps because our captures were performed in the early spring. It is noteworthy that these and previous findings indicate the presence of several putative vectors of leishmaniasis in Bhutan. However, to date none of the captured sand flies has been shown to be infected by Leishmania and the vector remains unidentified.
The remarkable difference in prevalence of positive LST results between Ozorong and Sunkosh is also intriguing but not fully elucidated by this analysis. We hypothesized that the much higher bed net usage in Sunkosh, where the malaria control program had distributed nets to all houses, might account for the difference in transmission rates. However, we could not test this hypothesis in the data set, because the systematic difference in bed net usage between the two sites was tantamount to epidemiological confounding. Further investigation is required to evaluate other possible risk factors, such as environmental determinants of vector populations and housing conditions, in addition to direct assessment of the potential protective effect of nets.
Many questions remain to be answered regarding transmission of VL in Bhutan. Actions that could help to clarify the situation include establishment of an appropriate surveillance system and a more in-depth retrospective analysis of reported cases. Because the potential for false positive results by rapid test is especially worrisome in a low incidence setting such as Bhutan, parasitological confirmation should be recommended in all cases, followed by a rapid epidemiological assessment of each case. This should include a search for more cases nearby, evaluation of possible reservoirs, isolation and characterization of the parasite, and vector studies. The potential protective impact of bed nets should also be evaluated.
ACKNOWLEDGMENTS
This assessment was made thanks to the support that the Spanish Agency for International Cooperation for Development (AECID) provides to WHO for the control of visceral leishmaniasis. We are grateful to Rinzin Namgay from the Ministry of Health, Bhutan and the staff from Karuna House canine shelter in Trashigang, Bhutan, for their support during the field study.
Footnotes
Authors' addresses: Thinley Yangzom, JDWNR-Hospital and Vector Borne Diseases Control Programme, Timphu Ministry of Health, Timphu, Bhutan, E-mail: thinleyangzom@gmail.com. Israel Cruz and Ricardo Molina, WHO Collaborating Center for Leishmaniasis, Servicio de Parasitología, Centro Nacional de Microbiología, Instituto de Salud Carlos III, Madrid, Spain, E-mails: cruzi@isciii.es and rmolina@isciii.es. Caryn Bern, Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, CA, E-mail: Caryn.Bern2@ucsf.edu. Daniel Argaw, Margriet den Boer, and Jorge Alvar, Department for the Control of Neglected Tropical Diseases (CDS/NTD/IDM), Leishmaniasis Control Program, World Health Organization, Geneva, Switzerland, E-mails: daniel@who.int, margrietdenboer@gmail.com, and alvarj@who.int. Iván Dario Vélez, Programa de Estudio y Control de Enfermedades Tropicales, Universidad de Antioquia, Medellín, Colombia, E-mail: id_velez@yahoo.com. Sujit K. Bhattacharya, WHO/South-East Asia Regional Office, New Delhi, India, E-mail: bhattacharyas@searo.who.int.
References
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, Den Boer M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kar B. The Assam fever. Wellcome History. 2003;23:2–4. [Google Scholar]
- 3.Donovan C. The etiology of heterogeneous fevers in India. Lancet. 1903;2:1401. [Google Scholar]
- 4.Mahajan SK, Machhan P, Kanga A, Thakur S, Sharma A, Prasher BS, Pal LS. Kala-Azar at high altitude. J Commun Dis. 2004;36:117–120. [PubMed] [Google Scholar]
- 5.Mathur P, Samantaray JC, Mangraj S. Smouldering focus of kala-azar in Assam. Indian J Med Res. 2004;120:56. [PubMed] [Google Scholar]
- 6.Bhattacharya SK, Rinzin N, Chusak P, Dash AP, Chowdhury R, Tobgay T, Narain JP. Occurrence and significance of kala-azar in Bhutan. Indian J Med Res. 2010;132:337–338. [PubMed] [Google Scholar]
- 7.Sokal JE. Editorial: measurement of delayed skin-test responses. N Engl J Med. 1975;293:501–502. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- 8.Gramiccia M, Bettini S, Gradoni L, Ciarmoli P, Verrilli ML, Loddo S, Cicalo C. Leishmaniasis in Sardinia. IV. Leishmanin reaction in the human population of a focus of low endemicity of canine leishmaniasis. Trans R Soc Trop Med Hyg. 1990;84:371–374. doi: 10.1016/0035-9203(90)90322-6. [DOI] [PubMed] [Google Scholar]
- 9.Weigle KA, Valderrama L, Arias AL, Santrich C, Saravia NG. Leishmanin skin test standardization and evaluation of safety, dose, storage, longevity of reaction and sensitization. Am J Trop Med Hyg. 1991;44:260–271. doi: 10.4269/ajtmh.1991.44.260. [DOI] [PubMed] [Google Scholar]
- 10.Bray RS. Immunodiagnosis of leishmaniasis. In: Chang KP, Bray RS, editors. Leishmaniasis. Amsterdam: Elsevier; 1985. pp. 177–182. [Google Scholar]
- 11.Lewis D. A taxonomic review of the genus Phlebotomus (Diptera: Psychodidae) Bull Br Mus. 1982;45:121–209. [Google Scholar]
- 12.Lewis DJ. The phlebotomine sandflies (Diptera: Psychodidae) of the oriental region. Bull Br Mus. 1978;37:217–343. [Google Scholar]
- 13.Kuhls K, Mauricio IL, Pratlong F, Presber W, Schönian G. Analysis of ribosomal DNA internal transcribed spacer sequences of the Leishmania donovani complex. Microbes Infect. 2005;7:1224–1234. doi: 10.1016/j.micinf.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Fraga J, Montalvo AM, De Doncker S, Dujardin JC, Van der Auwera G. Phylogeny of Leishmania species based on the heat-shock protein 70 gene. Infect Genet Evol. 2010;10:238–245. doi: 10.1016/j.meegid.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Barroso PA, Marco JD, Kato H, Tarama R, Rueda P, Cajal SP, Basombrio MA, Korenaga M, Taranto NJ, Hashiguchi Y. The identification of sandfly species, from an area of Argentina with endemic leishmaniasis, by the PCR-based analysis of the gene coding for 18S ribosomal RNA. Ann Trop Med Parasitol. 2007;101:247–253. doi: 10.1179/136485907X156988. [DOI] [PubMed] [Google Scholar]
- 16.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 17.Felsenstein J. PHYLIP-Phylogeny Inference Package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 18.InBios International Inc . Kalazar Detect Product Insert. Seattle, WA: InBios International Inc; 2012. [Google Scholar]
- 19.Pappas MG, Hajkowski R, Hockmeyer WT. Dot enzyme-linked immunosorbent assay (Dot-ELISA): a micro technique for the rapid diagnosis of visceral leishmaniasis. J Immunol Methods. 1983;64:205–214. doi: 10.1016/0022-1759(83)90399-x. [DOI] [PubMed] [Google Scholar]
- 20.Kuhls K, Keilonat L, Ochsenreither S, Schaar M, Schweynoch C, Presber W, Schonian G. Multilocus microsatellite typing (MLMT) reveals genetically isolated populations between and within the main endemic regions of visceral leishmaniasis. Microbes Infect. 2007;9:334–343. doi: 10.1016/j.micinf.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Alam MZ, Kuhls K, Schweynoch C, Sundar S, Rijal S, Shamsuzzaman AK, Raju BV, Salotra P, Dujardin JC, Schonian G. Multilocus microsatellite typing (MLMT) reveals genetic homogeneity of Leishmania donovani strains in the Indian subcontinent. Infect Genet Evol. 2009;9:24–31. doi: 10.1016/j.meegid.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Bhattarai NR, Dujardin JC, Rijal S, De Doncker S, Boelaert M, Van der Auwera G. Development and evaluation of different PCR-based typing methods for discrimination of Leishmania donovani isolates from Nepal. Parasitology. 2010;137:947–957. doi: 10.1017/S0031182009991752. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava P, Singh T, Sundar S. Genetic heterogeneity in clinical isolates of Leishmania donovani from India. J Clin Microbiol. 2011;49:3687–3690. doi: 10.1128/JCM.00729-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Jawabreh A, Schoenian G, Hamarsheh O, Presber W. Clinical diagnosis of cutaneous leishmaniasis: a comparison study between standardized graded direct microscopy and ITS1-PCR of Giemsa-stained smears. Acta Trop. 2006;99:55–61. doi: 10.1016/j.actatropica.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Volpini AC, Marques MJ, Lopes dos Santos S, Machado-Coelho GL, Mayrink W, Romanha AJ. Leishmania identification by PCR of Giemsa-stained lesion imprint slides stored for up to 36 years. Clin Microbiol Infect. 2006;12:815–818. doi: 10.1111/j.1469-0691.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- 26.Ali A, Ashford RW. Visceral leishmaniasis in Ethiopia. I. Cross-sectional leishmanin skin test in an endemic locality. Ann Trop Med Parasitol. 1993;87:157–161. doi: 10.1080/00034983.1993.11812749. [DOI] [PubMed] [Google Scholar]
- 27.Bern C, Amann J, Haque R, Chowdhury R, Ali M, Kurkjian KM, Vaz L, Wagatsuma Y, Breiman RF, Secor WE, Maguire JH. Loss of leishmanin skin test antigen sensitivity and potency in a longitudinal study of visceral leishmaniasis in Bangladesh. Am J Trop Med Hyg. 2006;75:744–748. [PubMed] [Google Scholar]
- 28.Bettini S, Gramiccia M, Gradoni L, Pozio E, Mugnai S, Maroli M. Leishmaniasis in Tuscany (Italy): VIII. Human population response to leishmanin in the focus of Monte Argentario (Grosseto) and epidemiological evaluation. Ann Parasitol Hum Comp. 1983;58:539–547. doi: 10.1051/parasite/1983586539. [DOI] [PubMed] [Google Scholar]
- 29.Davies CR, Llanos-Cuentas EA, Pyke SD, Dye C. Cutaneous leishmaniasis in the Peruvian Andes: an epidemiological study of infection and immunity. Epidemiol Infect. 1995;114:297–318. doi: 10.1017/s0950268800057964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bern C, Chowdhury R. The epidemiology of visceral leishmaniasis in Bangladesh: prospects for improved control. Indian J Med Res. 2006;123:275–288. [PubMed] [Google Scholar]
- 31.Das P, Samuels S, Desjeux P, Mittal A, Topno R, Siddiqui NA, Sur D, Pandey A, Sarnoff R. Annual incidence of visceral leishmaniasis in an endemic area of Bihar, India. Trop Med Int Health. 2010;15((Suppl 2)):4–11. doi: 10.1111/j.1365-3156.2010.02517.x. [DOI] [PubMed] [Google Scholar]
- 32.Topno RK, Das VN, Ranjan A, Pandey K, Singh D, Kumar N, Siddiqui NA, Singh VP, Kesari S, Bimal S, Kumar AJ, Meena C, Kumar R, Das P. Asymptomatic infection with visceral leishmaniasis in a disease-endemic area in Bihar, India. Am J Trop Med Hyg. 2010;83:502–506. doi: 10.4269/ajtmh.2010.09-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization Control of the leishmaniases. World Health Organ Tech Rep Ser. 2010;xii–xiii:1–186. back cover. [PubMed] [Google Scholar]
- 34.Sharma NL, Mahajan VK, Ranjan N, Verma GK, Negi AK, Mehta KI. The sandflies of the Satluj river valley, Himachal Pradesh (India): some possible vectors of the parasite causing human cutaneous and visceral leishmaniases in this endemic focus. J Vector Borne Dis. 2009;46:136–140. [PubMed] [Google Scholar]
- 35.Sharma NL, Mahajan VK, Negi AK. Epidemiology of a new focus of localized cutaneous leishmaniasis in Himachal Pradesh. J Commun Dis. 2005;37:275–279. [PubMed] [Google Scholar]
- 36.Sharma NL, Mahajan VK, Kanga A, Sood A, Katoch VM, Mauricio I, Singh CD, Parwan UC, Sharma VK, Sharma RC. Localized cutaneous leishmaniasis due to Leishmania donovani and Leishmania tropica: preliminary findings of the study of 161 new cases from a new endemic focus in Himachal Pradesh, India. Am J Trop Med Hyg. 2005;72:819–824. [PubMed] [Google Scholar]
- 37.Guan L, Yang Y, Xu Y, Qu J, Zho X, Wang G, Lu H, Zhong L, Chang KP. Leishmaniasis in Karamay. XIV. Identification of promastigote isolates from naturally infected Phlebotomus major wui. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 1994;12:257–261. [PubMed] [Google Scholar]
- 38.Leng YJ, Wang HB, Ge NL. A survey of phlebotomine sandflies (Diptera: Psychodidae) in Hubei Province, China. Parassitologia. 1991;33((Suppl)):377–379. [PubMed] [Google Scholar]
- 39.Wei F, Shang L, Jin H, Lian H, Liu W, Li Z, Gao H, Liu Q. Molecular detection and genetic diversity of Leishmania donovani in naturally infected Phlebotomus chinensis from southwestern China. Vector Borne Zoonotic Dis. 2011;11:849–852. doi: 10.1089/vbz.2010.0148. [DOI] [PubMed] [Google Scholar]