Abstract
Sensitive Schistosoma japonicum detection methods are needed to progress from schistosomiasis control to elimination. The sensitivity of the Kato-Katz thick smear and miracidium hatching tests decrease with infection intensity and serological tests cannot always identify current infections. We evaluated a fecal polymerase chain reaction (PCR) assay to detect S. japonicum infection in 106 humans and 8 bovines in China. PCR was highly sensitive, detecting S. japonicum DNA at 0.5 eggs/g of stool. Comparing PCR examination of a single stool sample to the miracidium hatching test using three consecutive stool samples, more humans were hatching test positive (20%) than PCR positive (15%). However, two individuals were PCR positive in a village where no infections were detected by coprological methods. The sensitivity of PCR makes it a promising tool for schistosomiasis diagnostics and screening, although egg shedding variability and stool sample size present challenges for any detection method in low-transmission areas.
Introduction
The changing epidemiology of schistosomiasis in countries such as China has fueled the need for more sensitive diagnostic and surveillance methods.1–4 Infection with the causative species in China, Schistosoma japonicum, is typically diagnosed using one of two coprological methods, the Kato-Katz thick smear procedure or the miracidium hatching test, or by immunoassays performed on serum. Both the Kato-Katz and hatching tests are inexpensive, require limited technical training, and are effective in situations of high prevalence and high-intensity infections, but their value is reduced in low-infection intensity settings as the sensitivity of the tests decrease with decreasing schistosome infection intensity.5–7 The performance of immunoassays such as indirect hemagglutination assay, enzyme-linked immunosorbent assay, and dipstick dye immunoassay has recently been assessed.8–10 These studies have shown that immunoassays have high sensitivities (> 90%) and ease of use when compared with coprological methods, but issues of specificity and the inability of antibody detection methods to distinguish between current and past infections are still of concern. Moreover, the sensitivity of serological testing, like coprological testing, declines as infection intensity decreases.11 An expert committee convened by the World Health Organization (WHO) to discuss schistosomiasis elimination concluded “standard parasitological testing…is particularly insensitive for detecting low-level or light infections…”4 A highly sensitive test is required to accurately assess prevalence of infection and propel the elimination effort forward.
This work evaluates the use of polymerase chain reaction (PCR) as a diagnostic and screening method for detection of Schistosoma japonicum infections in humans and bovines in four villages where schistosomiasis has reemerged. The use of PCR as a screening tool is advantageous because of its sensitivity and ubiquity in modern clinical settings. Earlier studies have described PCR methods for detection of Schistosoma mansoni and Schistosoma haematobium DNA in animal models and in human samples.12–16 Schistosoma japonicum PCR techniques have also been described.17–22 However, the use of PCR techniques to screen field-collected samples has been limited.19,21 Conventional PCR rather than quantitative real-time PCR (qPCR) was tested because of the limited need for a quantitative description of infections in a low-prevalence setting and because conventional PCR requires fewer resources than qPCR, making it a more accessible tool for resource-constrained settings. The PCR assay in this study was used to evaluate human and bovine samples and compared with Kato-Katz and miracidium hatching test results, providing, to our knowledge, the first evaluation of a PCR-based S. japonicum diagnostic method using field-collected samples from multiple S. japonicum host species.
Materials and Methods
Study location and sample collection.
This study was conducted in four rural, agricultural villages located in regions of Sichuan, China where schistosomiasis reemergence has been documented23,24; we define a village as the smallest organizational unit in a region, also referred to as a production group or natural village. Because of the sensitive nature of conducting infection surveys in regions where schistosomiasis transmission control criteria have officially been met, the names and exact locations of the counties and study villages have been withheld.
In November and December 2010, all residents in the study villages, 6–68 years of age, were invited to submit three stool samples on three consecutive days. Bovines in the village were identified by interviewing each head of household. Bovine samples were obtained by confining the animal to a pen or tying the animal until a sample was produced on three consecutive days. Human and bovine samples were collected and transferred to a county laboratory daily and examined using the miracidium hatching test and the Kato-Katz thick smear procedure upon arrival. Approximately 15 g of stool from one sample per person and bovine were transferred to a capped tube, frozen for transfer to Chengdu, and stored at −80°C at the Institute of Parasitic Diseases, Sichuan Center for Disease Control and Prevention, Chengdu, China (SIPD) for analysis using PCR in the summer of 2011.
Miracidium hatching test.
All human and bovine stool samples were examined using the miracidium hatching test.25 Approximately 30 g of stool were filtered through 80 head mesh to remove large particles, and then strained with 260 head nylon mesh to concentrate schistosome eggs. Tap water was used to wash the sample until rinse water ran clear. The sediment was then transferred to a flask and water was added to reach the narrow neck of the container for improved visualization. Because the miracidium hatching is sensitive to chlorine and pH, we resuspended sediment using water with pH between 6.5 and 7.5 and free chlorine < 1 ppm, and confirmed using a colorimeter (Colorimeter C301, NovaTech, Houston, TX). Samples were left undisturbed in a room with ambient temperatures between 28 and 30°C. Human samples were examined for the presence of miracidia 2, 5, and 8 hours after preparation. Because we have observed that S. japonicum eggs may hatch more rapidly in bovine stool because of the higher water volume, bovine samples were examined 1, 3, and 5 hours after sample preparation. Samples were examined for the presence of miracidia for at least 2 minutes at each observation point. A sample was classified as hatching test positive if any miracidia were observed.
Kato-Katz thick smear procedure.
Using the Kato-Katz thick smear procedure, three slides were prepared using 41.7 mg of homogenized stool for each slide from the first stool sample submitted by each person.26 Each slide was also examined for the presence of S. japonicum and the soil-transmitted helminthes (STHs), e.g., Ascaris lumbricoides, Trichuris trichiura, and Ancylostoma spp., to check for cross-reactivity. Because hookworm eggs degrade rapidly,27 slides were examined 2 hours after preparation, using a light microscope, for the presence of hookworm eggs. Slides were then stored for 24 hours out of direct sunlight before examination for S. japonicum, A. lumbricoides, and T. trichiura. Infection intensity, expressed in eggs per gram of stool (EPG), was calculated as the total number of S. japonicum eggs divided by the total sample weight. A sample was classified as Kato-Katz positive for schistosomiasis if at least one S. japonicum egg was detected.
DNA extraction.
DNA was extracted from one stool sample per person and bovine using the “isolation of DNA from larger volumes of stool” protocol from the QIAamp DNA Mini Stool Kit (QIAGEN, Valencia, CA), with slight modifications. A stool lysis buffer composed of 10 mM Tris pH 8.0, 300 mM EDTA pH 8.0, and 1% w/v sodium dodecyl sulfate was used for the first lysis step and the first incubation was conducted at 95°C. Extracted DNA was eluted with 50 μL of QIAGEN AE buffer. The DNA was extracted from 1 g of stool from each person and bovine.
PCR.
PCR was performed on all extractions using previously designed primers22; the primers targeted the highly repetitive retrotransposon SjR2 of S. japonicum and resulted in a 230 bp product. The sequences of the primers were: 5′-TCTAATGCTATTGGTTTGAGT-3′ (forward) and 5′-TTCCTTATTTTCACAAGGTGA-3′ (reverse). The reaction mixture was composed of 0.2 μL of each 25 μM primer, 0.2 μL 10 mM dNTP, 0.04 μL 5 Units/μL GoTaq DNA polymerase (Promega, Madison, WI), 2 μL 5× buffer with 7.5 mM MgCl2, 0.4 μL bovine serum albumin, 1.5 μL template DNA, and distilled water to a final volume of 10 μL. The initial denature step was run at 95°C for 5 minutes, followed by 35 cycles of 94, 53, and 72°C for 1 minute each. The final extension was run at 72° for 7 minutes. Amplified products were run on a 2% agarose gel with GoldView (SBS Genetech Co., Ltd., Beijing) for visualization.
Validating protocol and assessing sensitivity.
Schistosoma japonicum worms from an infected rabbit were provided by SIPD and had been stored at 4°C in 96% EtOH. Each worm was washed three times in distilled water and then suspended in 10 μL of lysis solution (0.02M NaOH, pH 12) and incubated at 95°C for at least 30 minutes. The resulting lysate was cooled to 4°C and 10 μL of neutralizing solution (40 mM Tris-HCl, pH 5) was added. The expected 230 bp PCR product was seen when worm lysate was used as the DNA template. The DNA extracted from the stool sample of an individual from an S. japonicum non-endemic population was used to test the primer specificity.
Sensitivity of the PCR assay was assessed by spiking stool samples from the non-endemic individual with S. japonicum eggs. Eggs from rabbit liver were provided by SIPD and had been stored at −20°C in 96% EtOH. Each spiked sample was prepared in triplicate with 1, 2, 3, 4, 5, 10, and 20 egg(s) in 1 g of stool, respectively. The three 0.5 EPG samples were prepared by spiking 2 g of stool with 1 egg. The full 2 g of spiked stool was used for DNA extraction. All DNA was extracted using the previously described DNA extraction protocol.
Ethical approval and treatment.
All participants provided written informed consent before participating in this study. The research protocol was approved by the Sichuan Institutional Review Board and the University of California, Berkeley, Committee for the Protection of Human Subjects. Each person who tested positive for S. japonicum was provided treatment with praziquantel by the county Anti-Schistosomiasis Control Station. All bovines testing positive were referred to the county veterinary station for treatment with praziquantel.
Results
The PCR assay was shown to be highly sensitive in detecting S. japonicum DNA in spiked stool samples. Schistosoma japonicum DNA was detected at 0.5 EPG as well as 1–5, 10, and 20 EPG.
A total of 142 people from the four study villages submitted stool samples. Individuals who submitted stool samples were, on average, older (mean age 49) and more likely to be female (50% female) than those who did not participate in the infection survey (mean age 39, 43% female), as many young adults, particularly males, leave the study villages to work or attend school in urban areas. We tested 106 people using all three parasitological testing methods (because of inadequate stool sample size, 26 individuals lacked results from all three tests). The prevalence of infection for these 106 individuals was 10.4%, 19.8%, and 15.1% according to the Kato-Katz thick smear, miracidium hatching test, and PCR assay, respectively. The miracidium hatching test was positive for 13.2% of individuals based on their first stool sample, 9.4% based on the second stool sample, and 13.9% based on the final stool sample. Mean infection intensity was 3.0 EPG (range 0 –88). Of the 11 Kato-Katz positive individuals, seven had only 1–3 eggs detected (8–24 EPG). At the village level, S. japonicum infections were detected by Kato-Katz and the hatching test in two of the four villages, and by PCR in three of the four villages (Table 1).
Table 1.
Detection of human Schistosoma japonicum infection by three different testing methods in four villages located in regions where schistosomiasis has reemerged in China
| Village | Tested | Number infected by | ||
|---|---|---|---|---|
| Hatching test | Kato-Katz | PCR | ||
| A | 40 | 8 | 4 | 7 |
| B | 35 | 13 | 7 | 7 |
| C | 20 | 0 | 0 | 2 |
| D | 11 | 0 | 0 | 0 |
| Total (%) | 106 | 21 (19.8) | 11 (10.4) | 16 (15.1) |
Each participant was requested to submit three stool samples, one per day from three consecutive days. Each sample was examined using the miracidium hatching test. One sample per person was examined using the Kato-Katz thick smear procedure (preparing three slides per person) and polymerase chain reaction (PCR).
Table 2 shows the concordance between the three tests. Two samples were PCR positive only, four samples were hatching test positive only, and zero samples were Kato-Katz positive only. When we examined only the first miracidium hatching test, four individuals were classified as hatching test positive, but PCR negative. When all stool samples were analyzed using the miracidium hatching test, eight individuals were classified as hatching test positive but PCR negative.
Table 2.
Comparison of three different diagnostic tests for Schistosoma japonicum infection in human stool, the Kato-Katz thick smear, the miracidium hatching test, and a PCR assay, conducted in regions where schistosomiasis has reemerged in China
| PCR+ | PCR− | |
|---|---|---|
| Miracidia hatching test (1 sample) and Kato-Katz | ||
| Kato-Katz+ and Hatch+ | 5 | 3 |
| Kato-Katz+ and Hatch− | 2 | 1 |
| Kato-Katz− and Hatch+ | 5 | 1 |
| Kato-Katz− and Hatch− | 4 | 85 |
| Miracidia hatching test (all samples) and Kato-Katz | ||
| Kato-Katz+ and Hatch+ | 6 | 4 |
| Kato-Katz+ and Hatch− | 1 | 0 |
| Kato-Katz− and Hatch+ | 7 | 4 |
| Kato-Katz− and Hatch− | 2 | 82 |
Each participant was requested to submit three stool samples, one per day from three consecutive days. Each sample was examined using the miracidium hatching test. One sample per person was examined using the Kato-Katz thick smear procedure (preparing three slides per person) and polymerase chain reaction (PCR). The table presents the results of the miracidium hatch test performed on the first stool sample (“1 sample”) and the combined results of the miracidium hatching tests from all samples from an individual (“all samples”).
We tested eight bovines using PCR and the miracidium hatching test. Six bovines were positive by both tests. One bovine was PCR negative and hatching test positive, and one bovine was PCR negative and hatching test negative. All eight bovines were from the same village.
Concomitant STH infections were seen, with a prevalence of 16% for hookworm infection, 6.6% for A. lumbricoides, and 3.8% for T. Trichuris infection among the 106 participants. No additional amplified bands were observed in the samples from individuals with STH infections.
Discussion
PCR-based detection methods for S. japonicum have been evaluated using experimental animal models17,18,20,22; however, PCR-testing of clinical samples from humans and field-collected samples from other mammalian hosts has been limited.19,21 Here, we have demonstrated a highly stable and sensitive PCR method able to detect schistosomiasis infection in a region where schistosomiasis had reemerged and infection intensity was low (mean infection intensity: 3.0 EPG). The ability to detect S. japonicum infection in low-prevalence areas has important implications for the clinical management of schistosomiasis and public health surveillance.
Schistosomiasis morbidity is generally proportional to infection intensity, as the quantity of eggs can increase fibrosis and the risk of anemia.28,29 However, low-intensity schistosomiasis infection when combined with low-intensity STH infection may synergistically increase the risk of anemia,30 underscoring the importance of sensitive diagnostics in areas where multiple helminth species are present, as in the case in our study region. In addition, the ability to detect low-intensity infections will be a critical tool in the determination of a morbidity profile of such infections, allowing us to more fully characterize their clinical effects.
A highly sensitive and stable assay for the detection of S. japonicum infections in humans and bovines is needed to attain long-term interruption of schistosomiasis. Our study demonstrates a viable detection method for S. japonicum infections in two host species. The bovine results are preliminary and suggestive, rather than definitive, because of the limited number of samples that were used. Future studies testing this method on a larger sample size of both humans and bovines will be worthwhile.
Schistosoma japonicum transmission is persistent even in regions with very low parasite concentrations, and models suggest modest parasite inputs can lead to the initiation of transmission in formerly parasite-free environments.31 Thus, identification and treatment of all infections, including those of animal hosts, is crucial to elimination efforts. The detection of infection by PCR in the village where infection prevalence was 0% according to Kato-Katz and hatching test demonstrates the value of PCR-based surveillance. Because control programs occur at the village level, more accurate village-level prevalence measures will better direct control efforts and provide insight to possible reasons for reemergence. The use of pooled samples may provide a method for cost-effective monitoring. The PCR method described here is also being piloted to detect S. japonicum DNA in snails and stool pits, which are candidates for environmental sampling.
Notably, the PCR results did not correspond exactly to the results of the two standard assays for S. japonicum egg detection. Two people were PCR positive for S. japonicum but negative according to the Kato-Katz and hatching test. We strongly believe these positive PCR results are true positives because of the absence of contamination in any of the negative control samples, which were analyzed with each set of field samples, underscoring the potential of the PCR method to offer a more sensitive diagnostic tool. In addition, eight samples were PCR negative for S. japonicum but hatching test positive. Based on the sensitivity of the PCR method in detecting S. japonicum DNA in the spiked stool samples, we do not believe that the fewer number of PCR positive results was caused by false negatives, but rather, to the absence of S. japonicum eggs in these samples. The hatching test results were based on the examination of a larger stool volume (30 g of stool per sample versus 1 g for the PCR assay) and the examination of stool from 3 days versus 1 day. When the results of the first miracidium hatching test, based on a single stool sample, were compared with PCR, the number of samples that were PCR negative but hatching test positive decreased to four, suggesting some individuals may have been infected with S. japonicum, however eggs may not be clinically detectable in every stool sample. Variability in egg shedding, caused by day-to-day variability in stool output, clustering of eggs within stool, and inter-individual variance,32–34 as well as stool sample size could explain why overall, more samples were found to be hatching test positive than PCR positive.
To illustrate, previous studies have shown that the number of eggs produced by one pair of worms, over time, follows the negative binomial distribution such that
where  is the mean egg output in EPG, and r is the aggregation parameter, a measure of the variability in egg output by a given worm pair. Assuming that egg output of each worm pair is independent (a reasonable assumption at low infection density), for an individual with N pairs of worms, the probability that no eggs are present in a one-gram stool sample is given by
is the mean egg output in EPG, and r is the aggregation parameter, a measure of the variability in egg output by a given worm pair. Assuming that egg output of each worm pair is independent (a reasonable assumption at low infection density), for an individual with N pairs of worms, the probability that no eggs are present in a one-gram stool sample is given by
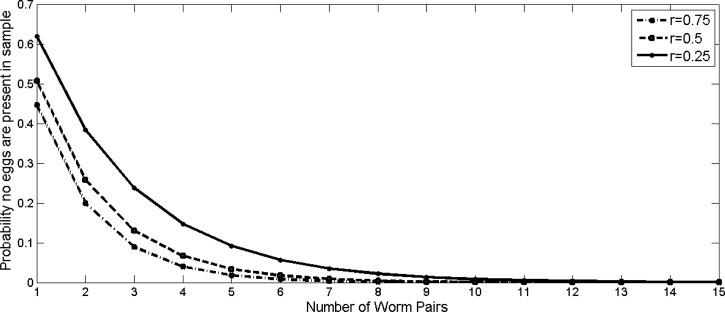
Following the estimations of Hubbard and others,34 we set 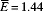 EPG and consider three scenarios corresponding to low, medium, and high levels of aggregation (Figure 1). In individuals infected with a single worm pair, the probability a one-gram stool sample contains zero eggs is 45–62%, depending on the aggregation parameter used. Although this probability decreases exponentially as the number of worm pairs increases, there is still a 10% chance of no egg production corresponding to N = 5 and r = 0.25. Therefore, one of the greatest challenges in correctly identifying infected individuals in low-prevalence regions may be simply obtaining a sample that contains S. japonicum DNA.
EPG and consider three scenarios corresponding to low, medium, and high levels of aggregation (Figure 1). In individuals infected with a single worm pair, the probability a one-gram stool sample contains zero eggs is 45–62%, depending on the aggregation parameter used. Although this probability decreases exponentially as the number of worm pairs increases, there is still a 10% chance of no egg production corresponding to N = 5 and r = 0.25. Therefore, one of the greatest challenges in correctly identifying infected individuals in low-prevalence regions may be simply obtaining a sample that contains S. japonicum DNA.
Figure 1.
Probability that no eggs are present in a one-gram stool sample in an individual infected with N worm pairs, estimated using three estimates of variability in egg output, (r). We assume egg shedding follows a negative binomial distribution, with a mean egg output 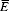 , set to 1.44,34 and aggregation (a measure of variability in egg output), r. Probabilities were calculated for high, medium, and low estimates of r.
, set to 1.44,34 and aggregation (a measure of variability in egg output), r. Probabilities were calculated for high, medium, and low estimates of r.
Collecting samples from multiple days can increase the probability that eggs are present in at least one sample. Equation 2 can also be used to calculate the probability that no eggs are present in stool samples obtained from an individual infected with one pair of worms on N different days, by substituting the number of worm pairs (Pw) with the number of days of sampling (Ds). If samples are collected on three different days, there is a 9–24% chance that no eggs will be present in any sample. For PCR analysis to be more accurately assessed as a possible diagnostic or screening method in very low infection intensity areas, it may be necessary to explore PCR assays that use DNA extracted from more than one gram of stool, or to collect samples on multiple days.
Though more expensive than the Kato-Katz and hatching test, PCR-based detection is a viable option in China because of the resources currently available at the province level and the willingness of the Chinese government to invest in public health infrastructure. At provincial anti-schistosomiasis control stations, there is both the equipment and technical knowledge necessary to perform PCR assays. At the time of our study, the county level anti-schistosomiasis control stations did not have the capacity to conduct PCR assays, but recent construction of new, standardized laboratories for these control stations and increased budget allowances may soon allow PCR capability. Although PCR requires the capital investment in a thermal cycler, the operating cost and process is relatively inexpensive and simple, especially with the use of master mixes made in batches and a small final mix volume. qPCR, although valuable for quantification, is more resource intensive than qualitative PCR and is not critical in a setting that only requires a binary outcome.
Countries that operate at a more low-resource setting than China may need detection methods that require less equipment than PCR. Loop-mediated isothermal amplification (LAMP) is another DNA amplification method that has been shown to be even more sensitive than PCR in the detection of S. japonicum DNA35 and does not require the use of a thermal cycler or gel visualization. Tools such as LAMP have the potential to be very valuable in detecting low-intensity infections in low-resource settings, but because many of China's laboratory facilities are already able to perform PCR, this stable and validated assay is currently the most promising option for a highly sensitive diagnostic and screening method.
ACKNOWLEDGMENTS
We thank Dong-chuan Qiu, Bo Zhong, Yang Lei, Rong-zhi Li, Hong Ye, Li-na Cui, the staff at SIPD, and the county Anti-Schistosomiasis control station leaders and staff for their hard work in the field and laboratory. We are grateful to the residents in our study villages who participated in this research. We thank Lee Riley and Eva Raphael from the University of California, Berkeley for providing laboratory resources and guidance. We also thank Fred Lewis from the Schistosomiasis Resource Center at the Biomedical Research Institute, Rockville, Maryland for providing S. japonicum-infected murine fecal samples for the pilot phases of this research.
Footnotes
Financial support: This work was made possible by a grant from the Center for Global Public Health at the University of California, Berkeley and a grant from the National Institute for Allergy and Infectious Diseases (R01AI068854).
Authors' addresses: Mai S. Fung, Shuo Wang, and Elizabeth J. Carlton, Environmental Health Sciences, School of Public Health, University of California, Berkeley, Berkeley, CA, E-mails: maifung@berkeley.edu, shuowang@berkeley.edu, and ejcarlton@berkeley.edu. Ning Xiao, Institute of Parasitic Diseases, Sichuan Center for Disease Control and Prevention, Chengdu, Sichuan, China (Ning Xiao's current address is National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention, Laboratory of Parasite and Vector Biology, Ministry of Health; WHO Collaborating Center for Malaria, Schistosomiasis and Filariasis, Shanghai, China), E-mail: ningxiao116@yahoo.com.cn.
References
- 1.Zhou XN, Bergquist R, Leonardo L, Yang GJ, Yang K, Sudomo M, Olveda R. Schistosomiasis japonica control and research needs. Adv Parasitol. 2010;72:145–178. doi: 10.1016/S0065-308X(10)72006-6. [DOI] [PubMed] [Google Scholar]
- 2.Wang LD, Guo JG, Wu XH, Chen HG, Wang TP, Zhu SP, Zhang ZH, Steinmann P, Yang GJ, Wang SP, Wu ZD, Wang LY, Hao Y, Bergquist R, Utzinger J, Zhou XN. China's new strategy to block Schistosoma japonicum transmission: experiences and impact beyond schistosomiasis. Trop Med Int Health. 2009;14:1475–1483. doi: 10.1111/j.1365-3156.2009.02403.x. [DOI] [PubMed] [Google Scholar]
- 3.Savioli L, Gabrielli AF, Montresor A, Chitsulo L, Engels D. Schistosomiasis control in Africa: 8 years after World Health Assembly Resolution 54.19. Parasitology. 2009;136:1677–1681. doi: 10.1017/S0031182009991181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO . Elimination of Schistosomiasis from Low-Transmission Areas: Report of a WHO Informal Consultation. Salvador, Brazil: WHO; 2009. [Google Scholar]
- 5.Lin DD, Liu JX, Liu YM, Hu F, Zhang YY, Xu JM, Li JY, Ji MJ, Bergquist R, Wu GL, Wu HW. Routine Kato-Katz technique underestimates the prevalence of Schistosoma japonicum: a case study in an endemic area of the People's Republic of China. Parasitol Int. 2008;57:281–286. doi: 10.1016/j.parint.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Yu JM, de Vlas SJ, Jiang QW, Gryseels B. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol Int. 2007;56:45–49. doi: 10.1016/j.parint.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Zhang YY, Luo JP, Liu YM, Wang QZ, Chen JH, Xu MX, Xu JM, Wu J, Tu XM, Wu GL, Zhang ZS, Wu HW. Evaluation of Kato-Katz examination method in three areas with low-level endemicity of schistosomiasis japonica in China: a Bayesian modeling approach. Acta Trop. 2009;112:16–22. doi: 10.1016/j.actatropica.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Peeling RW, Chen JX, Wu XH, Wu ZD, Wang SP, Feng T, Chen SH, Li H, Guo JG, Zhou XN. Evaluation of immunoassays for the diagnosis of Schistosoma japonicum infection using archived sera. PLoS Negl Trop Dis. 2011;5:e949. doi: 10.1371/journal.pntd.0000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou XN, Xu J, Chen HG, Wang TP, Huang XB, Lin DD, Wang QZ, Tang L, Guo JG, Wu XH, Feng T, Chen JX, Guo J, Chen SH, Li H, Wu ZD, Peeling RW. Tools to support policy decisions related to treatment strategies and surveillance of schistosomiasis japonica towards elimination. PLoS Negl Trop Dis. 2011;5:e1408. doi: 10.1371/journal.pntd.0001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu YC. Immunodiagnosis and its role in schistosomiasis control in China: a review. Acta Trop. 2005;96:130–136. doi: 10.1016/j.actatropica.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Li Y, Li H, Xing Y, Qu G, Dai J, Liang Y. Immunodiagnostic efficacy of detection of Schistosoma japonicum human infections in China: a meta analysis. Asian Pac J Trop Med. 2012;5:15–23. doi: 10.1016/S1995-7645(11)60238-1. [DOI] [PubMed] [Google Scholar]
- 12.Gomes LI, Marques LH, Enk MJ, Coelho PM, Rabello A. Further evaluation of an updated PCR assay for the detection of Schistosoma mansoni DNA in human stool samples. Mem Inst Oswaldo Cruz. 2009;104:1194–1196. doi: 10.1590/s0074-02762009000800021. [DOI] [PubMed] [Google Scholar]
- 13.Hamburger J, He N, Abbasi I, Ramzy RM, Jourdane J, Ruppel A. Polymerase chain reaction assay based on a highly repeated sequence of Schistosoma haematobium: a potential tool for monitoring schistosome-infested water. Am J Trop Med Hyg. 2001;65:907–911. doi: 10.4269/ajtmh.2001.65.907. [DOI] [PubMed] [Google Scholar]
- 14.Pontes LA, Dias-Neto E, Rabello A. Detection by polymerase chain reaction of Schistosoma mansoni DNA in human serum and feces. Am J Trop Med Hyg. 2002;66:157–162. doi: 10.4269/ajtmh.2002.66.157. [DOI] [PubMed] [Google Scholar]
- 15.Pontes LA, Oliveira MC, Katz N, Dias-Neto E, Rabello A. Comparison of a polymerase chain reaction and the Kato-Katz technique for diagnosing infection with Schistosoma mansoni. Am J Trop Med Hyg. 2003;68:652–656. [PubMed] [Google Scholar]
- 16.Sandoval N, Siles-Lucas M, Perez-Arellano JL, Carranza C, Puente S, Lopez-Aban J, Muro A. A new PCR-based approach for the specific amplification of DNA from different Schistosoma species applicable to human urine samples. Parasitology. 2006;133:581–587. doi: 10.1017/S0031182006000898. [DOI] [PubMed] [Google Scholar]
- 17.Lier T, Johansen MV, Hjelmevoll SO, Vennervald BJ, Simonsen GS. Real-time PCR for detection of low intensity Schistosoma japonicum infections in a pig model. Acta Trop. 2008;105:74–80. doi: 10.1016/j.actatropica.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Lier T, Simonsen GS, Haaheim H, Hjelmevoll SO, Vennervald BJ, Johansen MV. Novel real-time PCR for detection of Schistosoma japonicum in stool. Southeast Asian J Trop Med Public Health. 2006;37:257–264. [PubMed] [Google Scholar]
- 19.Lier T, Simonsen GS, Wang T, Lu D, Haukland HH, Vennervald BJ, Hegstad J, Johansen MV. Real-time polymerase chain reaction for detection of low-intensity Schistosoma japonicum infections in China. Am J Trop Med Hyg. 2009;81:428–432. [PubMed] [Google Scholar]
- 20.Thanchomnang T, Intapan P, Sri-Aroon P, Lulitanond V, Janwan P, Sanpool O, Maleewong W. Molecular detection of Schistosoma japonicum in infected snails and mouse feces using a real-time PCR assay with FRET hybridization probes. Mem Inst Oswaldo Cruz. 2011;106:831–836. doi: 10.1590/s0074-02762011000700008. [DOI] [PubMed] [Google Scholar]
- 21.Wu HW, Qin YF, Chu K, Meng R, Liu Y, McGarvey ST, Olveda R, Acosta L, Ji MJ, Fernandez T, Friedman JF, Kurtis JD. High prevalence of Schistosoma japonicum infection in water buffaloes in the Philippines assessed by real-time polymerase chain reaction. Am J Trop Med Hyg. 2010;82:646–652. doi: 10.4269/ajtmh.2010.09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia CM, Rong R, Lu ZX, Shi CJ, Xu J, Zhang HQ, Gong W, Luo W. Schistosoma japonicum: a PCR assay for the early detection and evaluation of treatment in a rabbit model. Exp Parasitol. 2009;121:175–179. doi: 10.1016/j.exppara.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Liang S, Yang C, Zhong B, Qiu D. Re-emerging schistosomiasis in hilly and mountainous areas of Sichuan, China. Bull World Health Organ. 2006;84:139–144. doi: 10.2471/blt.05.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlton EJ, Bates MN, Zhong B, Seto EY, Spear RC. Evaluation of mammalian and intermediate host surveillance methods for detecting schistosomiasis reemergence in southwest China. PLoS Negl Trop Dis. 2011;5:e987. doi: 10.1371/journal.pntd.0000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Department of Diseases Control . Textbook for Schistosomiasis Control. Shanghai: Shanghai Publishing House for Science and Technology; 2000. [Google Scholar]
- 26.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 27.Dacombe RJ, Crampin AC, Floyd S, Randall A, Ndhlovu R, Bickle Q, Fine PE. Time delays between patient and laboratory selectively affect accuracy of helminth diagnosis. Trans R Soc Trop Med Hyg. 2007;101:140–145. doi: 10.1016/j.trstmh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 28.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 29.Carlton EJ, Hsiang M, Zhang Y, Johnson S, Hubbard A, Spear RC. The impact of Schistosoma japonicum infection and treatment on ultrasound-detectable morbidity: a five-year cohort study in Southwest China. PLoS Negl Trop Dis. 2010;4:e685. doi: 10.1371/journal.pntd.0000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ezeamama AE, Friedman JF, Olveda RM, Acosta LP, Kurtis JD, Mor V, McGarvey ST. Functional significance of low-intensity polyparasite helminth infections in anemia. J Infect Dis. 2005;192:2160–2170. doi: 10.1086/498219. [DOI] [PubMed] [Google Scholar]
- 31.Spear RC, Seto EY, Carlton EJ, Liang S, Remais JV, Zhong B, Qiu D. The challenge of effective surveillance in moving from low transmission to elimination of schistosomiasis in China. Int J Parasitol. 2011;41:1243–1247. doi: 10.1016/j.ijpara.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu JM, de Vlas SJ, Yuan HC, Gryseels B. Variations in fecal Schistosoma japonicum egg counts. Am J Trop Med Hyg. 1998;59:370–375. doi: 10.4269/ajtmh.1998.59.370. [DOI] [PubMed] [Google Scholar]
- 33.Ross AG, Li Y, Sleigh AC, Williams GM, McManus DP. Fecal egg aggregation in humans infected with Schistosoma japonicum in China. Acta Trop. 1998;70:205–210. doi: 10.1016/s0001-706x(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 34.Hubbard A, Liang S, Maszle D, Qiu D, Gu X, Spear RC. Estimating the distribution of worm burden and egg excretion of Schistosoma japonicum by risk group in Sichuan province, China. Parasitology. 2002;125:221–231. doi: 10.1017/s003118200200207x. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, Rong R, Zhang HQ, Shi CJ, Zhu XQ, Xia CM. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP) Int J Parasitol. 2010;40:327–331. doi: 10.1016/j.ijpara.2009.08.010. [DOI] [PubMed] [Google Scholar]