Abstract
Japanese encephalitis virus (JEV) is transmitted to humans from pigs or birds by mosquitoes. In this study, the association between urban pig keeping and mosquito vectors was analyzed. A total of 7, 419 mosquitoes were collected overnight in urban households with and without pigs in Can Tho City, Vietnam. The most prevalent vectors were Culex tritaeniorhynchus (36%), Cx. gelidus (24%), and Cx. quinquefasciatus (15%), which were present in all parts of the city. Pigs were associated with increased numbers of Cx. tritaeniorhynchus. Traps close to pigs had higher numbers of Cx. tritaeniorhynchus and Cx. gelidus than traps close to humans. Increased number of persons in the household was associated with increased numbers of Cx. quinquefasciatus. We demonstrate that JEV vector species are present at urban households with and without pigs, and show that keeping pigs in an urban area increase the number of mosquitoes competent as vectors for JEV.
Introduction
Japanese encephalitis (JE) is a zoonotic disease caused by Japanese encephalitis virus (JEV), a flavivirus that is transmitted by mosquitoes, particularly Culex species. Wading birds, such as herons and egrets, are reservoir hosts for the virus.1,2 Pigs serve as amplifying hosts, and a high density of pigs in an area can be associated with increased number of cases of JE.3 In spite of the high viremia in swine, infection is usually asymptomatic, except for abortions and mummified fetuses in pregnant sows.2,4 Humans and horses are dead-end hosts for the virus but may occasionally develop encephalitis, with case fatality rates of up to 30%.5,6
The disease is present in southern and eastern Asia, and shows a tendency to emerge into new areas.7,8 In Vietnam, the first human case of JE was recognized in the 1960s9 and it has since become a serious health issue. During 1986–1994, there were more than 18,000 human cases reported,10 and JEV is currently endemic to the southern parts of the country.11–13 In southern Vietnam, the area used for irrigated rice production is increasing along with the pig production, which are factors likely to contribute further to the emergence of JEV.14 The Mekong delta region in Vietnam, has extensive pig farming and rice production,15,16 and is one of the regions in the country with the most JE cases.11,17 In Can Tho City Province, 60% of the pigs on farms have been shown to be seropositive for JEV.18
The transmission of vector-borne diseases are regulated by the host, the pathogen, and the biology of vectors and their vectorial capacity, which makes the epidemiology complex and also dependent on environmental factors, e.g., climate and anthropogenic influences, such as changes in agricultural practices, irrigation schemes, and urbanization.10,19,20
The most important vectors of JEV, such as Culex tritaeniorhynchus, commonly undergo larval development in rice fields in rural areas.2 Therefore, JE has been considered a rural disease, in contrast to the closely-related dengue fever virus, which is well known for its outbreaks in urban areas, and spread by an urban dwelling mosquito (Aedes aegypti).21 However, JE may also expand into densely populated areas because of rapid urbanization, coupled with growing demands for urban agriculture involving livestock.22,23 In the cities, poultry, pigs, or small ruminants are preferred.24,25 Thus, in such areas, the close proximity to animals may result in increasing risks for transmission of zoonotic diseases, including JE.
To date, the influence of population density and pig husbandry on JEV vector distribution in urban areas has not been extensively investigated, although it has been shown that JEV infections occur in humans in urban settings.26–28 Therefore, the aim of the present study was to assess the effect of urban pig rearing and other factors on the presence of JEV vectors at households in Can Tho City in southern Vietnam.
Material and Methods
Description of urban agriculture in study area.
Can Tho City is the central province of the Mekong delta in southern Vietnam. Data on the population of humans and animals and the area of rice fields and fish ponds were collected by visiting the local veterinary authorities in the different districts in the province during autumn of 2008, and using official government statistics.29 The study site in Ninh Kieu District is the most urban part of Can Tho City Province, and has a human population of approximately 217,000, which corresponded to a population density of 7,500 persons/km2 in 2008.29 The rest of the province is mainly rural, with population densities of 380–1,400 persons/km2 in the districts. The pig population in the rural districts varied between 65 and 123 pigs/km2. Although Ninh Kieu had a human population density five times as high as the second most densely populated district, it had 94 pigs/km2. The mean number of sows per pig holding, i.e., a household with domestic pigs, in Ninh Kieu was 1.4 (range = 0–4.5). Including piglets and boars, the mean number was 11 pigs per pig holding (range = 4.7–24. There were only nine hectares of rice paddies/km2 in the urban district, compared with the other districts, which had 35–205 hectares/km2.
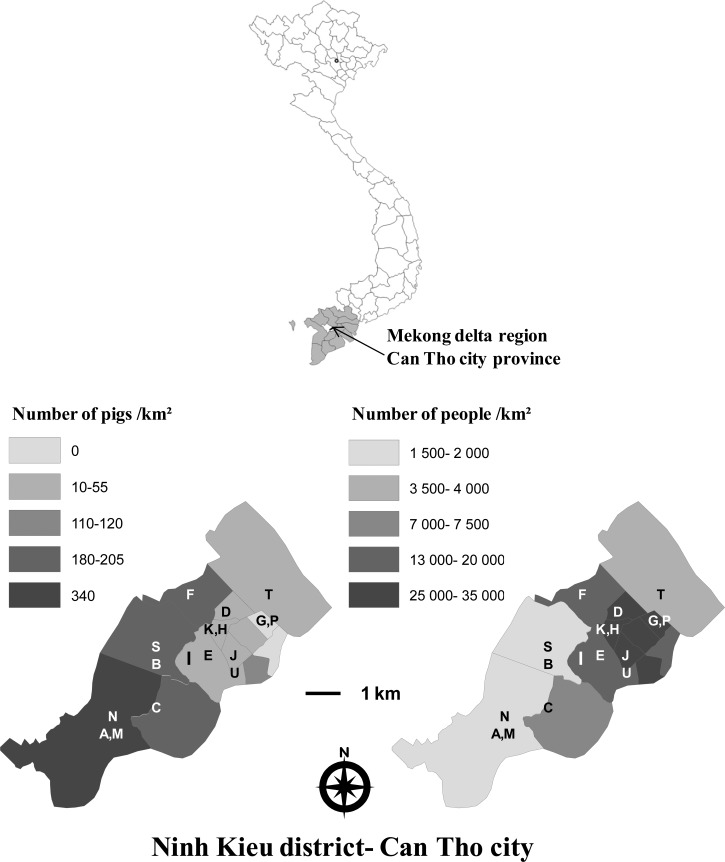
Ninh Kieu contains 13 wards. Seven of these wards had cattle or buffaloes, and the density of large ruminants was 0.6–29/km2. Only two of the most central wards, Tan An and An Hoi, had no records of pigs, and the ward reporting the most pigs, An Binh, had a density of 340 pigs/km2, the lowest human population density, and a ratio of 0.21 pigs/inhabitant. Pig and human populations are shown in Figure 1 (ArcMap software, ESRI, Redlands, CA).
Figure 1.
Location of the study area, Ninh Kieu District, Can Tho City, Vietnam. Pig and human population density is indicated for the wards of the Can Tho city (gray scale). Locations of the households included in the study are indicated by the letters A–U.
Study design and mosquito collection and identification.
Wards representing a transect of the city with human densities ranging from 1,598 to 31,447 persons/km2 and pig densities ranging from 0 to 340 pigs/km2 were selected. In these wards, households within the urban area with and without pigs, which enabled sampling and where mosquito traps could be affixed, were included.
Mosquito collection was conducted during three-week periods from the middle of February to the beginning of March and from the end of October to the middle of November in 2009. Collection of mosquitoes was planned to be conducted on a weekly basis with at least two repetitions during each sampling period. Because of circumstances, such as the household moving, or trap failures, all households could not be sampled at both sampling periods or sampling could not be repeated in two subsequent weeks. A total of 17 households in 10 wards were studied (Table 1 and Figure 1).
Table 1.
Households sampled during spring and fall of 2009 for Japanese encephalitis virus vectors in Ninh Kieu, Can Tho City, Vietnam
| Household | Ward | Pigs in the household | Sampling, no. occasions | |
|---|---|---|---|---|
| Spring 2009 | Fall 2009 | |||
| A | An Binh | Yes | 2 | 3 |
| M | An Binh | Yes | 0 | 1 |
| N | An Binh | Yes | 0 | 1 |
| F | An Hoa | Yes | 2 | 2 |
| G | An Hoi | No | 2 | 0 |
| P | An Hoi | No | 0 | 2 |
| B | An Khanh | Yes | 2 | 0 |
| S | An Khanh | Yes | 0 | 1 |
| H | An Nghiep | No | 1 | 0 |
| K | An Nghiep | No | 2 | 2 |
| J | An Phu | Yes | 2 | 4 |
| T | Cai Khe | Yes | 0 | 2 |
| C | Hung Loi | Yes | 2 | 2 |
| D | Thoi Binh | Yes | 2 | 2 |
| E | Xuan Khanh | No | 2 | 2 |
| I | Xuan Khanh | Yes | 2 | 3 |
| U | Xuan Khanh | Yes | 0 | 2 |
Data were collected on factors potentially important for presence of vectors, i.e., the number of persons in the household; the number of pigs, pets, poultry, and other livestock present; and the presence of rice fields or fish ponds on the property.
Mosquitoes were collected by using un-baited CDC mini light traps (Bioquip Products, Compton, CA) equipped with a 4-watt light bulb. At each household, two traps were operated between dusk and dawn (6:00 pm to 7:00 am). One trap was placed within two meters of the entrance to the human dwelling, or alternatively within two meters of the bed, if the bed was outside or in the same building as the pigs. In households with pigs, the second trap was placed either above or next to the pen. In households without pigs, the traps were placed 2–10 meters apart, where traps could be affixed. The traps were placed irrespective of other pets and livestock.
Mosquitoes were killed by freezing and identified according to the keys of Reuben and others.30 Because of difficulties in distinguishing Cx. vishnui and Cx. pseudovishnui within the Cx. vishnui subgroup,30 only Cx. tritaeniorhynchus was identified to species.
In collections containing more than 300 mosquitoes, a random sample of 300 specimens was identified, and the remaining specimens were counted. This amount always comprised more than 10% of the specimens in all the collections, and was thus estimated to be a representative sample size. The proportions of identified species and blood-filled specimens were then applied to the total number of mosquitoes to provide an extrapolated estimate of the composition of species in the total collection.
Statistical analyses.
Statistical analysis was performed by using SAS for Windows 9.2 (SAS Institute Inc., Cary, NC). To improve the normality of the data, the number of mosquitoes was logarithmically transformed (log (n + 0.01)). The number of pigs and people at the household level were treated as continuous variables, and close proximity to livestock, including poultry, and the presence of pets, were classified as two separate dichotomous variables. Univariable analyses for the association of rice fields or fish ponds with the number of mosquitoes were performed, and both variables were found to have similar associations. The two variables were merged into one variable, representing the presence of large water bodies adjacent to the household.
Data on ward level was calculated as densities. The density of persons, pigs, and large ruminants were calculated as individuals/km2 and used as continuous independent variables in the analyses. The area of rice fields and fish ponds was combined and divided by the total area of the ward.
To determine potential risk factors the dependent variables, the total number of mosquitoes, the total extrapolated number of Cx. tritaeniorhynchus, all members of the Cx. vishnui subgroup, Cx. gelidus, Cx. quinquefasciatus, Culex males, and the proportion of blood-filled females per trap per night were analyzed in two multivariable mixed effect models. Statistical analyses were performed for Cx. tritaeniorhynchus and the whole Cx. vishnui subgroup. The first set of models included only traps placed near human dwellings. A subset of models were also made that used pigs in the household as a categorical, present or not present, variable instead of being continuous. The second set of models included only households with pigs and also whether the collections were made close to pigs versus close to humans.
Mixed effects modeling was performed using the SAS Mixed procedure for all analyses, except for the proportion of blood-filled females, for which the Glimmix procedure was used. Mixed modeling was used to account for clustering of collections at the ward and household level. In both procedures, the household was a random effect within the ward, and sampling period was a random effect within the household. Satterthwaite denominator degrees of freedom were used. All ward and household variables were included and then backward elimination was used for factors with a P value < 0.05 were kept in the model. Factors with a confounding effect were only kept in the model if they had an effect of more than 25% on the coefficient β of another factor that continued to be significant. Interactions between the remaining factors were tested and discarded if the P value was > 0.05.
Results
Description of households.
There were 4–60 persons living in the investigated households without pigs (median = 4 persons). In the households with pigs, there were 1–10 persons (median = 6 persons). The latter households kept 1–110 pigs (median = 15 pigs). One of the households with pigs had no sows and seven households kept one or two sows. Four households had 6–13 sows. There was only one household where the distance between the nearest pigs and living quarters for humans was more than 10 meters. Only two households, I and S, had cattle, and household S also had crocodiles. Households A, F, I, M, N, and S had fish ponds by the house and two of these households (I and S) also had rice fields.
Mosquito collections.
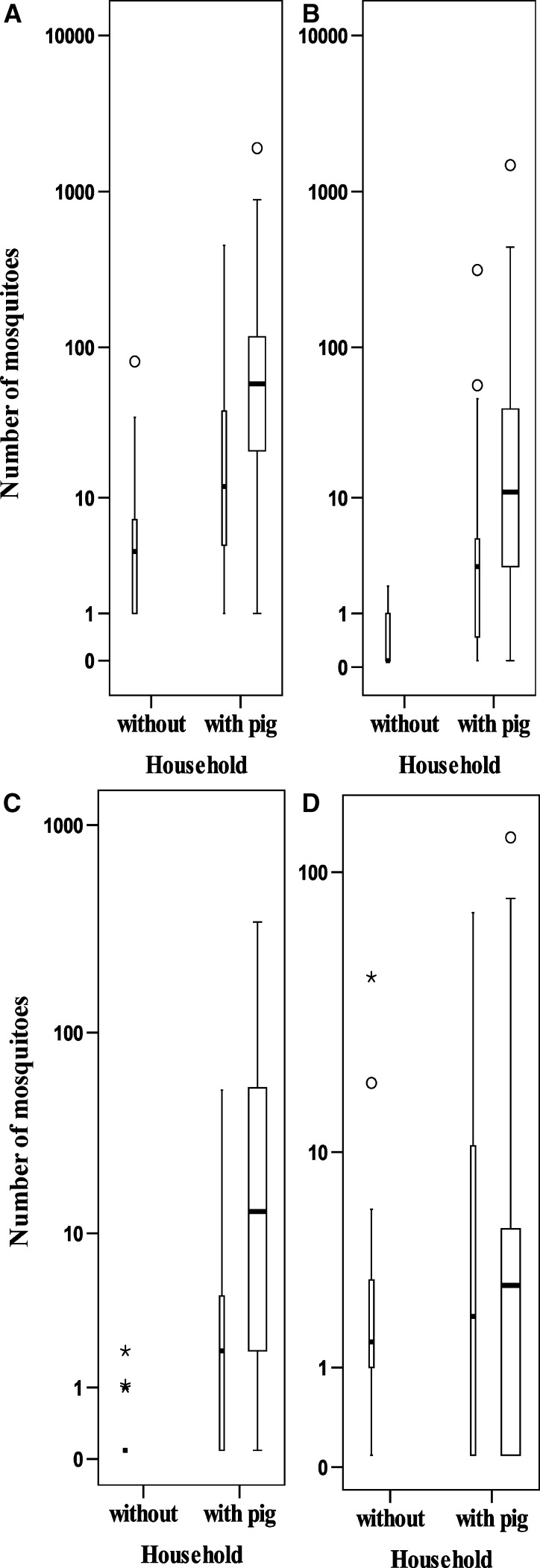
A total of 7 419 mosquitoes were collected (Table 2). The most prevalent species, Cx. tritaeniorhynchus (36%), Cx. gelidus (24%) and Cx. quinquefasciatus (15%), were found in all wards. The distribution of the number of these species at different collection sites is shown in Figure 2. Of the 4,872 identified specimens, 1,282 were blood filled. The proportion of blood-filled females was highest for Cx. gelidus (n = 421, 37%) and Cx. tritaeniorhynchus (n = 609, 35%) and was lowest for Cx. quinquefasciatus (n = 41, 5%). Other Culex mosquitoes identified were Cx. fuscocephala and members of the Cx. vishnui subgroup. The remaining mosquitoes consisted of the genera Mansonia, Aedes, Anopheles, and Uranotaenia. These mosquitoes and the Culex males were not identified to species.
Table 2.
Mosquito species collected in Ninh Kieu, Can Tho City, Vietnam, to determine the composition of mosquito vectors for Japanese encephalitis virus in an urban area
| Species/ward | An Binh | An Hoa | An Hoi | An Khanh | An Nghiep | An Phu | Cai Khe | Hung Loi | Thoi Binh | Xuan Khanh | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Culex tritaeniorhynchus | 278 | 75 | 4 | 548 | 5 | 72 | 124 | 179 | 110 | 344 | 1,739 |
| Culex vishnui subgroup* | 45 | 12 | 1 | 6 | – | 6 | – | 26 | 12 | 14 | 122 |
| Culex gelidus | 332 | 64 | 2 | 95 | 2 | 37 | 24 | 237 | 93 | 266 | 1,152 |
| Culex quinquefasciatus | 20 | 104 | 13 | 7 | 11 | 60 | 200 | 67 | 184 | 84 | 750 |
| Culex fuscocephala | 2 | – | – | 1 | – | – | – | 2 | – | – | 5 |
| Lutzia sp. | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Anopheles spp. | 63 | 7 | 1 | 85 | 1 | 4 | 28 | 53 | 16 | 36 | 294 |
| Uranotenia spp. | 22 | 1 | – | 13 | – | – | – | 4 | 1 | – | 41 |
| Masonia spp. | 20 | 3 | 1 | 20 | – | – | 1 | 56 | 3 | 48 | 152 |
| Aedes spp. | 1 | – | – | 1 | 1 | – | – | 8 | 1 | – | 12 |
| Culex males | 29 | 111 | 10 | 15 | 14 | 54 | 82 | 71 | 81 | 80 | 547 |
| Anopheles males | – | – | – | 3 | – | – | 2 | – | 1 | 2 | 8 |
| Masonia males | 5 | – | – | 6 | – | – | – | 7 | – | 26 | 44 |
| Aedes males | 1 | 1 | – | – | 1 | – | – | – | – | 2 | 5 |
| Unidentified | 22 | 1 | – | 1,605† | – | 1 | 15 | 6 | 20 | 877† | 2,547 |
| Total | 840 | 379 | 32 | 2,405 | 35 | 234 | 476 | 717 | 522 | 1,779 | 7,419 |
Excluding Culex tritaeniorhynchus.
At household S in An Khanh and household I in Xuan Khanh, more mosquitoes were collected per trap than could be identified in the local laboratory. A sample of 300 specimens were identified per trap, and the proportion of each species identified were then applied to the entire number collected to achieve an estimate of the mosquito population.
Figure 2.
Mosquitoes collected in households with and without pigs in Ninh Kieu District, Can Tho City, Vietnam. Collections made close to humans are shown by thin boxes and collections made close to pigs are shown by thick boxes. A, total number of mosquitoes, B, Culex tritaeniorhynchus. C, Cx. gelidus. D, Cx. quinquefasciatus. Circles indicate outliers > 1.5 × the interquartile range (error bars) and stars indicate extreme outliers > 3 × the interquartile range.
Analysis of risk factors associated with number of mosquitoes.
Collections near human dwellings in households with and without pigs.
There was no difference in the final models if the total number of pigs was used or if only the presence or non-presence of pigs were used. Therefore, only the results for the model with the number of pigs are presented (Table 3). For the total number of mosquitoes and the number of Cx. tritaeniorhynchus and all mosquitoes in the Cx. vishnui subgroup, pigs were the only risk factor with a significant effect (P = 0.021, 0.030, and 0.048, respectively). The number of Cx. gelidus collected was increased by the density of pigs in the ward (P = 0.016), but this effect was confounded by the presence of other livestock in the household and the presence of rice fields or fishponds. The number of Cx. quinquefasciatus was only significantly associated with the number of persons in the household (P = 0.035) and the density of large ruminants in the ward (P = 0.004). No factors were identified that significantly affected the number of Culex males collected or the proportion of blood-filled mosquitoes.
Table 3.
Factors associated with collection of Culex mosquito vectors for Japanese encephalitis virus near human dwellings in Ninh Kieu, Can Tho City, Vietnam*
| Risk factor | No. mosquitoes | Cx. vishnui subgroup | Cx. tritaeniorhynchus | Cx. gelidus | Cx. quinquefasciatus | Culex males | Proportion of blood-filled mosquitoes |
|---|---|---|---|---|---|---|---|
| No. pigs in household | 1.03† | 1.05† | 1.05† | ||||
| Pig density in ward | 1.02† | ||||||
| Rice field or fish pond | −9.65 | ||||||
| Rice fields and fish ponds in ward | |||||||
| Other livestock in household | 86.0 | ||||||
| Large ruminant density in ward | −1.28‡ | ||||||
| No. persons in household | 1.13† | ||||||
| Population density in ward | |||||||
| Pets in household |
Change in number of mosquitoes is the anti-logarithm of the coefficient β. Non-significant variables were confounders in the models.
P < 0.05.
P < 0.01.
Collections in households with pigs.
In the second analysis, the two collection sites in households with pigs were included (Table 4). The total numbers of pigs in the household and mosquito collections made close to pigs were associated with an increase in the total number of mosquitoes (P = 0.004 and P < 0.0001 respectively), as well as the number of Cx. tritaeniorhynchus (P = 0.024 and 0.0004, respectively) and all the mosquitoes in the Cx. vishnui subgroup (P = 0.035 and 0.0007, respectively). The number of Cx. gelidus was increased by collections close to pigs (P < 0.0001), number of persons in the household (P = 0.003), pig density (P = 0.010), and presence of other livestock (P = 0.010), and decreased by increased large ruminant density (P = 0.033), but again the associations were complex and confounded by presence of rice fields or fish ponds. None of the risk factors studied had any association with the number of Cx. quinquefasciatus, Culex males, and the proportion of blood-filled mosquitoes.
Table 4.
Factors associated with number of Culex mosquito vectors for Japanese encephalitis virus collected at households keeping pigs in Ninh Kieu, Can Tho City, Vietnam*
| Risk factor | No. mosquitoes | Cx. vishnui subgroup | Cx. tritaeniorhynchus | Cx. gelidus | Cx. quinquefasciatus | Culex males | Proportion of blood-filled mosquitoes |
|---|---|---|---|---|---|---|---|
| Catch near pigs | 3.89† | 7.77† | 8.96† | 17.1† | |||
| No. pigs in household | 1.04‡ | 1.05§ | 1.05§ | ||||
| Pig density in ward | 1.03§ | ||||||
| Rice field or fish pond | −118 | ||||||
| Rice fields and fish ponds in ward | |||||||
| Other livestock in household | 32,779‡ | ||||||
| Large ruminant density in ward | −1.86§ | ||||||
| No. persons in household | 1.25§ | 1.53‡ | |||||
| Population density in ward | |||||||
| Pets in household |
Change in number of mosquitoes is the anti-logarithm of the coefficient β.
P < 0.001.
P < 0.01.
P < 0.05.
Discussion
This study shows the association between JEV vector mosquitoes and the presence of pigs in a city. The presence of pigs per se and the number of pigs kept in the household were associated with an increase in the number of mosquitoes. Anthropophilic and zoophilic vector species were demonstrated to be associated differently with household risk factors.
Zoophilic Cx. tritaeniorhynchus was the most numerous mosquito species caught in the present study. The number of pigs per household was the only factor associated with a higher number of Cx. tritaeniorhynchus. This finding is in accordance with those of earlier studies, which have indicated that pigs are preferred feeding hosts.31,32 Rice fields were reported only in the peripheral wards of the study area, but Cx. tritaeniorhynchus could be found in all parts of the city. Our results may indicate that standing water collections, which are abundant within the city even during the dry season, could also provide larval habitats also Cx. tritaeniorhynchus, as has been shown for other mosquito species.33 Moreover, Cx. tritaeniorhynchus may disperse hundreds of kilometers during one night,34,35 which enables mosquitoes to be found in households far away from their larval habitats. The entire Cx. vishnui subgroup and Cx. tritaeniorhynchus displayed corresponding associations with the analyzed risk factors. The Cx. vishnui subgroup is known as the most important vectors for JEV36 and the virus has been isolated from such a mosquito in the Can Tho City Province.37
Culex tritaeniorhynchus and Cx. gelidus comprised 60% of the mosquitoes collected. Culex gelidus was associated with increasing overall pig densities in the ward and the presence of non-porcine livestock in the household. Because ruminants do not have clinical signs or amplify the virus, and could divert mosquitoes away from humans and pigs, they have been proposed as a zooprophylaxis.38,39 In a suburb of Bangkok, with population densities less than 3,000 persons/km2, but with more rural characteristics than in our study, more than 90% of the mosquitoes were Cx. tritaeniorhynchus and Cx. gelidus.40 Gingrich and others40,41 isolated JEV from Cx. tritaeniorhynchus and Cx. gelidus collected in the same Bangkok suburb. Culex gelidus may breed in a variety of water collections,42 and it is therefore not surprising that rice fields and fish ponds did not have a direct effect on the abundance.
There was no association between the numbers of Cx. quinquefasciatus and pigs. However, the number of persons in the household was associated with a significant increase in the number of the mosquito, whereas the density of large ruminants had a negative association. Culex quinquefasciatus is known to be anthrophilic,43 and the negative association with large ruminants could be result from the fact that large ruminant densities may be expected to be correlated to more rural, i.e., less urban, characteristics. Although anthropophilic Cx. quinquefasciatus commonly feed on humans,44 it may be opportunistic, and it has been demonstrated that the same individual mosquito may feed on pigs and humans.43,45 The potential of Cx. quinquefasciatus as a vector in urban areas is further indicated by its typical breeding underground in sewers and drains33,46 and because of the fact that JEV previously has been isolated from Cx. quinquefasciatus in southern Vietnam.11
There were a higher number of blood-filled mosquitoes collected near pigs, in accordance with previous findings.47 However, there was no significant difference in the proportion of blood-filled mosquitoes dependent on the locality where the traps were operated. The total number of mosquitoes collected in the traps varied considerably for unknown reasons. Reisen and others48 recommended light traps for standardized collection of JEV vectors because of the phototactic behavior of Cx. tritaeniorhynchus, but multiple conflicting sources of light in a city may interfere, resulting in an increased variation of mosquitoes collected.
It is estimated that 1.9 billion persons live in rural areas where JEV is transmitted,49 but the number of persons considered at risk would be increased if JEV would also be considered an urban disease. Today, more than half of the world's urban population live in Asia, and more than 60% of urban inhabitants are estimated to be poor.50,51 In low-income countries with poor infrastructure, the increasing problem of providing food for an expanding urban population at affordable prices23,52 creates a need for urban animal keeping. The urban areas of Can Tho City have a high pig density, and the presence of sows in the households indicates that there is a more permanent urban farming practice and not only a temporary keeping of pigs for slaughter.
To further elucidate the risk for human health associated to urban JEV vectors it would be important to determine the mosquitoes urban larval habitats, feeding habits, possible seasonal differences and infection rates. For instance, blood meal analysis may give some indications to which extent different mosquito species are feeding on pigs and humans, although most mosquitoes are opportunistic and may feed on hosts depending on their availability.53,54 When arboviruses circulate in mosquitoes, it is usually only a small proportion of the vectors that are infected. In a previous study in Can Tho City Province, it was possible to find one JEV-positive mosquito pool in 22,000 mosquitoes collected.37 No virus detection was performed in this study, but we showed that all components necessary for transmission of JEV can be present in a tropic urban environment because of the presence of pigs and zoophilic and anthropophilic vectors. Our results indicate that the anthropophilic Cx. quinquefasciatus may become more important for the transmission of JEV in areas with fewer pigs and more humans as an urban bridge vector between animals and humans, as hypothesized by Do and others.11 The recent detection of JEV RNA in Cx. pipiens, a sibling species of Cx. quinquefasciatus, in Europe also implies the importance of the Cx. pipiens complex, not only for urban transmission in the tropics, but also for the emergence outside Asia.55 In conclusion, our study emphasizes the increased risks for human exposure to JEV vector mosquitoes in cities with pigs, implying that JEV is not only a rural concern as has traditionally been claimed.21,56
ACKNOWLEDGMENTS
We thank Dr. Manh (Can Tho University) for providing excellent working facilities in Can Tho; Nguyen Thanh Thu, Truong Van Nho, and Huynh Ngoc Trang for assistance with the field work in Can Tho and translations and practical issues; and Dr. Ulf Emanuelson (Swedish University of Agricultural Sciences) for helpful comments on epidemiologic analyses.
Footnotes
Financial support: This study was supported by the Swedish International Development Cooperation Agency/Department of Research Cooperation.
Disclosure: None of the authors had any conflicts of interest during the study.
Authors' addresses: Johanna Lindahl and Ulf Magnusson, Department of Clinical Sciences, Division of Reproduction, Swedish University of Agricultural Sciences, Uppsala, Sweden, E-mails: johanna.lindahl@slu.se and ulf.magnusson@slu.se. Jan Chirico, Department of Virology, Immunobiology and Parasitology, National Veterinary Institute, Uppsala, Sweden, E-mail: jan.chirico@sva.se. Sofia Boqvist, Department of Biomedicine and Veterinary Public Health, Swedish University of Agricultural Sciences, Uppsala, Sweden, E-mail: sofia.boqvist@slu.se. Ho Thi Viet Thu, Department of Veterinary Medicine, Campus II, Can Tho University, Can Tho, Vietnam, E-mail: htvthu@ctu.edu.vn.
References
- 1.Buescher EL, Scherer WF, McClure HE, Moyer JT, Rosenberg MZ, Yoshii M, Okada Y. Ecologic studies of Japanese encephalitis virus in Japan. 4. Avian infection. Am J Trop Med Hyg. 1959;8:678–688. doi: 10.4269/ajtmh.1959.8.678. [DOI] [PubMed] [Google Scholar]
- 2.Rosen L. The natural history of Japanese encephalitis virus. Annu Rev Microbiol. 1986;40:395–414. doi: 10.1146/annurev.mi.40.100186.002143. [DOI] [PubMed] [Google Scholar]
- 3.Hsu SM, Yen AMF, Chen THH. The impact of climate on Japanese encephalitis. Epidemiol Infect. 2008;136:980–987. doi: 10.1017/S0950268807009454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joo HS. Japanese encephalitis virus. In: Pensaert MB, editor. Virus Infections of Porcines. Amsterdam: Elsevier Science Publishers B.V; 1989. pp. 131–136. [Google Scholar]
- 5.Calisher CH, Walton TE. Japanese, western, eastern and Venezuelan encephalitides. In: Studdert MJ, editor. Virus Infections in Equines. Amsterdam: Elsevier Science BV; 1996. pp. 141–155. [Google Scholar]
- 6.Solomon T, Dung NM, Kneen R, Gainsborough M, Vaughn DW, Khanh VT. Japanese encephalitis. J Neurol Neurosurg Psychiatry. 2000;68:405–415. doi: 10.1136/jnnp.68.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endy TP, Nisalak A. Japanese encephalitis virus: ecology and epidemiology. Curr Top Microbiol Immunol. 2002;267:11–48. doi: 10.1007/978-3-642-59403-8_2. [DOI] [PubMed] [Google Scholar]
- 8.Mackenzie JS, Williams DT, Smith DW. Japanese encephalitis virus: the geographic distribution, incidence, and spread of a virus with a propensity to emerge in new areas. In: Edward T, editor. Perspectives in Medical Virology. Amsterdam: Elsevier; 2006. pp. 201–268. [Google Scholar]
- 9.Igarashi A, Takagi M. Current situation of Japanese encephalitis and production of preventive vaccine in Vietnam. Jpn J Trop Med Hyg. 1992;20:55–56. [Google Scholar]
- 10.Tsai TF. Factors in the changing epidemiology of Japanese encephalitis and West Nile fever. In: Saluzzo J, editor. Factors in the Emergence of Arbovirus Diseases. Paris: Elsevier; 1997. pp. 179–189. [Google Scholar]
- 11.Do QH, Vu TQ, Huynh TK, Dinh QT, Deubel V. Current situation of Japanese encephalitis in the south of Vietnam, 1976–1992. Trop Med. 1994;36:202–214. [Google Scholar]
- 12.Bartley LM, Carabin H, Vinh Chau N, Ho V, Luxemburger C, Hien TT, Garnett GP, Farrar J. Assessment of the factors associated with flavivirus seroprevalence in a population in southern Vietnam. Epidemiol Infect. 2002;128:213–220. doi: 10.1017/s0950268801006495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen NT, Duffy MR, Hong NM, Hien NT, Fischer M, Hills SL. Surveillance for Japanese encephalitis in Vietnam, 1998–2007. Am J Trop Med Hyg. 2010;83:816–819. doi: 10.4269/ajtmh.2010.10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erlanger T, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009;15:1–7. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hai LT, Nguyen NH. Outlines of pig production in Vietnam. Pig News and Information. 1997;18:91–94. [Google Scholar]
- 16.Vo-Tong X, Le Than H, Chau BL. Research priorities for improving animal production by agro-ecological zone in Vietnam. In: Devendra C, Gardiner P, editors. Global Agenda for Livestock Research. IRRI, Los Banos, The Philippines: International Livestock Research Institute; 1995. Proceedings of the Consultation for the South-East Asia Region. May 10–13, 1995. [Google Scholar]
- 17.Nguyen HT, Nguyen TY. Japanese encephalitis in Vietnam 1985–1993. Southeast Asian J Trop Med Public Health. 1995;26:47–50. [Google Scholar]
- 18.Lindahl J, Boqvist S, Ståhl K, Thu H, Magnusson U. Reproductive performance in sows in relation to Japanese encephalitis virus seropositivity in an endemic area. Trop Anim Health Prod. 2012;44:239–245. doi: 10.1007/s11250-011-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer LD, Ebel GD. Dynamics of flavivirus infection in mosquitoes. In: Chambers TJ, Monath TP, editors. Flaviviruses: Pathogenesis and Immunity. San Diego, CA: Academic Press Inc.; 2003. pp. 187–232. [Google Scholar]
- 20.Cleaveland S, Haydon DT, Taylor L. Overviews of pathogen emergence: which pathogens emerge, when and why? Curr Top Microbiol Immunol. 2007;315:85–111. doi: 10.1007/978-3-540-70962-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 22.Rae AN. The effects of expenditure growth and urbanisation on food consumption in east Asia: a note on animal products. Agric Econ. 1998;18:291–299. [Google Scholar]
- 23.Nugent R. The impact of urban agriculture on the household and local economies. In: Bakker N, editor. Growing Cities, Growing Food: Urban Agriculture on the Policy Agenda. Feldafing, Germany: Deutsche Stiftung fuer Internationale Entwicklung; 2000. pp. 67–97. [Google Scholar]
- 24.Armar-Klemesu M. Urban agriculture and food security, nutrition and health. In: Bakker N, editor. Growing Cities, Growing Food: Urban Agriculture on the Policy Aagenda. A Reader on Urban Agriculture. Feldafing, Germany: Deutsche Stiftung fuer Internationale Entwicklung; 2000. pp. 99–117. [Google Scholar]
- 25.Thys E, Oueadraogo M, Speybroeck N, Geerts S. Socio-economic determinants of urban household livestock keeping in semi-arid western Africa. J Arid Environ. 2005;63:475–496. [Google Scholar]
- 26.Tseng H-F, Tan H-F, Chang C-K, Huang W-L, Ho W-C. Seroepidemiology study of Japanese encephalitis neutralizing antibodies in southern Taiwan: a comparative study between urban city and country townships. Am J Infect Control. 2003;31:435–440. doi: 10.1067/mic.2003.73. [DOI] [PubMed] [Google Scholar]
- 27.Bi P, Zhang Y, Parton KA. Weather variables and Japanese encephalitis in the metropolitan area of Jinan city, China. J Infect. 2007;55:551–556. doi: 10.1016/j.jinf.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Vallée J, Dubot-Pérès A, Ounaphom P, Sayavong C, Bryant JE, Gonzalez J-P. Spatial distribution and risk factors of dengue and Japanese encephalitis virus infection in urban settings: the case of Vientiane, Lao PDR. Trop Med Int Health. 2009;14:1134–1142. doi: 10.1111/j.1365-3156.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 29.Anh PT. Statistical Yearbook of Ninh Kieu Urban District in 2008. Can Tho City, Vietnam: Statistical Office of Ninh Kieu; 2009. [Google Scholar]
- 30.Reuben R, Tewari S, Hiriyan J, Akiyama J. Illustrated keys to species of Culex (Culex) associated with Japanese encephalitis in southeast Asia (Diptera: Culicidae) Mosq Systematics. 1994;26:75–96. [Google Scholar]
- 31.Bhattacharyya DR, Handique R, Dutta LP, Dutta P, Doloi P, Goswami BK, Sharma CK, Mahanta J. Host feeding patterns of Culex vishnui sub group of mosquitoes in Dibrugarh district of Assam. J Commun Dis. 1994;26:133–138. [PubMed] [Google Scholar]
- 32.Mwandawiro C, Tuno N, Suwonkerd W, Tsuda Y, Yanagi T, Takagi M. Host preference of Japanese encephalitis vectors in Chiangmai, northern Thailand. Med Entomol Zool. 1999;50:323–333. doi: 10.1016/s0035-9203(00)90303-1. [DOI] [PubMed] [Google Scholar]
- 33.Lillibridge KM, Parsons RAY, Randle Y, Travassos Da Rosa AP, Guzman H, Siirin M, Wuithiranyagool T, Hailey C, Higgs S, Bala AA, Pascua R, Meyer T, Vanlandingham DL, Tesh RB. The 2002 introduction of West Nile virus into Harris County, Texas, an area historically endemic for St. Louis encephalitis. Am J Trop Med Hyg. 2004;70:676–681. [PubMed] [Google Scholar]
- 34.Wada Y, Kawai S, Oda T, Miyagi I, Suenaga O, Nishigaki J, Omori N, Takahashi K, Matsuo R, Itoh T. Dispersal experiment of Culex tritaeniorhynchus in Nagasaki area (preliminary report) Trop Med. 1969;11:37–44. [Google Scholar]
- 35.Kay BH, Farrow RA. Mosquito (Diptera: Culicidae) dispersal: implications for the epidemiology of Japanese and Murray Valley encephalitis viruses in Australia. J Med Entomol. 2000;37:797–801. doi: 10.1603/0022-2585-37.6.797. [DOI] [PubMed] [Google Scholar]
- 36.van den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 37.Thu HT, Loan HK, Thao HTP, Tu TD. Isolation of Japanese Encephalitis Virus from Mosquitoes collected in Can Tho City. Nong Lam University; Ho Chi Minh City: 2006. International Workshop on Biotechnology in Agriculture. [Google Scholar]
- 38.Service MW. Agricultural development and arthropod-borne diseases: a review. Rev Saude Publica. 1991;25:165–178. doi: 10.1590/s0034-89101991000300002. [DOI] [PubMed] [Google Scholar]
- 39.Arunachalam N, Samuel PP, Hiriyan J, Rajendran R, Dash AP. Short report: observations on the multiple feeding behavior of Culex tritaeniorhynchus (Diptera: culicidae), the vector of Japanese encephalitis in Kerala in southern India. Am J Trop Med Hyg. 2005;72:198–200. [PubMed] [Google Scholar]
- 40.Gingrich JB, Nisalak A, Latendresse JR, Sattabongkot J, Hoke CH, Pomsdhit J, Chantalakana C, Satayaphanta C, Uechiewcharnkit K, Innis BL. Japanese encephalitis virus in Bangkok: factors influencing vector infections in three suburban communities. J Med Entomol. 1992;29:436–444. doi: 10.1093/jmedent/29.3.436. [DOI] [PubMed] [Google Scholar]
- 41.Gingrich JB, Nisalak A, Latendresse JR, Pomsdhit J, Paisansilp S, Hoke CH, Chantalakana C, Satayaphantha C, Uechiewcharnkit K. A longitudinal study of Japanese encephalitis in suburban Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 1987;18:558–566. [PubMed] [Google Scholar]
- 42.Whelan P, Hayes G, Carter J, Wilson A, Haigh B. Detection of the exotic mosquito Culex gelidus in the Northern Territory. Commun Dis Intell. 2000;24:74–75. doi: 10.33321/cdi.2000.24.11. [DOI] [PubMed] [Google Scholar]
- 43.Nitatpattana N, Apiwathnasorn C, Barbazan P, Leemingsawat S, Yoksan S, Gonzalez J-P. First isolation of Japanese encephalitis from Culex quinquefasciatus in Thailand. Southeast Asian J Trop Med Public Health. 2005;36:875–878. [PubMed] [Google Scholar]
- 44.Zinser M, Ramberg F, Willott E. Culex quinquefasciatus (Diptera: Culicidae) as a potential West Nile virus vector in Tucson, Arizona: blood meal analysis indicates feeding on both humans and birds. J Insect Sci. 2004;4:1–3. doi: 10.1093/jis/4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasegawa M, Tuno N, Yen NT, Nam VS, Takagi M. Influence of the distribution of host species on adult abundance of Japanese encephalitis vectors Culex vishnui subgroup and Culex gelidus in a rice-cultivating village in Northern Vietnam. Am J Trop Med Hyg. 2008;78:159–168. [PubMed] [Google Scholar]
- 46.Epstein P. West Nile virus and the climate. J Urban Health. 2001;78:367–371. doi: 10.1093/jurban/78.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baik D, Joo C. Epidemio-entomological survey of Japanese encephalitis in Korea. Korean J Parasitol. 1991;29:67–85. doi: 10.3347/kjp.1991.29.1.67. [DOI] [PubMed] [Google Scholar]
- 48.Reisen WK, Aslamkhan M, Basio RG. The effects of climatic patterns and agricultural practices on the population dynamics of Culex tritaeniorhynchus in Asia. Southeast Asian J Trop Med Public Health. 1976;7:61–71. [PubMed] [Google Scholar]
- 49.Keiser J, Maltese MF, Erlanger TE, Bos R, Tanner M, Singer BH, Utzinger J. Effect of irrigated rice agriculture on Japanese encephalitis, including challenges and opportunities for integrated vector management. Acta Trop. 2005;95:40–57. doi: 10.1016/j.actatropica.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Mougeot L. AGROPOLIS: the Social, Political and Environmental Dimensions of Urban Agriculture. London: IDRC/CRDI; 2005. p. 305. [Google Scholar]
- 51.Satterthwaite D, McGranahan G, Tacoli C. Urbanization and its implications for food and farming. Phil Trans R Soc B Biol Sci. 2010;365:2809–2820. doi: 10.1098/rstb.2010.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Veenhuizen R, Danso G. Profitability and Sustainability of Urban and Peri-urban Agriculture. Rome: United Nations Food and Agricultural Organization; 2007. [Google Scholar]
- 53.Kuno G, Chang G-JJ. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin Microbiol Rev. 2005;18:608–637. doi: 10.1128/CMR.18.4.608-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Den Hurk A, Nisbet D, Johansen C, Foley P, Ritchie S, Mackenzie J. Japanese encephalitis on Badu Island, Australia: the first isolation of Japanese encephalitis virus from Culex gelidus in the Australasian region and the role of mosquito host-feeding patterns in virus transmission cycles. Trans R Soc Trop Med H. 2001;95:595–600. doi: 10.1016/s0035-9203(01)90090-2. [DOI] [PubMed] [Google Scholar]
- 55.Ravanini P, Huhtamo E, Ilaria V, Crobu MG, Nicosia AM, Servino L, Rivasi F, Allegrini S, Miglio U, Magri A, Minisini R, Vapalahti O, Boldorini R. Japanese encephalitis virus RNA detected in Culex pipiens mosquitoes in Italy. Euro Surveill. 2012;17(pii):20221. doi: 10.2807/ese.17.28.20221-en. [DOI] [PubMed] [Google Scholar]
- 56.Shlim DR, Solomon T. Japanese encephalitis vaccine for travelers: exploring the limits of risk. Clin Infect Dis. 2002;35:183–188. doi: 10.1086/341247. [DOI] [PubMed] [Google Scholar]