Abstract
A study was conducted in the city of Salto, Uruguay, to identify mosquito-producing containers, the spatial distribution of mosquitoes and the relationship between the different population indices of Aedes aegypti. On each of 312 premises visited, water-filled containers and immature Ae. aegypti mosquitoes were identified. The containers were counted and classified into six categories. Pupae per person and Stegomyia indices were calculated. Pupae per person were represented spatially. The number of each type of container and number of mosquitoes in each were analyzed and compared, and their spatial distribution was analyzed. No significant differences in the number of the different types of containers with mosquitoes or in the number of mosquitoes in each were found. The distribution of the containers with mosquito was random and the distribution of mosquitoes by type of container was aggregated or highly aggregated.
Introduction
Dengue fever, the most important arboviral disease affecting humans, is an increasingly significant cause of morbidity and mortality in tropical and subtropical regions worldwide. In the past 50 years, the reported average annual number of cases of dengue infections has increased 30-fold.1 Dengue is of particular importance in Asia, the Americas, and the Western Pacific, where it has become increasingly endemic with epidemic outbreaks.2,3 In the 21st century, an estimated 40–50% of the world's population lives in countries endemic for dengue, emphasizing the urgency for finding solutions to control this vector-borne disease.4,5
In the absence of a vaccine, most efforts to control dengue are based on suppression, not eradication, of the peridomestic vector mosquito Aedes aegypti (L.) (Diptera, Culicidae); increasingly these efforts rely on reducing the number of larval breeding habitats.6 How should such efforts be monitored? The commonly used Stegomyia indices have a number of serious shortcomings as epidemiologic indicators of dengue transmission and should thus be viewed with caution. The container index (CI), house (premises) index (HI), and Breteau Index (BI) fail to take into account variances in adult Ae. aegypti production of different containers, and to adequately provide information on per area or a per person densities, factors known to relate to levels of transmission. A pupal and demographic survey providing an estimate of the number of pupae per person (PPI) in a community is more appropriate for assessing risk and devising strategies for the mosquitoes' control. The number of pupae counted is used as a measure of adult productivity in the different containers because mortality among pupae and emerging adults of Ae. aegypti is usually low.7,8 However, Stegomyia indices continue to be commonly used.
In South America, Ae. aegypti has a geographic extension reaching the 15°C annual isotherm with 10°C in the coldest month (July).9 Uruguay has been the southern limit of the dengue vector's distribution since 1997. Before this date, dengue had been absent from Uruguay for almost 40 years because it had been eradicated in 1958.10 Because of its geographic location, Uruguay has long periods during which temperatures fall below oviposition and activity thresholds, and into the lethal range for the insects, as suggested by Christophers11 and Focks and others.12,13 This finding sets apart the situation in Uruguay from situations in tropical countries where dengue fever is endemic.
Despite the efforts of the Uruguayan authorities (Ministry of Public Health, Municipalities) which involve public education, container disposal, monitoring with ovitraps and use of insecticides,14 the vector's distribution has steadily increased and now occupies much of the Uruguay.15,16 Although no cases of indigenous dengue have been recorded, there is great concern that this situation may change. This survey was designed to identify, for the first time in Uruguay, the important Ae. aegypti-producing containers (their importance being a function of a container's abundance and its productivity) in peridomestic households to characterize the spatial distribution of Ae. aegypti populations in the residences of Salto, Uruguay, and to analyze the relationship between Stegomyia and PPIs and by survey dates. This information will be of critical importance for defining an intervention strategy to reduce Ae. aegypti populations with neighborhood participation. Because of exclusion of commercial properties from the survey, we were not able to identify other mosquito sources in the city. Further studies are needed to complement the information gathered in the present study.
Materials and Methods
Study area and scientific team.
The survey was carried out in the urban area of Salto, located in the northwestern part of Uruguay (31°23′S, 57°58′W). Salto has a population of 123,000 persons, an average annual temperature of 18.1°C (62.2°F) and 1,040 mm (41 inches) of rainfall. Aedes aegypti has been present in Salto since 1999.17
The study involved researchers from the University of the Republic of Uruguay and the Ministry of Public Health (MSP according to its Spanish acronym), part of a multidisciplinary team that used an ecosystem approach based on the principles of Ecohealth18 in Uruguay (this approach formally connect ideas of environmental and social determinants of health with those of ecology and systems thinking in an action-research framework applied mostly within a context of social and economic development)18,19 to prevent and control the dengue vector.20,21 The results from this study will be integrated with the results from social research carried out by other researchers of the team, to formulate a multidisciplinary proposal to help prevent dengue fever in Uruguay.
Study design and sampling.
On a digitized street-and-building map mounted in a geographic information system using ArcMap version 10.0 (ESRI, Redlands, CA), we defined 52 sites located along seven North-South transects of the streets of Salto. Each site was separated by approximately 500 meters from its neighbors (Figure 1). In each site, 6 homes were randomly chosen, 3 on each side of the street within the same city block, for a total of 312 premises. Commercial buildings and vacant lots were systematically excluded. Three rounds of sampling were accomplished, each one lasting 5 consecutive days (February 18–22, March 10–14, and April 21–25, 2008). Ten teams of two people each, from the MSP, Ministry of Livestock, Agriculture and Fishing, Departmental Government of Salto, the Army and the Police participated in this sampling.
Figure 1.
Map of Salto, Uruguay, showing 52 sampling sites located along seven North-South transects of the streets.
Each premise was inspected for artificial water-filled containers and immature Ae. aegypti mosquitoes (pupae and larvae). The containers detected were counted and classified into six categories based on their size and use in Uruguayan households: tanks (capacity ≥ 200 L, used to collect water), buckets (capacity: 5–20 L, used as troughs or not used), jars (capacity: 10 L, used for chores around the house), bottles (capacity: 1–2 L), tires and others (miscellaneous small containers, not used). All pupae and larvae detected were collected, placed in labeled vials, and transported to the laboratories of the Dirección Departamental de Salud de Salto for taxonomic identification using the key of Darsie22 and counting. The analyses only included larvae and pupae identified as Ae. aegypti. The pupal counts were used to calculate PPI.
To calculate the traditional Stegomyia indices (HI, CI and BI) for comparison with the PPI, each container was also scored positive or negative for Ae. aegypti larvae and/or pupae. According to the procedure of Focks,8 the number of persons who had slept at the house the preceding night was also recorded.
Spatial representation and spatial analysis.
To represent the pattern of spatial distribution of events (PPI) based on the corresponding coordinates, we used data interpolation and data smoothing using the Gaussian kernel.23 This method enables estimating the probability of the occurrence of an event in each cell of a regular grid, with each cell of this grid being the weighted average of all values for that site. These values are assigned using a probability distribution function, in this case, Gaussian. The degree of smoothing is controlled by choosing a bandwidth that indicates the area to be considered in the calculation. This area should be related to the geographic scale of the hypothesis of interest or to prior knowledge about the problem under study.24 In agreement with Souza-Santos and Carvalho23 this analysis used a bandwidth of 300 meters based on dispersion of the female A. aegypti when they are not able to find suitable containers for breeding.25
Next, the urban and geographic characteristics of the sites located in the city of Salto, the environmental conditions of the homes, the areas around houses and open spaces, and the characteristics of the population were described and analyzed. We used data from the Geographic Information System Arc-View detailing density and type of vegetation and construction, land size, sanitation systems, street and sidewalk paving, storm sewer systems, topography, floodplains, access roads to the city, transport terminals and the socioeconomic level of the population. This information was provided by the Departmental Government of Salto and was used in addition to our own calculations from cartography and photograph interpretation of satellite photographs from Google Earth and photographs taken in the city.
Statistical analyses.
To investigate whether no significant differences existed between different sampling dates, the number of each type of container containing Ae. aegypti larvae and/or pupae (caused by the small number of immature mosquitoes found, larvae and pupae were used), and the number of mosquitoes in different containers was analyzed by using non-parametric Kruskal-Wallis analysis of variance. When significant differences were found, a Mann-Whitney test with a Bonferroni correction was used to verify between which dates differences existed. The relationship between the number of each type of positive container and the number of mosquitoes (larvae and pupae) in each for the total sampled data was analyzed by using a nonparametric Spearman's correlation coefficient.26
The spatial distribution of the different types of containers identified in Salto (in the 52 sampling sites), and larvae and pupae of Ae. aegypti found in different containers was analyzed by using the Green's aggregation index27 by date of sampling. Zero values or values close to zero, indicate a random distribution. Values less than zero (negative ≥ –0.10) indicate a uniform distribution, and positive values > 0 indicate an aggregated distribution.
The CI, HI, BI and PPI values obtained on the different sampling dates were compared by using non-parametric Kruskal-Wallis analysis. We tested the null hypothesis that there were no significant differences between dates for each of the indices. When significant differences were found (P < 0.05), the non-parametric Mann-Whitney test with Bonferroni correction was used to verify between which dates differences existed.
For the PPI, the non-parametric Mann-Whitney statistic was used to verify the null hypothesis that no significant difference existed between the second and third dates (the PPI for the first sampling date was excluded because information collected on the first day of sampling was not recorded correctly by the monitors). Correlations between the different indices calculated on different dates for each one of the 52 sampling sites (PPI not available for the first date) were analyzed by using the non-parametric Spearman's correlation coefficient. A significance level of P = 0.05 was used in all statistical analysis. All statistical analysis was conducted Past version 2.16, a free statistical software package.28
Results
Aedes aegypti per type of container.
No significant differences were found between different types of positive containers (Kruskal-Wallis = 14.56, P = 0.993) (tank, n = 24; buckets, n = 13; jars, n = 8; bottles, n = 8; tires, n = 13; others, n = 13), or between the number of mosquitoes (larvae and pupae) present in different containers (Kruskal-Wallis = 12.06, P = 0.997) (tank, n = 156; buckets, n = 254; jars, n = 91; bottles, n = 142; tires, n = 170; others, n = 255). No significant difference was evident between the three dates in the number of different types of positive containers (Kruskal-Wallis = 14.190, P = 0.064), or the number of mosquitoes (larvae and pupae) present in the different containers (Kruskal-Wallis = 3.399, P = 0.658).
Spatial distribution.
In Salto, the number of different containers with mosquitoes generally showed a random spatial distribution (the index was lower or close to zero). Only bottles and other containers had an aggregated distribution on the second date of sampling (0.331 and 0.164, respectively).
When the number of mosquitoes (larvae and pupae) was considered by type of container, an aggregated or highly aggregated distribution was evident in all cases (index values = 0.16 and 1). The highly aggregated distribution was a result of the number of immature mosquitoes in jars (first and third sampling date = 0.703 and 1, respectively), bottles (third sampling date = 1) and other containers (first sampling date = 1).
Variation and relationship between indices.
Comparison of Stegomyia indices for the three sampling dates showed significantly lower values for the CI, HI, and BI on the third sampling date compared with the second sampling date. For the first date, the indices showed intermediate values (Table 1). We found significant differences between March 10–14 and April 21–25 sampling dates for CI, HI, and BI. The PPI showed significant differences (Z = 3.75, P = 0.0001, by Mann-Whitney test) between March 10–14 (PPI = 0.12) compared with April 21–25 (PPI = 0.01).
Table 1.
Estimated values of container index (CI), house index (HI), Breteau Index (BI), and no. pupae per person index (PPI) for three sampling dates, Salto, Uruguay
| Dates in 2008 | CI | HI | BI | PPI |
|---|---|---|---|---|
| February 18–22 | 8.9 | 6.2 | 6.5 | 0.04 |
| March 10–14 | 9.0 | 11.6 | 18.8 | 0.12 |
| April 21–25 | 3.1 | 2.3 | 2.9 | 0.01 |
The BI was significantly correlated (Spearman's nonparametric correlation) with the HI on the second (r = 0.482, P = 0.017) and the third (r = 0.935, P = 0.025) sampling dates, even when data for the three sampling dates (r = 0.479, P = 0.001) or the last two samples (r = 0.508, P = 0.004) were considered together. The PPI showed a significant correlation with the BI in the second sampling date (r = 0.683, P = 0.000) and when data from the second and third sampling dates were analyzed together (r = 0.602, P = 0.000). Also, the PPI showed a significant correlation with the HI in the second sampling date (r = 0.599, P = 0.002). The CI was not correlated with any of the other indices.
Spatial representation of PPI.
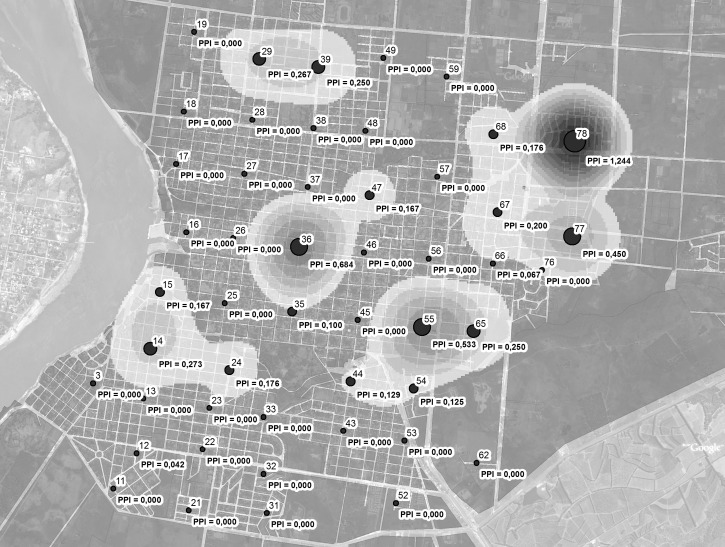
To describe the urban and geographic characteristics of the sites located in Salto, thematic maps were created using data from Geographic Information System Arc View (Figure 2). The highest PPI values were concentrated in sites 78 (PPI = 1.244), 36 (PPI = 0.684), 55 (PPI = 0.533), 77 (PPI = 0.450) and 65 (PPI = 0.250) (Figure 3).
Figure 2.
Spatial representation of the variation of different urban characteristics in Salto, Uruguay. 1 = contours; 2 = vegetation density; 3 = road network and flood areas; 4 = property size. Prepared by using a geographic information system.
Figure 3.
Values and Kernel map of number of Aedes aegypti pupae per person index from 52 sampling sites in Salto, Uruguay.
Discussion
Despite the intensive efforts of the program in Uruguay to minimize the population of Ae. aegypti (public education, container disposal, monitoring with ovitraps, and use of insecticides) this study clearly confirms that the species is abundant in Salto. The vector did not show a preference for key epidemiologic containers when considering the number of different containers with mosquitoes, or the number of immature mosquitoes found inside containers. This finding would have been a useful tool for prioritizing vector-control interventions as has been suggested by other authors.7,29–35 The low population numbers of Ae. aegypti in Salto, reported in this study, may explain these results.
The aggregated or highly aggregated distribution of the number of mosquitoes (larvae plus pupae) by container type reported in this study is in agreement with results obtained by Reuben and others36 and Barrera and others.31 This finding indicates that mosquitoes were present in a small number of total containers, most samples did not contain mosquitoes, and few containers contained most mosquitoes according to the phenomenon of overpopulation in the containers. This type of mosquito distribution has important ramifications for determining sample size in future studies. This is important if the vector population estimate is to be representative. The sample size should be smaller if the pupae are heavily concentrated in a small number of types of containers. Conversely, a larger sample size is needed if mosquitoes are scattered over a greater number of container types.32 However, the spatial distribution of the containers with larvae and/or pupae was mostly random (except for bottles and other containers in the second sampling). Because it is well known that in nature many species of insect tend to increase in numbers in favorable places,37 our results indicate that the distribution of Ae. aegypti in Salto may be more heavily influenced by effects of micro-environmental conditions on container productivity, rather than on differential spatial distribution of the containers.
The highest PPI values found in sites 36, 55, 65, 77 and 78 represent a specific risk situation because in each of these locations there would be a greater possibility of dengue transmission if the virus were present. Site 36 is located in the center of the city, in the true urban sector with old and emblematic buildings, institutional buildings, homes that have backyards with lush vegetation, stores, and commercial regions. Sites 55 and 65 are in southeastern sector of the city, which has a suburban character, separated houses with gardens, and plenty of vegetation around the houses and in public spaces. The predominant homes are of middle and middle-lower socioeconomic level. Sites 77 and 78 are on the northeastern and eastern edge of the city, on main access routes (where goods and persons arrive and the old and new rail terminal and passenger terminal are located), in an area with large, poorly cared for housing estates with abundant vegetation, warehouses, sports fields, houses for lower and medium socioeconomic class families and industry sites.
The highest PPI values at the edges and center of the city in areas with abundant vegetation, coincides with those of Barrera and others,31,38 who found that container productivity was positively and significantly associated with the number of trees per premise and water volume. Other conditions beneficial to Ae. aegypti, as mentioned elsewhere, were present at some of these areas, such as poorly-maintained houses,30 neighborhoods along or contiguous with the principal roads and points of access (leading to an influx of people and goods),39 unplanned urbanization,40 and warehouses that create conditions for risk areas. Such relationships should be the subject of future studies in Uruguay and used for selecting for disposal those containers that are exposed to environmental conditions conducive to greater Ae. aegypti productivity.
Higher indices (CI, HI, BI, and PPI) recorded during the second sampling compared with the third sampling may have been caused by the fact that after a previous extensive drought, 58.8 mm of rainfall occurred in the 15 days before the start of the second sampling versus the 1.7 mm before the third sampling.41 Rain is one of the abiotic factors that influence the existence of breeding sites and the emergence of Ae. aegypti adults when water-filled containers are present.38
Only BI and HI showed a significant correlation between them, as did the BI and PPI, although only in some samples and only when data from several sampling dates were considered together. This result differs from that reported by Bisset and others,42 who indicated that although each of the three Stegomyia indices was closely correlated with the other two indices (P < 0.05 for each), no statistically significant correlations were observed between any of the pupal indices calculated (the numbers of pupae per person, per container inspected, and per hectare) and any of the Stegomyia indices.
If one considers that non-quantitative studies have been performed in Uruguay, this study of the relative abundance of containers with immature Ae. aegypti mosquitoes, their spatial characterization, and the relationship between indices (especially PPI) contributed to generate information that would make it possible to implement in the future a strategy based on the elimination of containers to reduce the risk of dengue epidemics. This implementation would help to direct health interventions and create more financially sustainable campaigns against Ae. aegypti.
The location of Uruguay at the southern edge of the distribution of Ae. aegypti in South America, with climatic conditions that limit but do not prevent increasing abundance of this vector during the non-winter period of the year, indicates that this country is well situated for studies of Ae. aegypti ecology, particularly with respect to increases in distribution as a result of climate change.
ACKNOWLEDGMENTS
We thank the MSP authorities and the field team for conducting the surveys; the Ministry of Livestock, Agriculture and Fishing, the Departmental Government of Salto, and the Army and Police of Salto for assistance; all persons who allowed us onto their property for collecting data; and Rodrigo Fernandez for creating the figures.
Footnotes
Financial support: This study was supported by a grant from Pan American Health Organization.
Authors' addresses: César Basso, Unidad de Entomología, Facultad de Agronomía, Universidad de la República, Av. E. Garzón 780, 12900 Montevideo, Uruguay, E-mail: cbasso@adinet.com.uy. Ruben M. Caffera, Unidad de Sistemas Ambientales, Facultad de Agronomía, Universidad de la República, Av. Garzón 780, 12900 Montevideo, Uruguay, E-mail: rmcaffera@gmail.com. Elsa García da Rosa and Rosario Lairihoy, Departamento de Parasitología Veterinaria, Facultad de Veterinaria, Regional Norte, Universidad de la República, Gral Rivera 1350, 50.000 Salto, Uruguay, E-mails: elsagdr@yahoo.com.ar and barbieri363@gmail.com. Cristina González, Dirección Departamental de Salud, Ministerio de Salud Pública, Uruguay 364, Salto, 50.000 Uruguay, E-mail: cristinagonzalezdiaz@hotmail.com. Walter Norbis, Departamento de Biología Animal, Instituto de Biología, Facultad de Ciencias, Universidad de la República, Iguá 4225, 11400 Montevideo, Uruguay, E-mail: walter.norbis@gmail.com. Ingrid Roche, Instituto de Teoría de la Arquitectura y Urbanismo, Facultad de Arquitectura, Universidad de la República, Br. Artigas 1031, 11200 Montevideo, Uruguay, E-mail: ingridroc@gmail.com.
References
- 1.UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, 2007 . Report of the Scientific Working Group. Geneva: World Health Organization; Meeting on Dengue, Geneva, October 1–5, 2006. [Google Scholar]
- 2.Guzmán M, Kouri G, Díaz M, Llop A, Vazquez S, Gonzalez D, Castro O, Alvarez A, Fuentes O, Montada D, Padmanabha H, Sierra B, Perez A, Rosario D, Pupo M, Diaz C, Sanchez L. Dengue, one of the great emerging health challenges of the 21st century. Expert Rev Vaccines. 2004;3:511–520. doi: 10.1586/14760584.3.5.511. [DOI] [PubMed] [Google Scholar]
- 3.Kroeger A, Nathan M, Hombach J. Dengue. Nat Rev Microbiol. 2004;2:360–361. doi: 10.1038/nrmicro890. [DOI] [PubMed] [Google Scholar]
- 4.Heintze C, Garrido V, Kroeger A. What do community-based dengue control programmes achieve? A systematic review of published evaluations. Trans R Soc Trop Med Hyg. 2007;101:317–325. doi: 10.1016/j.trstmh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez L, Cortinas J, Pelaez O, Gutierrez H, Concepción D, Van der Stuyft P. Breteau Index threshold levels indicating risk for dengue transmission in areas with low Aedes infestation. Trop Med Int Health. 2010;15:173–175. doi: 10.1111/j.1365-3156.2009.02437.x. [DOI] [PubMed] [Google Scholar]
- 6.Nathan MB, Knudsen AB. Aedes aegypti infestastion characteristics in several Caribbean countries and implications for integrated community-based control. J Am Mosq Control Assoc. 1991;7:400–404. [PubMed] [Google Scholar]
- 7.Focks DA, Chadee DD. Pupal survey: an epidemiologically significant surveillance method for Aedes aegypti: an example using data from Trinidad. Am J Trop Med Hyg. 1997;56:159–167. doi: 10.4269/ajtmh.1997.56.159. [DOI] [PubMed] [Google Scholar]
- 8.Focks DA. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors. Special Program for Research and Training in Tropical Diseasess (TDR), UNICEF, UNDP, World Bank, World Health Organization; 2003. http.//www.who.inr/tdr/publications/publications/pdf/dengue_vectors.pdf Available at. Accessed May 2, 2009. [Google Scholar]
- 9.Otero M, Solari HG, Schweigmann N. A stochastic population dynamics model for Aedes aegypti: formulation and application to a city with temperate climate. Bull Math Biol. 2006;68:1945–1974. doi: 10.1007/s11538-006-9067-y. [DOI] [PubMed] [Google Scholar]
- 10.Salvatella R. Aedes aegypti (Diptera, Culicidae). Notificación de su presencia en Uruguay. Rev Med Uruguay. 1997;13:118–121. [Google Scholar]
- 11.Christophers R. Aedes aegypti (L.). The Yellow Fever Mosquito. Cambridge, UK: Cambridge University Press; 1960. [Google Scholar]
- 12.Focks D, Haile D, Daniels E, Mount G. Dynamic life table model for Aedes aegypti (L.) (Diptera: Culicidae). Simulation results and validation. J Med Entomol. 1993;30:1018–1028. doi: 10.1093/jmedent/30.6.1018. [DOI] [PubMed] [Google Scholar]
- 13.Focks D, Haile D, Mount G. Dynamic life table model for Aedes aegypti (L.) (Diptera: Culicidae). Analysis of the literature and model development. J Med Entomol. 1993;30:1003–1017. doi: 10.1093/jmedent/30.6.1003. [DOI] [PubMed] [Google Scholar]
- 14.García da Rosa E, Lairihoy R, Leivas JC, González W, Paulino D. Monitoreo de Aedes aegypti mediante el uso de ovitrampas. Entomol Vect. 2003;10:451–456. [Google Scholar]
- 15.Willat G, Basmadjián Y, Martínez M, Rosa R. Dispersión de Aedes aegypti en el Uruguay. Biol Acuat. 2007;23:79. [Google Scholar]
- 16.Ministerio de Salud Pública 2011. http://www.msp.gub.uy Available at. Accessed April 12, 2011.
- 17.Willat G, Capdevila A, Martínez M, Boga A. Evolución de Aedes aegypti en Uruguay, 1997–2003. Entomol Vect. 2003;10:437–444. [Google Scholar]
- 18.Lebel J. La Santé. Une Appproche Écosystématique. Ottawa, Canada: International Development Research Centre; 2003. [Google Scholar]
- 19.Charron DF. Ecohealth Research in Practice. Innovative Applications of an Ecosystem Approach to Health. Ottawa, Canada: Springer-IDRC; 2012. [Google Scholar]
- 20.Basso C, Romero S, Martínez M, Roche I, Gómez M, Detomasi S, Pereira J. Prevención y control del vector del dengue, Aedes aegypti (L.), en Uruguay acudiendo a un enfoque ecosistemático. In: Augusto LG, Carneiro RM, Martins PH, editors. Abordagem Ecossistêmica em Saúde. Ensaios Para o Controle de Dengue. Recife, Brazil: Universitária da UFPE; 2005. pp. 175–185. [Google Scholar]
- 21.Basso C. Abordaje Ecosistémico para Prevenir y Controlar al Vector del Dengue en Uruguay. Montevideo, Uruguay: Universidad de la República; 2010. [Google Scholar]
- 22.Darsie RF., Jr Mosquitoes of Argentina. Part I. Keys for identification of adult females and fourth stage larvae in English and Spanish (Diptera: Culicidae) Mosq Syst. 1985;17:153–253. [Google Scholar]
- 23.Souza-Santos R, Carvalho MS. Análise da distribuiçao espacial de larvas de Aedes aegypti na Ilha do Governador, Río de Janeiro, Brasil. Cad Saude Publica. 2000;16:31–42. doi: 10.1590/s0102-311x2000000100004. [DOI] [PubMed] [Google Scholar]
- 24.Santos SM, Barcellos C, Carvalho MS, Flôres R. Detecção de aglomerados espaciais de óbitos por causas violentas em Porto Alegre, Rio Grande do Sul, Brasil, 1996. Cad Saude Publica. 2001;17:1141–1151. doi: 10.1590/s0102-311x2001000500015. [DOI] [PubMed] [Google Scholar]
- 25.Trpis M, Häusermann W, Craig GB., Jr Estimates of population size, dispersal, and longevity of domestic Aedes aegypti (Diptera: Culicidae) by mark-release-recapture in the village of Shauri Moyo in eastern Kenya. J Med Entomol. 1995;32:27–33. doi: 10.1093/jmedent/32.1.27. [DOI] [PubMed] [Google Scholar]
- 26.Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. New York: W.H. Freeman and Company; 1998. [Google Scholar]
- 27.Krebs CJ. Ecological Methodology. Menlo Park, CA: Addison and Wesley Longman; 1989. [Google Scholar]
- 28.Hammer ´Ø, Harper DA, Ryan PD. PAST: paleontological statistic software package for education and data analysis. Palaeontol Electronica. 2001;4:1–9. [Google Scholar]
- 29.Chadee DD. Key premises, a guide to Aedes aegypti (Diptera: Culicidae) surveillance and control. Bull Entomol Res. 2004;94:201–207. doi: 10.1079/ber2004297. [DOI] [PubMed] [Google Scholar]
- 30.Arredondo-Jiménez JI, Valdez-Delgado KM. Aedes aegypti pupal/demographic surveys in southern Mexico: consistency and practicality. Ann Trop Med Parasitol. 2006;100:17–32. doi: 10.1179/136485906X105480. [DOI] [PubMed] [Google Scholar]
- 31.Barrera R, Amador M, Clark GG. Use of the pupal survey technique for measuring Aedes aegypti (Diptera: Culicidae) productivity in Puerto Rico. Am J Trop Med Hyg. 2006;74:290–302. [PubMed] [Google Scholar]
- 32.Focks DA, Alexander N. Multicounty Study of Aedes aegypti Pupal Productivity Survey Methodology: Findings and Recommendations. Geneva: World Health Organization/Special Programme for Research and Training in Tropical Diseases; 2006. [Google Scholar]
- 33.Midega JT, Nzovu J, Kahindi S, Sang RC, Mbogo C. Application of the pupal/demographic-survey methodology to identify the key container habitats of Aedes aegypti (L.) in Malindi district, Kenya. Ann Trop Med Parasitol. 2006;100:61–72. doi: 10.1179/136485906X105525. [DOI] [PubMed] [Google Scholar]
- 34.Lenhart AE, Castillo CE, Oviedo M, Villegas E. Use of the pupal/demographic-survey technique to identify the epidemiologically important types of containers producing Aedes aegypti (L.) in a dengue-endemic area of Venezuela. Ann Trop Med Parasitol. 2006;100:53–59. doi: 10.1179/136485906X105516. [DOI] [PubMed] [Google Scholar]
- 35.Chadee DD, Huntley S, Focks DA, Chen AA. Aedes aegypti in Jamaica, West Indies: container productivity profiles to inform control strategies. Trop Med Int Health. 2009;14:220–227. doi: 10.1111/j.1365-3156.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- 36.Reuben R, Das PK, Samuel GD, Brooks GD. Estimation of daily emergence of Aedes aegypti (Diptera: Culicidae) in Sonepat, India. J Med Entomol. 1978;14:705–714. [Google Scholar]
- 37.Birch LC. Experimental background to the study of the distribution and abundance of insects: I. The influence of temperature, moisture and food on the innate capacity for increase of three grain beetles. Ecology. 1953;34:698–711. [Google Scholar]
- 38.Barrera R, Amador M, Clark GG. Ecological factors influencing Aedes aegypti (Diptera: Culicidae) productivity in artificial containers in Salinas, Puerto Rico. J Med Entomol. 2006;43:484–492. doi: 10.1603/0022-2585(2006)43[484:efiaad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Lagrotta MT, Silva WC, Souza-Santos R. Identification of key areas for Aedes aegypti control through geoprocessing in Nova Iguaçu, Rio de Janeiro State, Brazil. Cad Saude Publica. 2008;24:70–80. doi: 10.1590/s0102-311x2008000100007. [DOI] [PubMed] [Google Scholar]
- 40.Tauil PL. Urbanização e Ecologia do dengue. Cad Saude Publica. 2001;17:99–102. [PubMed] [Google Scholar]
- 41.Dirección Nacional de Meteorología 2011. http://www.meteorologia.gub.uy/index.php/pluviometria Available at. Accessed July 8, 2011.
- 42.Bisset JA, Marquetti MC, Suárez S, Rodríguez MM, Padmanabha H. Application of the pupal/demographic-survey methodology in an area of Havana, Cuba, with low densities of Aedes aegypti (L.) Ann Trop Med Parasitol. 2006;100:45–51. doi: 10.1179/136485906X105507. [DOI] [PubMed] [Google Scholar]