Abstract
To elucidate the epidemic status, clinical profile, and current diagnostic issues of scrub typhus in Shandong Province, we analyzed the surveillance data of scrub typhus from 2006 to 2011 and conducted a hospital-based disease survey in 2010. Scrub typhus was clustered in mountainous and coastal areas in Shandong Province, with an epidemic period from September to November. The most common manifestations were fever (100%), eschar or skin ulcer (86.3%), fatigue (71.6%), anorexia (71.6%), and rash (68.6%). Predominant complications included bronchopneumonia, toxic hepatitis, and acute cholecystitis in 21.6%, 3.9%, and 2.9% of the cases, respectively. Severe complications including toxic myocarditis, heart failure, pneumonedema, pleural effusion, and emphysema were first reported in Shandong. Missed and delayed diagnosis of scrub typhus was common in local medical institutions. Alarm should be raised for changes of clinical features and current diagnostic issues of scrub typhus in newly developed endemic areas.
Introduction
Scrub typhus, a mite-borne rickettsiosis caused by Orientia tsutsugamushi, is widely endemic in the Asia–Pacific region, and it threatens a population of 1 billion people.1 Although effective drug therapy is widely available, scrub typhus remains a severe public health problem, with increasing reports of drug-resistant strains of O. tsutsugamushi and travel-acquired cases.2–7 In China, the disease was once endemic only in the tropics and subtropics; however, it emerged in the temperate zone of northern China, with the first outbreak in Shandong Province (latitude 35°42′ N) in 1986,8 and it rapidly reached to latitude 49°30′ N.9 Hitherto, infection of O. tsutsugamushi has been found in at least 15 provinces, municipalities, and autonomous regions of northern China.10 Because scrub typhus is not included in the list of notified infectious diseases in mainland China, lack of availability of nationwide surveillance data of the disease results in underestimation of its prevalence and hazard.
Shandong Province is considered as a typical endemic area of scrub typhus in northern China. As an emerging infectious disease, scrub typhus presented a trend of wide and fast spread in the past two decades. To heighten the surveillance of scrub typhus, direct network report of the disease was initiated by Shandong Diseases Reporting Information System (SDRIS) in 2006. The predominant genotype of O. tsutsugamushi was proved to be Kawasaki-like in mountainous inland.11 A novel strain, which formed an independent clade in the phylogenetic tree based on partial coding sequences of 56-kDa antigen, was found in a recent study.12 The vigilance of genetic variation of O. tsutsugamushi and development of ecotourism in Shandong have become new impetuses for better identification of epidemiological and clinical characteristics of scrub typhus. Drafting the geographic distribution and temporal trend and identifying new clinical characteristics are imperative for control and prevention of the disease in newly developed endemic areas.
Deaths caused by delay in diagnosis and treatment were not uncommon in scrub typhus patients.13,14 To prompt early diagnosis, guide subsequent therapy, and minimize the mortality of scrub typhus, current diagnostic issues are required to be identified, and effective strategies should be established.
The purpose of the present study was to identify changes in epidemiology and clinical profile, clarify current diagnostic issues of scrub typhus, arouse clinical awareness, and facilitate its diagnosis and prevention.
Materials and Methods
Study site.
Shandong Province, with an area of 157,000 km2 and a total population of over 95 million, is located on the eastern coast of China (longitude 114°19′ E to 122°43′ E, latitude 34°22′ N to 38°23′ N). It is in the lower reaches of the Yellow River, and it extends out to the Pacific Ocean in the form of the Shandong Peninsula, with a coastline of 3,121 km. It is mountainous in the center but mostly flat in the periphery. Shandong has a temperate and monsoonal climate (average annual temperature is 13.6–14.3°C; average annual precipitation is 543–845 mm). Considering the incidence and case distribution of scrub typhus in Shandong, stratified cluster sampling was adopted for questionnaire survey. In total, five districts (Gangcheng, Laicheng, Xintai, Yinan, and Yiyuan) from the inland and four districts (Jimo, Jiaonan, Donggang, and Wendeng) from coastal areas were randomly selected in this study.
Case definition.
Coexistence of more than or equal to three of the following items can be used to diagnose a clinical case of scrub typhus: (1) a field exposure history 1–3 weeks before onset; (2) symptoms including high fever, lymphadenopathy, skin rash, splenomegaly, hepatomegaly, or multiorgan dysfunction; (3) typical cutaneous leisions (eschars or ulcers); (4) rapid defervescence with appropriate antibiotics; and (5) Weil–Felix OX-K agglutination titer ≥ 1:80. Confirmed cases were clinical cases with a positive result in immunoglobulin M (IgM) or IgG using a rapid immunochromatographic immunoassay or nested polymerase chain reaction (PCR) test targeting 56-kDa gene of O. tsutsugamushi.
Data sources.
Surveillance data, including demographic information and onset place and date of reported cases of scrub typhus from 2006 to 2011, were obtained through SDRIS to describe the epidemiological characteristics of scrub typhus.
A structured questionnaire was used for scrub typhus cases that occurred during 2010 in the nine districts selected in the study. Four parts of content were included in the questionnaire: (1) demographic and socioeconomic information: sex, age, occupation, education level, and residential environment; (2) basic information on the course of disease: date and place of disease onset, main complaint, date and medical institution of the initial attendance and correct diagnosis, method for diagnosis, and outcome of the disease; (3) clinical manifestations and complications; and (4) laboratory findings. Medical records were retrospectively reviewed to get detailed information of disease progress.
Statistical analysis.
A database was set up using the software EpiData 3.1 (Jens M. Lauritsen, Odense, Syddanmark, Denmark) after quality check of the original data. The accuracy was ensured with double data entry and logistic consistency check. SPSS 16.0 (SPSS Inc., Chicago, IL) was used for statistical processing. Continuous data were described as mean ± SD and analyzed by Student t tests in this study. Statistical significance of difference in the proportions among different groups was determined by χ2 test or Fisher exact test. P values < 0.05 were considered to be statistically significant (two-tailed).
Ethical statement.
The study was approved by the Ethics Committee on Preventive Medicine of Shandong University. Informed oral consent was obtained from all the adult participants or the legal guardians of minors.
Results
Distribution of scrub typhus cases.
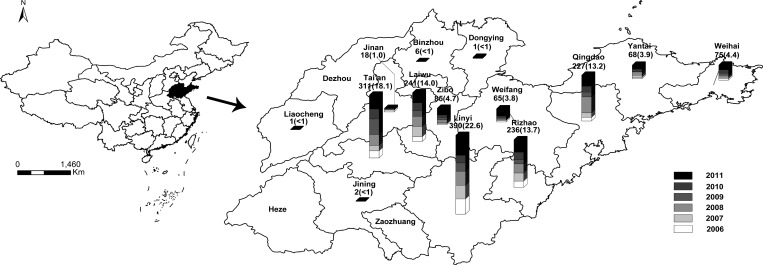
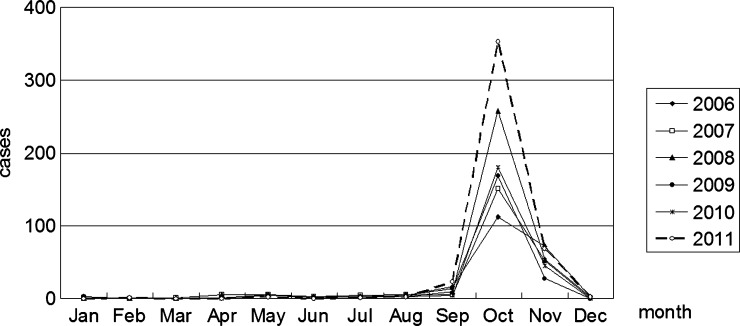
A total of 1,722 clinical cases were reported from 2006 to 2011 in Shandong Province, with a fluctuation of annual incidence between 0.23 and 0.47 per 100,000 people. The distribution of cases annually reported in Shandong is shown in Figure 1. Linyi, Tai'an, Laiwu, Rizhao, Qingdao, Zibo, and Weihai were the top seven regions in reported case numbers, which accounted for 90.7% of all cases in Shandong. Monthly changes in the number of scrub typhus cases in Shandong revealed an epidemic period from September to November, with a peak in October (Figure 2).
Figure 1.
Spatial distribution of annual reported scrub typhus cases in Shandong. Reported case number (%) per year during 2006–2011 in each region is shown above each bar.
Figure 2.
Seasonal distribution of scrub typhus cases during 2006–2011 in Shandong.
Of all the reported cases of scrub typhus, 84.6% were farmers; however, workers, preschoolers, students, retirees, and staff from other occupations were also involved. Among the total cases, 826 cases (48.0%) were males, and 896 cases (52.0%) were females. The age of patients ranged from 7 months to 91 years, with a median age of 54 years. Patients above the age of 40 years made up 80.0% of the total patients. The sex ratio (male:female) of patients ≤ 19 years was 1.6:1, whereas the ratio of patients > 19 years was 0.9:1. Significant difference was shown in sex between the two age groups (P = 0.003).
Clinical findings.
A total of 102 confirmed cases (43 cases from coastal areas and 59 cases from inland areas) was recruited in the study, of which 90 cases (88.2%) had been admitted to the hospital; 100 patients had IgM antibodies for scrub typhus, and 15 patients had IgG antibodies.
All the cases responded well to chlorampenicol, tetracycline, doxycycline, azithromycin, and fluoroquinolones, and they all recovered from illness. All patients had fever, of which 36 patients (35.3%) had continued fever, 24 patients (23.5%) had remittent fever, 35 patients (34.3%) had irregular fever, and 7 patients (6.9%) were unclear of the fever type. The duration of fever varied from 1 to 17 days (average = 6.6 days), with the average highest body temperature of 39.2°C. The six major manifestations were eschar or skin ulcer (86.3%), anorexia (71.6%), fatigue (71.6%), rash (68.6%), headache (62.7%), and generalized myalgia (47.1%) (Table 1).
Table 1.
Clinical manifestations of scrub typhus cases
| Clinical characteristics | N (%) | P value | ||
|---|---|---|---|---|
| Total | Coastal | Mountainous inland | ||
| Symptoms | ||||
| Fever | 102 (100) | 43 (100) | 59 (100) | – |
| Fatigue | 73 (71.6) | 31 (72.1) | 42 (71.2) | 0.920* |
| Anorexia | 73 (71.6) | 33 (76.7) | 40 (67.8) | 0.323* |
| Headache | 64 (62.7) | 27 (62.8) | 37 (62.7) | 0.994* |
| Generalized mylagia | 48 (47.1) | 21 (48.8) | 27 (45.8) | 0.759* |
| Dizziness | 44 (43.1) | 21 (48.8) | 23 (39.0) | 0.321* |
| Chill† | 35 (34.3) | 21 (48.8) | 14 (23.7) | 0.008* |
| Abdominal tenderness | 17 (16.7) | 9 (20.9) | 8 (13.6) | 0.324* |
| Pharyngeal congestion | 19 (18.6) | 8 (18.6) | 11 (18.6) | 0.996* |
| Nausea | 13 (12.7) | 4 (9.3) | 9 (15.3) | 0.373* |
| Vomiting | 15 (14.7) | 6 (14.0) | 9 (15.3) | 0.855* |
| Cough† | 14 (13.7) | 2 (4.7) | 12 (20.3) | 0.023* |
| Conjunctival congestion | 8 (7.8) | 6 (14.0) | 2 (3.4) | 0.067‡ |
| Phlegm | 3 (2.9) | 0 (0.0) | 3 (5.1) | 0.261‡ |
| Signs | ||||
| Eschar or skin ulcer† | 88 (86.3) | 41 (95.3) | 47 (79.7) | 0.023* |
| Rash | 70 (68.6) | 29 (67.4) | 41 (69.5) | 0.826* |
| Facial flushing | 44 (43.1) | 18 (41.9) | 26 (44.1) | 0.824* |
| Liver percussion pain† | 23 (22.5) | 5 (11.6) | 18 (30.5) | 0.024* |
| Kidney percussion pain | 2 (2.0) | 0 (0.0) | 2 (3.4) | 0.507‡ |
| Lymphadenopathy | 27 (26.5) | 10 (23.3) | 17 (28.8) | 0.530* |
| Hepatomegaly | 7 (6.9) | 3 (7.0) | 4 (6.8) | 1.000‡ |
| Splenomegaly | 14 (13.7) | 9 (20.9) | 5 (8.5) | 0.071* |
| Complications† | 34 (33.3) | 20 (46.5) | 14 (23.7) | 0.016* |
χ2 test.
There was significant difference in characteristics between the two groups (P < 0.05).
Fisher exact test.
Eschar distribution of scrub typhus patients is shown in Table 2. Front chest, superior abdomen, and axilla were the most preferential sites for eschar formation. The preferential sites for eschar formation in males and females in coastal areas are different from those sites in males and females in mountainous inland areas. The average diameter of the eschars was 7.05 (±4.23) mm. Scattered erythroic or bolarious maculopapulae were observed in 68.6% of the patients with or without itching.
Table 2.
Eschar distribution of scrub typhus cases
| Location of eschars* | Total N (%) | Coastal N (%) | Mountainous inland N (%) | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Head and neck | 0 (0.0) | 5 (10.6) | 0 (0.0) | 1 (6.7) | 0 (0.0) | 4 (12.5) |
| Front chest, superior abdomen, and axilla | 12 (35.3) | 19 (40.4) | 4 (18.2) | 7 (46.7) | 8 (66.7) | 12 (37.5) |
| Inferior abdomen, buttocks, and area around perineum | 14 (41.2) | 8 (17.0) | 12 (54.5) | 4 (26.7) | 2 (16.7) | 4 (12.5) |
| Back | 2 (5.9) | 6 (12.8) | 1 (4.5) | 2 (13.3) | 1 (8.3) | 4 (12.5) |
| Upper limbs | 2 (5.9) | 5 (10.6) | 2 (9.1) | 0 (0.0) | 0 (0.0) | 5 (15.6) |
| Lower limbs | 4 (11.8) | 4 (8.5) | 3 (13.6) | 1 (6.7) | 1 (8.3) | 3 (9.4) |
| Total | 34 (100.0) | 47 (100.0) | 22 (100) | 15 (100.0) | 12 (100.0) | 32 (100.0) |
Three patients who had more than or equal to two eschars were excluded from the description.
Thirty-six cases developed complications in the process of disease, with respiratory, alimentary, and circulatory systems involved (Table 3). Bronchopneumonia was the most common complication of scrub typhus in Shandong, which was observed in 21.6% of the total patients, followed by toxic hepatitis (3.9%) and acute cholecystitis (2.9%).
Table 3.
Complications associated with scrub typhus
| System involved | N (%) |
|---|---|
| Alimentary system | 9 (8.8) |
| Toxic hepatitis | 4 (3.9) |
| Acute gastritis | 2 (2.0) |
| Acute cholecystitis | 3 (2.9) |
| Respiratory system | 26 (25.5) |
| Bronchopneumonia | 22 (21.6) |
| Emphysema | 1 (1.0) |
| Pneumonedema | 1 (1.0) |
| Pleural effusion | 1 (1.0) |
| Pleuritis | 1 (1.0) |
| Circulatory system | 3 (2.9) |
| Toxic myocarditis | 2 (2.0) |
| Heart failure | 1 (1.0) |
Difference was significant in the presence of eschar or skin ulcer (P = 0.023), liver percussion pain (P = 0.024), chill (P = 0.008), cough (P = 0.023), and complications (P = 0.016) between patients from coastal and inland areas.
Laboratory findings.
Laboratory findings of scrub typhus cases are shown in Table 4. Over 17% of patients had abnormal white blood cell (WBC) count, and 26.8% of patients had abnormal platelet count. Elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were present in 61.5% and 86.4% of patients, respectively. Most patients had a normal serum creatinine (Scr) and blood urea nitrogen (BUN) levels. Abnormality in liver function was common. Increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT) occurred in 75.0% and 80.3% of the total cases, with the mean serum levels of 96.97 and 105.61 IU/L, respectively. Over one-half of patients had hypoalbuminemia, and 95.5% of patients had a decreased albumin/globulin (A/G) ratio.
Table 4.
Laboratory findings of scrub typhus cases
| Variable | Ni/Nt | Percent | Mean ± SD |
|---|---|---|---|
| WBC count (109/L) | 7.47 ± 2.90 | ||
| Leukocytosis | 10/74 | 13.5 | |
| Leukopenia | 3/74 | 4.1 | |
| Increased LYM% | 23/64 | 35.9 | |
| Decreased EO% | 37/47 | 78.7 | |
| Platelet count (109/L) | 147.51 ± 68.99 | ||
| Thrombocytosis | 1/71 | 1.4 | |
| Thrombocytopenia | 18/71 | 25.4 | |
| CRP (mg/dL) | 3.62 ± 3.18 | ||
| Elevated CRP | 19/22 | 86.4 | |
| ESR (mm/hour) | 19.38 ± 7.40 | ||
| Elevated ESR | 8/13 | 61.5 | |
| AST (IU/L) | 96.97 ± 136.85 | ||
| Elevated AST | 48/64 | 75.0 | |
| ALT (IU/L) | 105.61 ± 119.19 | ||
| Elevated ALT | 57/71 | 80.3 | |
| LDH (IU/L) | 394.18 ± 177.81 | ||
| Elevated LDH | 18/24 | 75.0 | |
| α-HBDH (IU/L) | 293.97 ± 151.56 | ||
| Elevated α-HBDH | 13/20 | 65.0 | |
| CK-MB (IU/L) | 16.71 ± 13.50 | ||
| Elevated CK-MB | 4/27 | 14.8 | |
| Albumin (g/dL) | 3.49 ± 0.41 | ||
| Hypoalbuminemia | 34/67 | 50.7 | |
| Decreased A/G ratio | 64/67 | 95.5 | |
| Scr (μmol/L) | 70.23 ± 19.61 | ||
| Elevated Scr | 2/54 | 3.7 | |
| BUN (mmol/L) | 4.90 ± 2.16 | ||
| Elevated BUN | 3/53 | 5.7 |
A/G ratio = albumin/globulin ratio; α-HBDH = hydroxybutyrate dehydrogenase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; BUN = blood urine nitrogen; CK-MB = creatinine kinase–MB; CRP = C-reactive protein; EO% = percentage of eosinophilic granulocyte; ESR = erythrocyte sedimentation rate; LDH = lactate dehydrogenase; LYM% = percentage of lymphocyte; Ni = case number with abnormal result; Nt = case number taking tests; Scr = serum creatinine; WBC = white blood cell.
Difference was statistically significant in ALT level and percentage of lymphocyte (LYM%) between patients from coastal areas and mountainous inland areas. The mean levels of ALT in patients from coastal areas and mountainous inland areas were 62.71 and 137.0 IU/L, respectively (Student t test, P = 0.003), and the mean levels of LYM% were 41.1% and 32.7%, respectively (Student t test, P = 0.040).
Increased bronchovascular shadows were observed in 15.7% of patients on chest X-rays or computed tomography. Fifteen patients had an abnormal electrocardiogram (ECG), of which 33.3% of patients had T-wave change, 26.7% of patients had ST-T change, 26.7% of patients had complete or partial bunch branch block, 13.3% of patients had sinus bradycardia, 6.7% of patients had premature ventricular extrasystole, and 6.7% of patients had abnormal q-wave.
Diagnostic issues.
According to SDRIS, 20 of the reported cases from 2006 to 2011 had been initially misdiagnosed as epidemic or murine typhus, hemorrhagic fever with renal syndrome, typhoid, and human granulocytic anaplasmosis before correct diagnosis of scrub typhus.
Weil–Felix OX-K agglutination reaction was performed on 27 (26.5%) subjects, and 10 of these subjects turned out to have an agglutination titer ≥ 1:80. No accurate diagnostic tests were available in hospitals in Shandong Province.
It took ≥ 4 days to seek medical service after the disease onset for over one-third of the patients. Less than one-quarter of cases received diagnosis of scrub typhus on the first day of their visit to the clinic/hospital, whereas it took ≥ 4 days for 58.0% of the patients to receive correct diagnosis. The average time lag between initial clinic/hospital visit and diagnosis was 3.8 days (range = 0–22 days). Approximately 68% of the patients received correct diagnosis and appropriate drug therapy in the first 1 week of disease progress. No difference was statistically significant in the time lag from disease onset to correct diagnosis between patients from coastal and inland areas.
We figured that 63.4% of the subjects went to clinics in villages as their first choice when they felt malaise; however, none of them got accurately diagnosed. The other 15.8%, 15.8%, and 5.0% of patients chose township health centers, county hospitals, and municipal hospitals, of which the accurate diagnostic rates were 37.5%, 87.5%, and 100%, respectively. Of all the subjects, 97 subjects (95.1%) knew little about scrub typhus, and none of them knew about prevention before the onset of disease; 66 cases (64.7%) were not informed of any preventive measures for scrub typhus by the doctors during their clinic/hospital visits.
Discussion
Scrub typhus is ubiquitous in rural areas, and it experienced a rapid increase in Shandong in the past two decades. Mountainous inland and coastal areas are two epidemic centers of scrub typhus. A similar pattern of geographic distribution was reported in Taiwan.15 It may be ascribed to the specific geographic and climatic conditions under which the transmission chain of scrub typhus works.
Scrub typhus has been recognized as one of the leading causes of fever of unknown origin (FUO) among farmers. It may result from the poor sanitary condition in rural areas and long-time field activities of farmers, especially during harvest time, which increased chances of infestation by chigger mites harboring O. tsutsugamushi. Seasonal distribution of scrub typhus cases was consistent with the fluctuation of Leptotrombidium scutellare, which was confirmed as the dominant mite during autumn in Shandong.16 We suggest that rodents and breeding areas of mites be eradicated and preventive measures be taken before and after outdoor activities in endemic areas to minimize the possibility of infestation by chiggers.
The clinical presentations of scrub typhus were nonspecific and similar to some other acute febrile diseases, including epidemic or murine typhus, hemorrhagic fever with renal syndrome, typhoid, and human granulocytic anaplasmosis, resulting in difficulties in differentiation. Eschar serves as a luminous clue for diagnosis of scrub typhus and differentiation from other causes of FUO mentioned above; however, some echars may be too small to be detected, and most of them form in skin folds and usually cause no abnormal sensation. Eschar was not found in 13.7% of the patients in this study. It is possible that the patients missed the optimal time for physical examination, and patients infected with some genotypes or low loads of O. tsutsugamushi that did not form eschar were reported.17,18 Additional research is needed to clarify the relationship between clinical features and genotypes and loads of the pathogen. The distribution pattern of eschar in scrub typhus patients in this study was different from the pattern in Mengyin County, but it was very similar to the pattern reported in South Korea.19,20 Patients without eschar have potential risk of developing severe complications for delayed medical care.21 Identification of preferential sites of eschar formation in different endemic areas and thorough physical examination could facilitate its detection, allow early diagnosis, and avoid severe outcomes.
Scrub typhus has been reported in many areas of northern China since the first outbreak in Shandong, including Jiangsu, Tianjin, Shanxi, Hebei, Henan, and Anhui.10 These cases mainly occurred in autumn and winter. Fever, headache, fatigue, anorexia, eschar or skin ulcer, and rash were common manifestations of scrub typhus. However, certain clinical differences were identifiable among these areas. Prevalence of eschar or skin ulcer was lower in Shandong than in Shanxi (100%) but higher than in Tianjin (62.8%) and Anhui (67.3%).22–24 The presence of lymphadenopathy was much less common in Shandong than in Shanxi (80.0%) and Tianjin (73.7%).22,23 However, a higher prevalence than the other areas in northern China of bronchopneumonia was noted in Shandong (3.9–16.7%).22,24 Additionally, elevated CRP was much more commonly detected in patients in Shandong than in patients in Anhui (28.0%).24 Yonchon was the predominant genotype of O. tsutsuagmushi in Shanxi.22 Anhui isolates had an nucleotide identity of 99% with Shandong isolates, all of which belonged to the Kawasaki genotype.12,24 Differences in clinical features among the endemic areas may be attributed to the prevalent genotypes of O. tsutsugamushi.
Clinical features between coastal areas and mountainous inland areas were different, but the causes are still unclear. The percentage of cases with complications was much higher in coastal areas than mountainous inland areas, suggesting that it is a more serious clinical problem in coastal areas. More severe scrub typhus in newly endemic areas may be associated with the higher virulence and loads of O. tsutsugamushi carried by chiggers, lack of immunity of populations, and delay in diagnosis and treatment. The predominant genotype of O. tsutsugamushi in patients from mountainous inland areas of Shandong was Kawasaki type,11 which exhibited a low virulence,25 and it was consistent with the less severe clinical outcomes. Thus, much information remains to be gathered about the transmission cycle of scrub typhus in newly developed epidemic foci in coastal areas.
A few complications were first reported in Shandong in the present study, including gastritis, pleuritis, cholecystitis, emphysema, pneumonedema, pleural effusion, toxic myocarditis, and heart failure, and some of these complications are life-threatening. Meningoencephalitis,26 gastrointestinal bleeding, acute renal failure, acute hepatic failure, acute respiratory distress syndrome,27 hearing impairment,28 opsoclonus,29 and pancreatic abscess30 were not found in our study. It was reported that serious complications often developed in the second week during the course of untreated cases.31,32 In the present study, 32.4% of the patients did not receive correct diagnosis and appropriate treatment until the second week after onset, which may partly explain the occurrence of severe complications in Shandong patients. Empirical treatment should be administered to the patients suspected of scrub typhus at their first visit to clinics or hospitals in case of poor prognosis.
Antimicrobial resistance in scrub typhus patients was reported in northern Thailand, and naturally occurring drug-resistant strains of O. tsutsugamushi were isolated thereafter.2 Drug refractory cases have not been reported in Shandong or other areas of northern China, which may be partly because of the widely combined use of antibiotics. However, the emergence of drug-resistant strains of O. tsutsugamushi is alarming under the condition of antibiotic abuse in clinical therapy. Although fluoroquinolone was effective for a few patients in the study, we suggest that it not be taken as a first choice; there is intrinsic resistance in O. tsutsugamushi, and there is the possibility of higher fatalities in treating severe cases.33,34
Weil–Felix OX-K agglutination reaction is the only test generally available for detecting scrub typhus in hospitals in Shandong Province, but low sensitivity hampers its clinical application. Methods of higher sensitivity and specificity, including indirect immunofluorescence assay,35 PCR,36–38 and immunohistochemistry,39 are limited in primary medical institutions, because they require expensive equipment or well-trained staff. A gold conjugate-based rapid diagnostic test was recommended in rural areas for its high sensitivity and convenient usage.40
The village clinic was the first choice for medical service for over one-half of the patients. However, the diagnostic accuracy for scrub typhus in village clinics and township health centers was much lower than the accuracy in county and municipal hospitals. It was implied that a substantial proportion of scrub typhus cases were misdiagnosed because of lack of awareness and unavailability of accurate diagnostic tests. When proper laboratory tests are provisionally unavailable, a training program for the medical staff is imperative. Patients in Shandong knew little about scrub typhus, and they were not informed of preventive measures of the disease by their doctors. This finding revealed a schism between clinical medicine and public health in local areas. Physicians should actively publicize effective preventive measures to scrub typhus patients to prevent them from reinfection and reduce the incidence in endemic areas.
The present study has some limitations. Hospital-based surveys could not avoid selection bias; patients with less severe clinical symptoms who did not attend clinics or hospitals were not included in the assessment of clinical profile. Additionally, clinical information was retrospectively retrieved from the medical records, and a few items concerning laboratory tests were not integrated in all of the subjects.
In summary, scrub typhus should be suspected in patients with fever and eschar, particularly those patients with a history of exposure to endemic areas during autumn and winter in Shandong. Alarm should be raised on scrub typhus patients in case of severe complications. The study emphasizes the need for a training program for medical staff and accurate and convenient laboratory tests in local medical institutions to prompt early diagnosis. Results of the present study are instructive to those newly endemic areas with similar geographic and climatic conditions as Shandong. Additional surveillance of changes of epidemiological and clinical features and investigation of pathogenic characteristics of scrub typhus are required, especially in unstable endemic areas that have newly developed and lack information.
ACKNOWLEDGMENTS
We are grateful to all the participants in the study and all the medical and administrative staff who assisted our investigation and sample collection.
Footnotes
Financial support: The work was supported by National Natural Science Foundation of China Grants 30972515 and 81273133 and Graduate Innovation Foundation of Shandong University Grant yyx10063.
Authors' addresses: Meng Zhang, Zhong-Tang Zhao, and Lei Ding, Department of Epidemiology and Health Statistics, School of Public Health, Shandong University, Jinan, People's Republic of China, E-mails: meng-zhang@mail.sdu.edu.cn, ztzhao@sdu.edu.cn, and 05gw@163.com. Xian-Jun Wang, Zhong Li, and Shu-Jun Ding, Institute for Viral Disease Control and Prevention, Shandong Center for Disease Control and Prevention, Jinan, People's Republic of China, E-mails: xjwang62@163.com, sdlz01@yahoo.com.cn, and dingshujun@sina.com.
References
- 1.Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429–436. doi: 10.1097/00001432-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, Jongsakul K, Teja-Isavadharm P, Bhodhidatta D, Corcoran KD, Dasch GA, Strickman D. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet. 1996;348:86–89. doi: 10.1016/s0140-6736(96)02501-9. [DOI] [PubMed] [Google Scholar]
- 3.Mathai E, Rolain JM, Verghese GM, Abraham OC, Mathai D, Mathai M, Raoult D. Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci. 2003;990:359–364. doi: 10.1111/j.1749-6632.2003.tb07391.x. [DOI] [PubMed] [Google Scholar]
- 4.Watt G, Strickman D. Life-threatening scrub typhus in a traveler returning from Thailand. Clin Infect Dis. 1994;18:624–626. doi: 10.1093/clinids/18.4.624. [DOI] [PubMed] [Google Scholar]
- 5.Nachega JB, Bottieau E, Zech F, Van Gompel A. Travel-acquired scrub typhus: emphasis on the differential diagnosis, treatment, and prevention strategies. J Travel Med. 2007;14:352–355. doi: 10.1111/j.1708-8305.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- 6.Ghorbani RP, Ghorbani AJ, Jain MK, Walker DH. A case of scrub typhus probably acquired in Africa. Clin Infect Dis. 1997;25:1473–1474. doi: 10.1086/516990. [DOI] [PubMed] [Google Scholar]
- 7.Jensenius M, Fournier PE, Raoult D. Rickettsioses and the international traveler. Clin Infect Dis. 2004;39:1493–1499. doi: 10.1086/425365. [DOI] [PubMed] [Google Scholar]
- 8.Yang YF, Wang JL, Yao YC. Investigation of the first scrub typhus epidemic in Shandong Province. Chin J Epidemiol. 1987;8:280. [Google Scholar]
- 9.Liu GP, Lu ZX. Chigger mites and scrub typhus in the northeast of China. Chin J Public Health. 2000;16:779–781. [Google Scholar]
- 10.Zhang M, Wang XJ, Zhao ZT. Current epidemic status and issues on prevention and control of scrub typhus. Chin J Epidemiol. 2011;32:419–423. [PubMed] [Google Scholar]
- 11.Liu YX, Zhao ZT, Gao Y, Jia CQ, Zhang JL, Yang ZQ, Wang SM, Jiang BF. Characterization of Orientia tsutsugamushi strains isolated in Shandong Province, China by immunofluorescence and restriction fragment length polymorphism (RFLP) analyses. Southeast Asian J Trop Med Public Health. 2004;35:353–357. [PubMed] [Google Scholar]
- 12.Yang LP, Zhao ZT, Li Z, Wang XJ, Liu YX, Bi P. Comparative analysis of nucleotide sequences of Orientia tsutsugamushi in different epidemic areas of scrub typhus in Shandong, China. Am J Trop Med Hyg. 2008;78:968–972. [PubMed] [Google Scholar]
- 13.Wang ZW. Investigation of the first outbreak in Feicheng. Dis Surveill. 2001;16:2. [Google Scholar]
- 14.Lee CS, Hwang JH, Lee HB, Kwon KS. Risk factors leading to fatal outcome in scrub typhus patients. Am J Trop Med Hyg. 2009;81:484–488. [PubMed] [Google Scholar]
- 15.Lee YS, Wang PH, Tseng SJ, Ko CF, Teng HJ. Epidemiology of scrub typhus in eastern Taiwan, 2000–2004. Jpn J Infect Dis. 2006;59:235–238. [PubMed] [Google Scholar]
- 16.Liu YX, Zhao ZT, Wu QY, Yang ZQ, Zhang JL, Xu JJ, Peng ZL, Miao ZS. Investigation on the vectors of scrub typhus in the foci of Shandong Province. Mod Prev Med. 2004;31:676–678, 684. [Google Scholar]
- 17.Sonthayanon P, Chierakul W, Wuthiekanun V, Phimda K, Pukrittayakamee S, Day NP, Peacock SJ. Association of high Orientia tsutsugamushi DNA loads with disease of greater severity in adults with scrub typhus. J Clin Microbiol. 2009;47:430–434. doi: 10.1128/JCM.01927-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DM, Yun NR, Neupane GP, Shin SH, Ryu SY, Yoon HJ, Wie SH, Kim WJ, Lee CY, Choi JS, Yang TY. Differences in clinical features according to Boryoung and Karp genotypes of Orientia tsutsugamushi. PLoS One. 2011;6:e22731. doi: 10.1371/journal.pone.0022731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu YX, Feng D, Suo JJ, Xing YB, Liu G, Liu LH, Xiao HJ, Jia N, Gao Y, Yang H, Zuo SQ, Zhang PH, Zhao ZT, Min JS, Feng PT, Ma SB, Liang S, Cao WC. Clinical characteristics of the autumn-winter type scrub typhus cases in south of Shandong Province, northern China. BMC Infect Dis. 2009;9:82. doi: 10.1186/1471-2334-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DM, Won KJ, Park CY, Yu KD, Kim HS, Yang TY, Lee JH, Kim HK, Song HJ, Lee SH, Shin H. Distribution of eschars on the body of scrub typhus patients: a prospective study. Am J Trop Med Hyg. 2007;76:806–809. [PubMed] [Google Scholar]
- 21.Kim DM, Kim SW, Choi SH, Yun NR. Clinical and laboratory findings associated with severe scrub typhus. BMC Infect Dis. 2010;10:108. doi: 10.1186/1471-2334-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen XR, Yu Q, Zhang YQ, Niu H, Zheng XZ, Zhang JT, Zhang XY, Duan GZ, Cheng C. Investigation of scrub typhus in Shanxi Province and study on its etiology. Bull Acad Mil Med Sci. 2000;24:275–281. [Google Scholar]
- 23.Yu CS, Zhang ZL, Luo YQ, Zhang Y, Liu ZY, Cong BQ, Liu ZY, Sun ZZ, Wang CL, Fan LH, Zhang CS, Chen LH. Report on first finding an epidemic of Scrub typhus in north rural areas. Chin J Epidemiol. 1992;13:212–215. [PubMed] [Google Scholar]
- 24.Zhang S, Song H, Liu Y, Li Q, Wang Y, Wu J, Wan J, Li G, Yu C, Li X, Yin W, Xu Z, Liu B, Zhang Q, Wan K, Li G, Fu X, Zhang J, He J, Hai R, Yu D, Walker DH, Xu J, Yu XJ. Scrub typhus in previously unrecognized areas of endemicity in China. J Clin Microbiol. 2010;48:1241–1244. doi: 10.1128/JCM.01784-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagano I, Kasuya S, Noda N, Yamashita T. Virulence in mice of Orientia tsutsugamushi isolated from patients in a new endemic area in Japan. Microbiol Immunol. 1996;40:743–747. doi: 10.1111/j.1348-0421.1996.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim DM, Kim YS, Cho HY, Lee YB. Scrub typhus meningoencephalitis occurring during doxycycline therapy for Orientia tsutsugamushi. Diagn Microbiol Infect Dis. 2011;69:271–274. doi: 10.1016/j.diagmicrobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Wang CC, Liu SF, Liu JW, Chung YH, Su MC, Lin MC. Acute respiratory distress syndrome in scrub typhus. Am J Trop Med Hyg. 2007;76:1148–1152. [PubMed] [Google Scholar]
- 28.Premaratna R, Chandrasena TG, Dassayake AS, Loftis AD, Dasch GA, de Silva HJ. Acute hearing loss due to scrub typhus: a forgotten complication of a reemerging disease. Clin Infect Dis. 2006;42:e6–e8. doi: 10.1086/498747. [DOI] [PubMed] [Google Scholar]
- 29.Nam TS, Choi SM, Park KH, Kim MK, Cho KH. Opsoclonus associated with scrub typhus. Neurology. 2010;74:1925. doi: 10.1212/WNL.0b013e3181e2438d. [DOI] [PubMed] [Google Scholar]
- 30.Yi SY, Tae JH. Pancreatic abscess following scrub typhus associated with multiorgan failure. World J Gastroenterol. 2007;13:3523–3525. doi: 10.3748/wjg.v13.i25.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berman SJ, Kundin WD. Scrub typhus in South Vietnam. A study of 87 cases. Ann Intern Med. 1973;79:26–30. doi: 10.7326/0003-4819-79-1-26. [DOI] [PubMed] [Google Scholar]
- 32.Tsay RW, Chang FY. Serious complications in scrub typhus. J Microbiol Immunol Infect. 1998;31:240–244. [PubMed] [Google Scholar]
- 33.Tantibhedhyangkul W, Angelakis E, Tongyoo N, Newton PN, Moore CE, Phetsouvanh R, Raoult D, Rolain JM. Intrinsic fluoroquinolone resistance in Orientia tsutsugamushi. Int J Antimicrob Agents. 2010;35:338–341. doi: 10.1016/j.ijantimicag.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai CC, Lay CJ, Wang CL, Ho YH, Wang LS, Chen LK. Levofloxacin versus tetracycline antibiotics for the treatment of scrub typhus. Int J Infect Dis. 2010;14:e62–e67. doi: 10.1016/j.ijid.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Blacksell SD, Bryant NJ, Paris DH, Doust JA, Sakoda Y, Day NP. Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: a lack of consensus leads to a lot of confusion. Clin Infect Dis. 2007;44:391–401. doi: 10.1086/510585. [DOI] [PubMed] [Google Scholar]
- 36.Furuya Y, Yoshida Y, Katayama T, Yamamoto S, Kawamura A., Jr Serotype-specific amplification of Rickettsia tsutsugamushi DNA by nested polymerase chain reaction. J Clin Microbiol. 1993;31:1637–1640. doi: 10.1128/jcm.31.6.1637-1640.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang J, Chan TC, Temenak JJ, Dasch GA, Ching WM, Richards AL. Development of a quantitative real-time polymerase chain reaction assay specific for Orientia tsutsugamushi. Am J Trop Med Hyg. 2004;70:351–356. [PubMed] [Google Scholar]
- 38.Paris DH, Blacksell SD, Newton PN, Day NP. Simple, rapid and sensitive detection of Orientia tsutsugamushi by loop-isothermal DNA amplification. Trans R Soc Trop Med Hyg. 2008;102:1239–1246. doi: 10.1016/j.trstmh.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 39.Kim DM, Park CJ, Lim SC, Park KH, Jang WJ, Lee SH. Diagnosis of scrub typhus by immunohistochemical staining of Orientia tsutsugamushi in cutaneous lesions. Am J Clin Pathol. 2008;130:543–551. doi: 10.1309/X17HNNJKMYGHT4HP. [DOI] [PubMed] [Google Scholar]
- 40.Zhang LJ, He S, Jin YM, Li L, Li XM, Liu LY, Yu HL, Yu Q, Chen CF, Wang SW. A rapid, sensitive and reliable diagnostic test for scrub typhus in China. Indian J Med Microbiol. 2011;29:368–371. doi: 10.4103/0255-0857.90166. [DOI] [PubMed] [Google Scholar]