Abstract
In Vietnam, Plasmodium falciparum and P. vivax are responsible for most malaria infections, and P. malariae and P. ovale infections are rarely reported. Nevertheless, species-specific polymerase chain reaction analysis on 2,303 blood samples collected during a cross-sectional survey conducted in a forest area of central Vietnam identified 223 (9.7%) P. falciparum, 170 (7.4%) P. vivax, 95 (4.1%) P. malariae, and 19 (0.8%) P. ovale mono-infections and 164 (7.1%) mixed infections. Of the 671 Plasmodium-positive samples by polymerase chain reaction, only 331 were detected by microscopy. Microscopy poorly diagnosed P. malariae, P. ovale, and mixed infections. Clinical and sub-clinical infections occurred in all age groups. The risk for infection and disease decreased with age, probably because of acquired partial immunity. The common occurrence of sub-patent infections seems to indicate that the malaria burden is underestimated and that diagnostic and therapeutic policies should be adapted accordingly.
Introduction
Since the 1990s, the malaria burden in Vietnam has been efficiently reduced (> 90%) by the national malaria control program and malaria is now confined to the remote and forest areas populated mainly by poor ethnic minorities in which a large proportions (≤ 80%) of asymptomatic and untreated infections with low parasite densities can be found.1,2 Plasmodium falciparum and P. vivax are the most commonly identified malaria species, with a few rare reports of P. malariae and P. ovale infections.1,3,4 Nevertheless, routine detection and management of malaria is based on microscopy (thick blood film) that has a limited sensitivity (±50 parasites/μL of blood). Consequently, low-density infections and mixed infections may be missed. Sub-patent malaria infections, detectable only by molecular techniques (polymerase chain reaction [PCR]), can be relatively common in areas of low malaria endemicity in Southeast Asia and South America and may substantially contribute to maintaining malaria transmission in these areas.5–10
Because Vietnam has reached a pre-elimination stage, with malaria having been eliminated in most of its territory, further successes and ultimately elimination require more sensitive diagnostic tools that are able to successfully detect residual foci of low transmission for better targeting of control/elimination efforts. However, because PCR-based diagnosis is limited by its cost, the need of specific equipment, and trained personnel, malaria cases reported by the national health information system are almost exclusively based on microscopy identification. To assess more precisely the burden and complexity of human malaria infections in central Vietnam, we retrospectively analyzed by PCR blood samples collected in a large cross-sectional survey conducted within the framework of a project evaluating the effectiveness of insecticide-treated hammocks.
Materials and Methods
Sample collection.
Filter paper blood samples collected during a large-scale malariometric survey conducted in November–December 2004 in a rural area of central Vietnam (Ninh Thuan Province) were retrospectively selected to be analyzed by species-specific PCR. Details on the survey methods and study site have been reported.1 In brief, the study population was composed mainly of persons of the Ra-glai ethnic minority, whose subsistence is based on forest farming and products exploitation.11 Malaria transmission is low and perennial in the study region, with two annual peaks (at the beginning and end of the rainy season), and is mainly caused by Anopheles dirus sensu stricto, a sylvatic species and an efficient malaria vector with exophagic and exophilic behavior.
The survey was conducted in the framework of a community-based cluster randomized trial on the effectiveness of long-lasting insecticide-treated hammocks in 20 clusters of approximately 1,000 persons each. The survey, which was part of the baseline study and was conducted just before implementation of the intervention, involved 4,090 persons randomly selected from the study population. After an interview and clinical examination, survey participants provided a finger prick blood sample for microscopy (thick and thin blood films) and for molecular and serologic tests. Blood samples were placed on Whatman grade 3 filter paper (Whatman, Springfield Mill, United Kingdom). After drying, filter paper blood samples were kept individually in plastic bags with silica gel and stored at room temperature in a dark and dry place.
Protocols and results of microscopic examination of blood smears have been reported.1 Slide reading and quality control (double reading) was performed by senior technicians at the National Institute for Malariology, Parasitology and Entomology (Hanoi, Vietnam). Unfortunately, for the present study, blood smears were not available for external quality control (post-PCR). Therefore, microscopy results are those reported originally.
Sample size.
The previously determined parasite rate by microscopy in the survey was estimated to be 17.7% with a precision of 4.5%.1 Thus, taking the lower limit of the confidence interval (CI) (13%) as a minimum prevalence by cluster, a minimum of 100 filter paper blood samples per cluster (plus a safety margin of 10%) would enable detecting a minimum parasite rate by PCR of 13% with a 6% precision at a 95% confidence level. A total of 2,303 filter paper blood samples were analyzed by species-specific PCR.
Species-specific semi-nested multiplex PCR.
DNA was extracted from filter paper by using the saponine-chelex method, and the PCR was performed according to a described method with primers specific for the 18S rDNA region.12,13 The PCR products were subjected to electrophoresis on a 2% agarose gel for 60 minutes at 5V/cm with 0.5× Tris-acetate EDTA buffer. The gels were stained with ethidium bromide, and visualized with ultraviolet light. The sizes of the PCR products were compared with a standard 100-basepair DNA ladder (Fermentas, Burlington, Ontario, Canada) and positive controls of each Plasmodium species. Precautions for cross-contamination during handling were taken by implementing negative controls in each step from extraction to the nested PCR step. Approximately 5% (n = 120) of the samples were repeated blindly and results were confirmed by a senior technician.
The following case definitions were used. Sub-patent malaria infections were defined as malaria infections detected by PCR but negative by microscopy (absence of trophozoites and gametocytes after examining 1,000 leukocytes. Asymptomatic malaria infections were defined as PCR-detected malaria infections (regardless of microscopy results) without fever at the time of sampling (body temperature < 37.5°C) or history of fever during the 3 days before sampling. Symptomatic malaria infections were defined as PCR-detected malaria infections (regardless of microscopic results) with fever at the time of sampling and/or history of fever during the 3 days before sampling). A microscopically positive slide was defined as any slide with either asexual or sexual Plasmodium stages, or both. For the purpose of our study, inclusion of gametocytes among positive slides was justified by the need of having the microscopy comparable to PCR because PCR detects asexual and sexual stages. Parasite density was defined as the density of the predominant species in each infection. Gametocyte prevalence was defined as the prevalence of malaria infections (detected by PCR) carrying gametocytes (identified by microscopy) of any of the four species.
Data analysis.
Data were entered into Excel (Microsoft, Redmond, WA) and analyzed by using Stata version 10 software (StataCorp LP, College Station, TX). Descriptive statistics were used to compute malariometric indices, and a survey χ2 test (svytab command in STATA) was used to test for significant differences in proportions (P < 0.05). The prevalence of all malaria infections (all PCR-detected infections), patent/sub-patent, symptomatic/asymptomatic infections, and gametocyte carriage were computed by age group. The effect of age on the risk for malaria infection was adjusted for previously defined confounders such as forest work, bed net use, and socioeconomic status in a multivariate survey logistic regression (svylogit command in STATA) to take into account the cluster design.14 Subsequently, among all malaria infected cases, the risk for patent infection (compared with sub-patent infection), the risk of symptomatic infection (compared with asymptomatic infection), the risk of infections with gametocytes (compared with those without gametocytes), and the risk of mixed infections (compared with mono-infections) were similarly examined by age groups and adjusted for the above mentioned confounders in four survey logistic regression models.
Ethical considerations.
The protocol of the cluster randomized trial was approved by the Institutional Review Board of the Institute of Tropical Medicine and by the Ethical Committee of the University Hospital (both in Antwerp, Belgium). In Vietnam, the protocol was approved by the National Institute of Malariology, Parasitology and Entomology (Hanoi) and the Ministry of Health.1
Results
Malaria species distribution and prevalence.
Among 2,303 filter paper blood samples analyzed, 671 were positive by PCR, resulting in an overall parasite prevalence of 29.1% (95% CI = 23.3–35.8). Most patients had a Plasmodium falciparum mono-infection (prevalence = 9.7%), followed by P. vivax (7.4%), P. malariae (4.1%), and P. ovale (0.8%) mono-infections (Table 1). Approximately 25% of all infections yielded more than one Plasmodium species (prevalence of mixed infections = 7.1%); ≤ 3 species could be identified in 16% of the mixed infections. Co-infections with P. malariae were the most common (66.5%), followed by those including P. vivax or P. falciparum. Interestingly, P. ovale was found in 30.5% (50 of 164) of all mixed infections. The overall prevalence of P. ovale was high (3%, 69 of 2,303), and this species was mostly observed in mixed infections (72%, 50 of 69 of the cases). This trend was shared by P. malariae, which also occurred in 53% of the cases (109 of 204) in co-infections, and P. falciparum and P. vivax preferentially occurred as mono-infections (70%, 223 of 319 and 63%, 170 of 269, respectively).
Table 1.
Malariometric indices based on PCR and microscopy detection, central Vietnam*
| Malaria indices (n = 2,303) | PCR, no. (%) | Microscopy,† no. (%) |
|---|---|---|
| Overall malaria prevalence | 671 (29.1) | 331 (14.4) |
| Mono-infections:mixed infections (%) | 507:164 (3.1) | 276:55 (5.0) |
| Malaria prevalence by species | ||
| P. falciparum | 223 (9.7) | 149 (6.5) |
| P. vivax | 170 (7.4) | 125 (5.4) |
| P. malariae | 95 (4.1) | 2 (0.1) |
| P. ovale | 19 (0.8) | 0 |
| Mixed | 164 (7.1) | 55 (2.4) |
| P. malariae | 109 (66.5) | 5 (9.10) |
| P. vivax | 99 (60.4) | 55 (100.0) |
| P. falciparum | 96 (58.5) | 50 (90.9) |
| P. ovale | 50 (30.5) | 0 |
| Prevalence of gametocytes‡ | – | 144 (6.3) |
| Prevalence of sub-patent infections | 340 (14.7) | – |
| Prevalence of asymptomatic infections | 547 (23.8) | 265 (11.5) |
PCR = polymerase chain reaction.
Microscopy examination results of the 671 PCR-positive samples (all PCR-negative samples were considered negative for microscopy for the purpose of this report).
Microscopically detected gametocytes.
Among the 671 samples positive by PCR, only in 331 (49%) was the malaria infection also detected by microscopy, suggesting that sub-patent malaria infections (14.8%, 340 of 2,303) were as least as prevalent as patent infections (14.4%) in this population. The microscopically determined species distribution showed a majority of P. falciparum mono-infections (n = 149) followed by P. vivax (n = 125); only two P. malariae and no P. ovale infections were identified. The prevalence of microscopically detected mixed infections was only 2.4% (n = 55), all of them P. vivax co-infections, mostly with P. falciparum (n = 50).
Discrepancies between results of microscopy and PCR were common. However, because blood smears were not available for an independent external re-examination, individual comparisons between microscopy and molecular results would be spurious. Therefore, the main characteristics of discrepant results were analyzed after grouping PCR results by mono-infections or mixed infections. Among PCR-identified P. malariae and P. ovale mono-infections (n = 114), only 28% (n = 32) were detected by microscopy but most of them were identified as P. falciparum or P. vivax mono-infections with a median parasite density of 56 parasites/μL (interquartile range [IQR] = 12–320 parasites/μL). Among PCR-identified P. falciparum and P. vivax mono-infections, 48% (187 of 393) were detected by microscopy and most (87%, 144 of 187) were identified as P. falciparum and P. vivax mono-infections, with a median parasite density of 160 parasites/μL (IQR = 40–960 parasites/μL).
Of the 164 mixed infections, 68% (112 of 164) were positive by microscopy, but only 23% (25 of 112) were identified as mixed infections; the remaining were classified as P. falciparum or P. vivax mono-infections. The median parasite density in mixed infections correctly identified as such by microscopy and PCR was 240/μL (IQR = 144–760 parasites/μL). Among those classified as either P. falciparum or P. vivax mono-infections, the median parasite density was lower (68 parasites/μL, IQR = 24–480 parasites/μL). Among PCR-negative samples, 82 (5%) were positive by microscopy, most of them either P. falciparum or P. vivax mono-infections and with a low parasite density (median density = 32 parasites/μL, IQR = 16–72 parasites/μL). Because slides results could not be cross-checked, for the purpose of the present analysis, these infections were considered as false-positives results, although the presence of an infection cannot be excluded with certainty because PCR analysis may have failed because of the presence of inhibitors, sample degradation, mutations at primer sites, or errors during handling.
Age-dependent risk for malaria infection and symptoms.
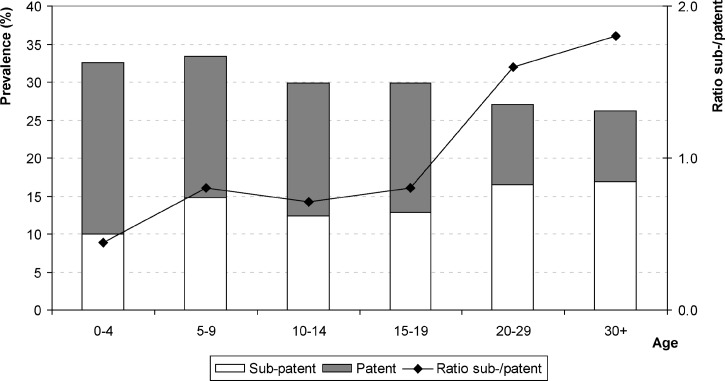
The prevalence of all PCR-detected malaria infections decreased from 32.5% in the group less than five years of age to 26.2% in adults ≥ 30 years of age, with a slow but significantly decreasing trend (P < 0.005, by score test for trend) (Figure 1). Interestingly, the prevalence of patent infections across age groups was reduced by more than 60% (from 22.5% to 9.4%), and the prevalence of sub-patent infections increased by 69% (from 10% to 16.9%). This finding is illustrated by the evolution of the sub-patent:patent infections ratio, which was 0.4 in persons less than five years of age, approximately 0.8 in persons ≤ 19 years of aged, and approximately 2 in adults.
Figure 1.
Prevalence of patent (gray bars) and sub-patent (white bars) malaria infections by age group, Central Vietnam.
Multivariate adjusted analysis for the risk of malaria infection (all PCR positive) (Table 2) by age group showed that the decreasing trend in the likelihood of malaria infection became significant from the age of 20 years with an odds of infections that was 35% lower in adults compared with children less than five years of age (adjusted odds ratio [AOR] = 0.65, 95% CI = 0.46–0.92). Similarly, compared with sub-patent infections, the likelihood of patent infections (Table 2) decreased with age although not significantly until the age of 19 years (AOR = 0.38, 95% CI = 0.12–1.18), but significantly from in those ≥ 20 years of age; adults were > 80% less at risk compared with persons less than five years of age (AOR = 0.18, 95% CI = 0.06–0.43).
Table 2.
Multivariate adjusted analysis for the risk of malaria infection by age group (survey logistic regression models), central Vietnam*
| Characteristic | No, positive/no. tested | AOR | P | 95% CI |
|---|---|---|---|---|
| All malaria infections† | ||||
| Age groups, years | ||||
| 0–4 | 68/209 | 1 | ||
| 5–9 | 142/425 | 1.17 | 0.26 | 0.88–1.54 |
| 10–14 | 94/315 | 0.89 | 0.53 | 0.59–1.32 |
| 15–19 | 70/235 | 0.77 | 0.27 | 0.47–1.26 |
| 20–29 | 126/467 | 0.65 | 0.02 | 0.46–0.92 |
| ≥ 30 | 171/652 | 0.65 | 0.03 | 0.45–0.96 |
| Patent infections†‡ | ||||
| Age groups, years | ||||
| 0–4 | 47/68 | 1 | ||
| 5–9 | 79/142 | 0.52 | 0.13 | 0.22–1.24 |
| 10–14 | 55/94 | 0.5 | 0.08 | 0.23–1.09 |
| 15–19 | 40/70 | 0.38 | 0.09 | 0.12–1.18 |
| 20–29 | 49/126 | 0.18 | 0.001 | 0.08–0.44 |
| ≥ 30 | 61/171 | 0.16 | 0.001 | 0.06–0.43 |
| Gametocyte carriage†‡ | ||||
| Age groups, years | ||||
| 0–4 | 29/68 | 1 | ||
| 5–9 | 40/142 | 0.49 | 0.03 | 0.26–0.93 |
| 10–14 | 22/94 | 0.31 | 0.00 | 0.16–0.63 |
| 15–19 | 15/70 | 0.25 | 0.01 | 0.1–0.70 |
| 20–29 | 18/128 | 0.15 | 0.00 | 0.06–0.35 |
| ≥ 30 | 20/171 | 0.12 | 0.00 | 0.06–0.24 |
| Mixed infections†‡ | ||||
| Age groups, years | ||||
| 0–4 | 21/68 | 1 | ||
| 5–9 | 38/142 | 0.8 | 0.49 | 0.42–1.55 |
| 10–14 | 22/94 | 0.48 | 0.09 | 0.21–1.12 |
| 15–19 | 15/70 | 0.41 | 0.01 | 0.21–0.79 |
| 20–29 | 34/126 | 0.53 | 0.1 | 0.25–1.13 |
| ≥ 30 | 34/171 | 0.34 | 0.02 | 0.14–0.80 |
| Symptomatic infection†‡ | ||||
| Age groups, years | ||||
| 0–4 | 19/68 | 1 | ||
| 5–9 | 25/142 | 0.58 | 0.196 | 0.245–1.362 |
| 10–14 | 19/94 | 0.54 | 0.088 | 0.266–1.105 |
| 15–19 | 15/70 | 0.51 | 0.119 | 0.210–1.214 |
| 20–29 | 23/126 | 0.37 | 0.015 | 0.171–0.809 |
| ≥ 30 | 23/171 | 0.26 | 0.003 | 0.111–0.594 |
AOR = adjusted odds ratio; CI = confidence interval.
Adjusted for forest work, socioeconomic level, and bed net use.
Among all infected persons.
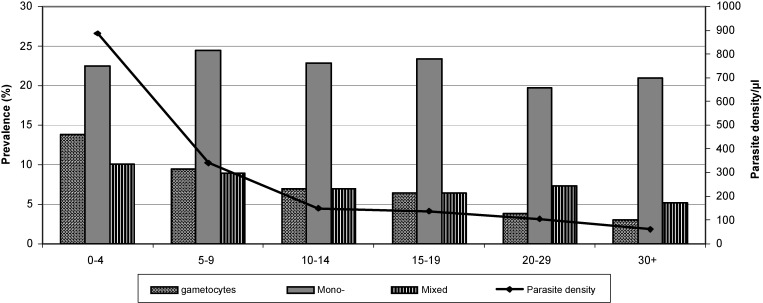
The marked decrease in age-specific prevalence of patent infections correlated with a similar decrease (77%) in the prevalence of gametocytes from 13.9% to 3.1% (Figure 2). In the multivariate adjusted model (Table 2), the odds of carrying gametocytes among infected persons was also > 80% lower in the adult groups (≥ 20 years of age) compared with children less than five years of age. Interestingly, the prevalence of mixed infections also decreased with age (from 10.1% to 5.2%), and the prevalence of mono-infections did not decrease. In the multivariate adjusted model (Table 2) infected adults (> 30 years old) were 66% less at risk of having a mixed infection (compared with a mono-infection) compared with infected children less than five years of age. The age-related decrease of all microscopically defined indices (patent, mixed species, and gametocyte carrying infections) correlated well with a significant reduction (more than 10-fold) of the mean parasite density (geometric mean), which decreased from 883 parasites/μL (95% CI = 518–1,519) in persons less than five years of age to 61 parasites/μL (95% CI = 42–88) in adults ≥ 30 years of age (Figure 2).
Figure 2.
Prevalence of gametocyte carriage and mixed malaria infections with mean parasite density (geometric mean) by age group, central Vietnam. Mono = mono-infections.
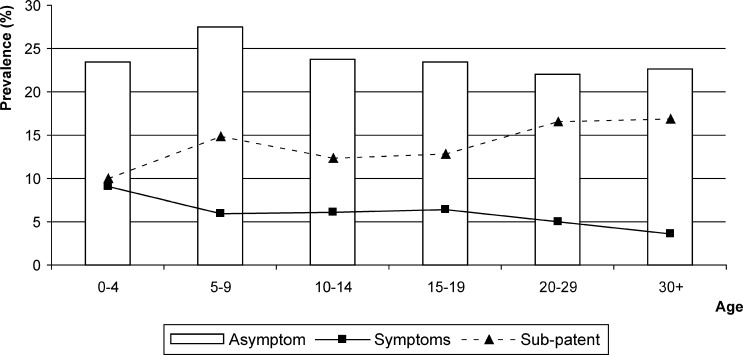
Most (81.5) malaria infections detected by PCR were asymptomatic at the time of the survey, and the proportion of symptomatic infections was similar between sub-patent and patent infections, i.e., 17.1% (58 of 340) and 19.9% (66 of 331), respectively. The prevalence of symptomatic infections decreased progressively, from 9.1% persons less than five years of age to 3.5% in adults, and the prevalence of asymptomatic infections remained stable at approximately 23% (Figure 3). The evolution of symptomatic infections with age mirrored the evolution of sub-patent infections. There was an overall decreasing trend with age in the proportion of symptomatic infections, i.e., from 28% in children less than five years of age to 12% in adults ≥ 30 years of age, similarly in sub-patent and patent infections.
Figure 3.
Prevalence of symptomatic, asymptomatic and sub-patent malaria infections by age group, central Vietnam.
The multivariate adjusted risk of symptomatic infection among infected persons (Table 2E) decreased by 63% in young adults (20–29 years of age; AOR = 0.37, 95% CI = 0.17–0.81) and by almost 75% in adults (≥ 30 years of age) compared with children less than five years of age.
Discussion
In Vietnam, the malaria control program has successfully relied on microscopic diagnosis to reduce the burden of malaria. As previously reported, in the remaining malaria-endemic areas located mainly in central Vietnam, asymptomatic infections are common.1 Our analysis by PCR showed a higher prevalence and more complex species distribution than expected by routine microscopy. Half of the infections detected by PCR were sub-patent, suggesting that the actual occurrence of malaria exceeds that usually estimated by microscopy. This finding was reported in a meta-analysis by Okell and others in 2009, which showed that the prevalence of P. falciparum infections by microscopy was on average 50% of that measured by PCR and that this difference was increasing with decreasing transmission.15 More precisely, the median parasite prevalence by PCR was 35.7% (IQR = 24.9–48.5) for areas where microscopic prevalence is 10.0–24.9%, which is consistent with our results, i.e., 29.1% and 14.4%, respectively.
More interestingly, P. malariae and P. ovale were not rare species, as detected by standard microscopy, and often occurred in mixed infections, which were commonly observed by PCR in our population. These findings confirm previous reports from other countries in Southeast Asia and Africa, where use of molecular techniques showed a high complexity of infections, i.e., high proportions of mixed infections and high occurrence of P. malariae and P. ovale compared with that detected by standard microscopy.7,8,16–18
Presumably, low parasite densities made it difficult to accurately identify the Plasmodium infection and to detect cryptic species by microscopy. This suggestion is supported by the higher parasite densities for the mixed infection identified by both techniques, and the constantly higher frequency of P. falciparum or P. vivax mono-infections identified at low-level densities. Because P. malariae and P. ovale have so far been considered rare species, technicians are more likely to recall the well-known P. falciparum and P. vivax mono-infections. In cases of low parasite counts, the higher sensitivity and specificity of PCR over microscopy lead to more accurate species identification.7,8,16
Because PCR cannot be routinely used for diagnosis, awareness of the species distribution and further training on the detection of non-falciparum malaria could improve control efforts. This information is particularly important for P. vivax and P. ovale, for which dormant liver forms can be reactivated months or years after the primary infection, but also for P. malariae, which has been reported to cause malaria attack years after the primary infection.19
The high proportion of asymptomatic and sub-patent infections indicates the development of partial immunity that is able to control parasitemia and maintain it at low and even undetectable densities.5,20 The degree of partial immunity can be estimated by analyzing the risk of infection by age groups under the assumption that it would increase with age because of longer exposure. The effect of age on the risk of infection was adjusted for the confounding effects of forest activity, bed net use, and socioeconomic level, which were shown to be strong risk factors for malaria infections, to get a better estimate of the independent effect of age.14 Thus, the adjusted effect of age showed that the odds of infection decreased with increased age. In persons ≥ 20 years of age, the odds of infection were significantly lower compared with children less than five years of age. This age dependent risk was also illustrated by the evolution the ratio sub-patent/patent infections. Patent infections were more common in the younger groups, and in persons ≥ 20 years of age sub-patent infections largely exceeded patent infections. This finding was confirmed in the multivariate adjusted analysis where the odds of patent infection was reduced by ≥ 80% and the odds of symptomatic infection was reduced by ≥ 66.7% in infected adults compared with infected children. A recent report by Proietti and others showed similar results from an area of high malaria endemicity in Uganda, where the proportion of sub-patent P. falciparum infections changed from 20% in children less than 15 years of age to approximately 50% in the older age groups.18
With increasing age, the prevalence of gametocyte carriage and mixed infections decreased markedly and their risks were significantly decreased by 75% and 60%, respectively, at the age of adolescence. The observed results of age dependency likely indicate the progressive acquisition of immunity. Other reports mentioning the age dependency of malaria prevalence and symptomatic disease also attributed this phenomenon to the acquisition of protective immunity.5,8,10,20 Two forms of immunity are likely to occur. One form is anti-disease immunity, which is illustrated by numerous asymptomatic infections even in young children and the significantly lower risk of symptomatic infection (compared with asymptomatic infection) in adults.20,21 The other form is immunity against the parasites, which develops more slowly, and results in lower prevalence with low densities infections in the adult population. The partial development of anti-parasite immunity might be responsible for lower risk of symptomatic and patent infection by age. The reduction of the risk for symptomatic and patent disease became significant in adults and corresponds to cumulative exposure during adult life when working in forest fields. In addition, as observed in Indonesia, immunity to infections may develop more rapidly in adults than in children and probably contributes to the lower risk of clinical malaria observed in individuals ≥ 20 years of age.22,23
Adults had lower gametocyte carriage, suggesting a lower transmissibility to the vector. However, the gametocytes identified by microscopy are likely to represent only the tip of the iceberg.24 In Kenya, gametocyte prevalence in children infected with P. falciparum was approximately four times higher by PCR compared with that determined by microscopy.25 The long duration of gametocytes carriage after infection (≤ 55 days) and the ability of sub-patent gametocyte infections to infect mosquitoes makes the adult population a potent reservoir for sustaining transmission.25,26 This suggestion is plausible if one considers the behavior of the vector (exophilic and exophagic) and exposure to mosquito bites of adults in forests, where these persons can easily infect mosquitoes. The contribution of asymptomatic and sub-patent infection to maintain malaria transmission within this context should be further clarified. Sub-patent and asymptomatic infections have been shown to be infective to mosquitoes, although at lower rates than patent and symptomatic infections, but because sub-patent and asymptomatic infections are more prevalent and remain undetected longer, their contribution might be substantial.9
If one considers that this is a cross-sectional study, the evolution of asymptomatic or sub-patent infections towards clinical malaria cannot be excluded because infected persons were not followed-up and may have shown development of a clinical attack after the survey. Nevertheless, asymptomatic carriage, as indicated by its prevalence and by several reports for the region, is probably a common occurrence.1,14,24
Therefore, the true parasite distribution of all five Plasmodium species infecting humans in malaria-endemic regions of Vietnam can be estimated only by conducting similar surveys in other districts or provinces to which malaria is endemic.27 Such information will be essential for guiding and monitoring future elimination efforts.
ACKNOWLEDGMENTS
Peter van den Eede was a research fellow of the Institute for the Promotion of Innovation by Science and Technology in Flanders.
Footnotes
Financial support: This study was supported by the Research Foundation, Flanders and the Directorate-General for Development Cooperation Framework Agreement III.
Authors' addresses: Hong Van Nguyen, Ngo Duc Thang, and Le Xuan Hung, National Institute of Malariology, Parasitology and Entomology, Tu Liem District, Hanoi, Vietnam, E-mails: nvhong1982@yahoo.com, thangnimpevn@yahoo.com, and lxhung1952@yahoo.com. Peter van den Eede, Chantal van Overmeir, Umberto D'Alessandro, and Annette Erhart, Department of Parasitology, Institute of Tropical Medicine, Antwerp, Belgium, E-mails: pvandeneede@itg.be, cvovermeir@itg.be, udalessandro@itg.be, and aerhart@itg.be.
References
- 1.Thang ND, Erhart A, Hung le X, Thuan le K, Xa NX, Thanh NN, Ky PV, Coosemans M, Speybroeck N, D'Alessandro U. Rapid decrease of malaria morbidity following the introduction of community-based monitoring in a rural area of central Vietnam. Malar J. 2009;8:3. doi: 10.1186/1475-2875-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erhart A, Ngo DT, Phan VK, Ta TT, van Overmeir C, Speybroeck N, Obsomer V, Le XH, Le KT, Coosemans M, D'Alessandro U. Epidemiology of forest malaria in central Vietnam: a large scale cross-sectional survey. Malar J. 2005;4:58. doi: 10.1186/1475-2875-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawamoto F, Miyake H, Kaneko O, Kimura M, Nguyen TD, Nguyen TD, Liu Q, Zhou M, Le DD, Kawai S, Isomura S, Wataya Y. Sequence variation in the 18S rRNA gene, a target for PCR-based malaria diagnosis, in Plasmodium ovale from southern Vietnam. J Clin Microbiol. 1996;34:2287–2289. doi: 10.1128/jcm.34.9.2287-2289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gleason NN, Fisher GU, Blumhardt R, Roth AE, Gaffney GW. Plasmodium ovale malaria acquired in Vietnam. Bull World Health Organ. 1970;42:399–403. [PMC free article] [PubMed] [Google Scholar]
- 5.Ladeia-Andrade S, Ferreira MU, de Carvalho ME, Curado I, Coura JR. Age-dependent acquisition of protective immunity to malaria in riverine populations of the Amazon Basin of Brazil. Am J Trop Med Hyg. 2009;80:452–459. [PubMed] [Google Scholar]
- 6.Alves FP, Durlacher RR, Menezes MJ, Krieger H, Silva LH, Camargo EP. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg. 2002;66:641–648. doi: 10.4269/ajtmh.2002.66.641. [DOI] [PubMed] [Google Scholar]
- 7.Steenkeste N, Rogers WO, Okell L, Jeanne I, Incardona S, Duval L, Chy S, Hewitt S, Chou M, Socheat D, Babin FX, Ariey F, Rogier C. Sub-microscopic malaria cases and mixed malaria infection in a remote area of high malaria endemicity in Rattanakiri Province, Cambodia: implication for malaria elimination. Malar J. 2010;9:108. doi: 10.1186/1475-2875-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Putaporntip C, Hongsrimuang T, Seethamchai S, Kobasa T, Limkittikul K, Cui L, Jongwutiwes S. Differential prevalence of Plasmodium infections and cryptic Plasmodium knowlesi malaria in humans in Thailand. J Infect Dis. 2009;199:1143–1150. doi: 10.1086/597414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alves FP, Gil LH, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LH. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42:777–779. doi: 10.1093/jmedent/42.5.777. [DOI] [PubMed] [Google Scholar]
- 10.Coura JR, Suárez-Mutis M, Ladeia-Andrade S. A new challenge for malaria control in Brazil: asymptomatic Plasmodium infection–a review. Mem Inst Oswaldo Cruz. 2006;101:229–237. doi: 10.1590/s0074-02762006000300001. [DOI] [PubMed] [Google Scholar]
- 11.Peeters Grietens K, Xuan XN, Van Bortel W, Duc TN, Ribera JM, Ba Nhat T, Van KP, Le Xuan H, D'Alessandro U, Erhart A. Low perception of malaria risk among the Ra-glai ethnic minority in south-central Vietnam: implications for forest malaria control. Malar J. 2010;9:23. doi: 10.1186/1475-2875-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–568. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 13.Rubio JM, Post RJ, van Leeuwen WM, Henry MC, Lindergard G, Hommel M. Alternative polymerase chain reaction method to identify Plasmodium species in human blood samples: the semi-nested multiplex malaria PCR (SnM-PCR) Trans R Soc Trop Med Hyg. 2002;96((Suppl)):S199–S204. doi: 10.1016/s0035-9203(02)90077-5. [DOI] [PubMed] [Google Scholar]
- 14.Thang ND, Erhart A, Speybroeck N, Hung le X, Thuan le K, Hung CT, Ky PV, Coosemans M, D'Alessandro U. Malaria in central Vietnam: analysis of risk factors by multivariate analysis and classification tree models. Malar J. 2008;7:28. doi: 10.1186/1475-2875-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis. 2009;200:1509–1517. doi: 10.1086/644781. [DOI] [PubMed] [Google Scholar]
- 16.Coleman RE, Sattabongkot J, Promstaporm S, Maneechai N, Tippayachai B, Kengluecha A, Rachapaew N, Zollner G, Miller RS, Vaughan JA, Thimasarn K, Khuntirat B. Comparison of PCR and microscopy for the detection of asymptomatic malaria in a Plasmodium falciparum/vivax endemic area in Thailand. Malar J. 2006;5:121. doi: 10.1186/1475-2875-5-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oguike MC, Betson M, Burke M, Nolder D, Stothard JR, Kleinschmidt I, Proietti C, Bousema T, Ndounga M, Tanabe K, Ntege E, Culleton R, Sutherland CJ. Plasmodium ovale curtisi and Plasmodium ovale wallikeri circulate simultaneously in African communities. Int J Parasitol. 2011;41:677–683. doi: 10.1016/j.ijpara.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proietti C, Pettinato DD, Kanoi BN, Ntege E, Crisanti A, Riley EM, Egwang TG, Drakeley C, Bousema T. Continuing intense malaria transmission in northern Uganda. Am J Trop Med Hyg. 2011;84:830–837. doi: 10.4269/ajtmh.2011.10-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins WE, Jeffery GM. Plasmodium malariae: parasite and disease. Clin Microbiol Rev. 2007;20:579–592. doi: 10.1128/CMR.00027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayor A, Aponte JJ, Fogg C, Saúte F, Greenwood B, Dgedge M, Menendez C, Alonso PL. The epidemiology of malaria in adults in a rural area of southern Mozambique. Malar J. 2007;6:3. doi: 10.1186/1475-2875-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baird JK, Purnomo Basri H, Bangs MJ, Andersen EM, Jones TR, Masbar S, Harjosuwarno S, Subianto B, Arbani PR. Age-specific prevalence of Plasmodium falciparum among six populations with limited histories of exposure to endemic malaria. Am J Trop Med Hyg. 1993;49:707–719. doi: 10.4269/ajtmh.1993.49.707. [DOI] [PubMed] [Google Scholar]
- 23.Baird JK, Krisin Barcus MJ, Elyazar IR, Bangs MJ, Maguire JD, Fryauff DJ, Richie TL, Sekartuti Kalalo W. Onset of clinical immunity to Plasmodium falciparum among Javanese migrants to Indonesian Papua. Ann Trop Med Parasitol. 2003;97:557–564. doi: 10.1179/000349803225001472. [DOI] [PubMed] [Google Scholar]
- 24.Erhart A, Ngo DT, Phan VK, Ta TT, Van Overmeir C, Speybroeck N, Obsomer V, Le XH, Le KT, Coosemans M, D'alessandro U. Epidemiology of forest malaria in central Vietnam: a large scale cross-sectional survey. Malar J. 2005;4:58. doi: 10.1186/1475-2875-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bousema JT, Schneider P, Gouagna LC, Drakeley CJ, Tostmann A, Houben R, Githure JI, Ord R, Sutherland CJ, Omar SA, Sauerwein RW. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J Infect Dis. 2006;193:1151–1159. doi: 10.1086/503051. [DOI] [PubMed] [Google Scholar]
- 26.Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, Sutherland C, Sauerwein R, Ghani AC, Drakeley C. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J. 2010;9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van den Eede P, Van HN, Van Overmeir C, Vythilingam I, Duc TN, Hung le X, Manh HN, Anné J, D'Alessandro U, Erhart A. Human Plasmodium knowlesi infections in young children in central Vietnam. Malar J. 2009;8:249. doi: 10.1186/1475-2875-8-249. [DOI] [PMC free article] [PubMed] [Google Scholar]