Abstract
Microtubules are essential components of axon guidance machinery. Among β-tubulin mutations, only those in TUBB3 have been shown to cause primary errors in axon guidance. All identified mutations in TUBB2B result in polymicrogyria, but it remains unclear whether TUBB2B mutations can cause axon dysinnervation as a primary phenotype. We have identified a novel inherited heterozygous missense mutation in TUBB2B that results in an E421K amino acid substitution in a family who segregates congenital fibrosis of the extraocular muscles (CFEOM) with polymicrogyria. Diffusion tensor imaging of brains of affected family members reveals aberrations in the trajectories of commissural projection neurons, implying a paucity of homotopic connections. These observations led us to ask whether axon dysinnervation is a primary phenotype, and why the E421K, but not other, TUBB2B substitutions cause CFEOM. Expression of exogenous Tubb2b-E421K in developing callosal projection neurons is sufficient to perturb homotopic connectivity, without affecting neuronal production or migration. Using in vitro biochemical assays and yeast genetics, we find that TUBB2B-E421K αβ-heterodimers are incorporated into the microtubule network where they alter microtubule dynamics and can reduce kinesin localization. These data provide evidence that TUBB2B mutations can cause primary axon dysinnervation. Interestingly, by incorporating into microtubules and altering their dynamic properties, the E421K substitution behaves differently than previously identified TUBB2B substitutions, providing mechanistic insight into the divergence between resulting phenotypes. Together with previous studies, these findings highlight that β-tubulin isotypes function in both conserved and divergent ways to support proper human nervous system development.
INTRODUCTION
The structural and functional integrity of the microtubule cytoskeleton is critical to human nervous system development. Microtubules are dynamic polymers that assemble from αβ-tubulin heterodimers and support diverse functions inside the cell. Humans harbor nine genes that encode distinct β-tubulin monomers, termed isotypes. Because isotype sequences are conserved across evolution (1–3) and isotypes differ in their spatial and temporal expression (2,4–7), it has been suggested that distinct isotype compositions may confer unique properties to the microtubule polymer (8,9). However, the role of isotype diversity in supporting the multitude of microtubule-related functions remains unclear.
Human genetics has begun to provide insight into the role of specific tubulin isotypes. Twenty-three unique human heterozygous missense mutations in genes that encode β-tubulin isotypes TUBB2B and TUBB3 have been reported (10–14). Phenotype–genotype analyses support correlations between the mutated isotype and the resulting neurological phenotypes (11,15). Mutations altering TUBB3, which is expressed in post-mitotic neurons but not glia, segregate into two distinct phenotypic groups (11,13,15). Eight reported amino acid substitutions in TUBB3 cause congenital fibrosis of the extraocular muscles (CFEOM), a congenital disorder in which maldevelopment of cranial nerve axons leads to ptosis (drooping eye lids) and restricted eye movements (11,16–18). A subset of mutations segregate with facial weakness, intellectual and social disabilities and/or progressive sensorimotor peripheral neuropathy. Human neuroimaging revealed cranial nerve hypoplasia and a mouse model of the TUBB3 R262C substitution suggests this is due to cranial axon misguidance (11,16). This CFEOM mouse model also recapitulates the human anterior commissure and corpus callosum (CC) hypoplasia (11). Neither human neuroimaging nor mouse pathology has identified cortical lamination defects, indicating that CFEOM-causing mutations in TUBB3 lead to primary axonal dysinnervation (11,15).
The second group of TUBB3 mutations includes six unique amino acid substitutions that cause a spectrum of cortical dysplasias collectively termed malformations of cortical development (MCD), including gyral disorganization and simplification or polymicrogyria (PMG), a cortical dysplasia resulting from impaired neuronal migration and characterized by shallow sulci and excessive numbers of gyri on the brain surface (19). Patients with MCD have intellectual and motor disabilities and can have comitant strabismus, but do not have CFEOM. Neuroimaging revealed CC hypoplasia and misguided fiber bundles in the internal capsule, suggesting additional axon pathfinding defects (13).
Eight amino acid substitutions in TUBB2B, which is highly expressed in both neurons and glia, have been reported to cause tubulin folding defects and PMG. Affected family members also present with variable degrees of CC dysplasia (10,12). The association of PMG with mutations that result in reduced TUBB2B levels suggests that TUBB2B may be essential for proper neuronal migration (10). A newly reported de novo TUBB2B mutation in a single patient associates with the developmental delay in the setting of PMG and open-lip schizencephaly, and unilateral ptosis and exotropia in the setting of a thin ipsilateral oculomotor nerve (14). These findings raise the possibility that TUBB2B, like TUBB3, has a critical role in axon guidance, but it remains unclear whether accompanying axon tract abnormalities are primary defects, or if they arise secondary to severe structural brain abnormalities.
We have now identified a novel heterozygous TUBB2B mutation that segregates with CFEOM, bilateral PMG and a paucity of homotopic callosal connections (20). Using this mutation, we ask whether mutant TUBB2B alleles can cause primary axonal dysinnervation, and why only a subset of TUBB2B mutations cause CFEOM. By introducing this mutation into developing mouse cortical neurons in a mosaic fashion, we do not induce migratory phenotypes and PMG, but do find disruptions in homotopic connectivity, thus providing evidence of a primary axonal phenotype. Using in vitro and in vivo cellular assays, we find that αβ-heterodimers containing the E421K substitution incorporate into microtubule polymers and alter dynamic instability and specific kinesin–microtubule interactions. These molecular phenotypes differ from those caused by TUBB2B mutations that do not result in CFEOM, providing an explanation for the divergence in their respective phenotypes.
RESULTS
A genetically undefined syndrome is characterized by CFEOM with intellectual disability
We previously reported and have now re-examined an Australian-based Caucasian family in which the mother (I:2) and two (II:2 and II:3) of four children have CFEOM and intellectual disability (20). The mother (44 years) and two affected daughters (21 and 20 years) display CFEOM with no significant changes since the initial report. All have bilateral ptosis (drooping eyelids), both eyes fixed in downgaze, and a compensatory chin-up head position. Vertical eye movements are severely limited with jerky convergent movements on attempted elevation, and horizontal movements are variably restricted. Both daughters showed significant intellectual disability (<1st percentile) in recent neuropsychological testing and had head circumferences <2nd percentile, whereas their heights and weights fell in the 10–50th percentiles. The mother, though not formally assessed, was previously reported to have low to average intellectual function (20). One daughter has difficulty modulating her behavior. The remaining exams including slit lamp, fundoscopy, pupillary reaction, non-oculomotor cranial nerve function, motor strength and tone, deep tendon reflexes, sensory modalities and coordination were normal. None have lost developmental milestones, experienced seizures or developed symptoms of peripheral neuropathy. Nerve conduction studies of the older daughter were normal (Fig. 1A).
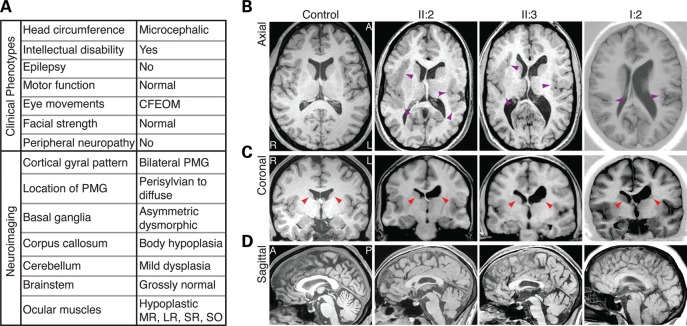
Figure 1.
A genetically undefined syndrome characterized by CFEOM and intellectual disability. (A) Chart summarizing clinical and neuroradiological findings of affected family members. (B) Axial views show the extent of PMG in patients. PMG is diffuse and bilateral in II:2 and II:3 and primarily perisylvian in I:2 (purple arrowheads). (B and C) Axial and coronal views show basal ganglia dysmorphisms. There is marked dysplasia of the left caudate head, and a poorly defined putamen when compared with the right (red arrowheads). In addition, there are asymmetric white matter tract abnormalities, with poor fasciculation of the left internal capsule and left ventricular dilation. (D) Sagittal views show thinning of the CC body, whereas the genu is comparably less hypoplastic. I:2 (affected mother), II:2 (affected older daughter) and II:3 (affected younger daughter) refer to pedigree positions in Figure 2. CFEOM, congenital fibrosis of the extraocular muscles; PMG, polymicrogyria; MR, medial rectus; LR, lateral rectus; SR, superior rectus; SO, superior oblique; L, left hemisphere; R, right hemisphere; A, anterior; P, posterior.
Previous magnetic resonance imaging (MRI) scans of the daughters at ages 4 and 9 showed variable hypoplasia of affected extraocular muscles, and imaging of the mother at the age of 26 had revealed thinned medial and lateral rectus muscles, and hypoplastic superior rectus and levator palpebrae superioris muscles, consistent with CFEOM (20). The daughters' earlier scans, as well as repeat brain MRI scans at the age of 19 (II:3) and 20 (II:2), revealed prominent diffuse bilateral PMG (Fig. 1B), dilation of the left lateral ventricle (Fig. 1B and C) with hypoplasia of the body of the ipsilateral caudate nucleus (Fig. 1C), and fusion of an enlarged caudate nucleus head with the underlying putamen (20). Both have a thin CC body with comparatively normal looking genu (Fig. 1D). Retrospective review of the mother's scan (I:2) also revealed mild bilateral perisylvian PMG in addition to the previously reported ventricular and caudate asymmetries (Fig. 1B and C).
CFEOM and intellectual disability segregate with a heterozygous mutation in TUBB2B
Haplotype analysis previously revealed co-segregation of the family's autosomal dominant phenotype with polymorphic markers flanking the FEOM1 and FEOM3 loci (20). Thus, we sequenced the disease genes at these loci, KIF21A and TUBB3, but did not identify disease-associated variants. Next, taking a candidate approach, we sequenced TUBA1A and TUBB2B and identified a heterozygous c.1261G>A nucleotide substitution in exon 4 of TUBB2B that co-segregated with the affliction status and with a haplotype flanking the TUBB2B locus (Fig. 2A and B). The TUBB2B variant was absent from 336 control individuals, dbSNP (http://www.ncbi.nih.gov/SNP), the 1000 Genomes Project (http://browser.1000genomes.org/index.html) and the NHLBI ESP Exome Variant Server (http://evs.gs.washington.edu/EVS/). No disease variants were found in TUBA1A.
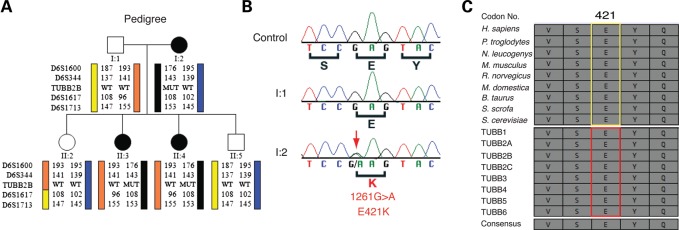
Figure 2.
Heterozygous TUBB2B 1261G>A mutation (E421K) segregates with CFEOM and PMG. (A) Schematic of pedigree and targeted linkage analysis on chromosome 6 identifies a haplotype flanking the TUBB2B locus that segregates with the disease with complete penetrance. Pedigree members are denoted by circles (females) and squares (males) and by generation and position. Solid shapes indicate clinically affected individuals. ‘MUT’ indicates presence of the TUBB2B mutation, whereas ‘WT’ indicates the wild-type TUBB2B allele. (B) Targeted sequencing of TUBB2B coding exons and splice sites reveals a 1261G>A (E421K) heterozygous missense mutation (red arrow) that is found in all affected and not in unaffected individuals from the pedigree. (C) The E421 residue is strictly conserved across eukaryotic Tubb2b homologues (top, yellow box) and human β-tubulin isotypes (bottom, red box).
The TUBB2B c.1261G>A mutation is predicted to substitute a positively charged lysine for a negatively charged glutamic acid at residue 421 of TUBB2B (E421K). E421 is evolutionarily conserved across β-tubulin isotypes from yeast to human (Fig. 2C). Along with E410 and D417, it is located in the C-terminal H12 α-helix of β-tubulin and is crucial for kinesin–microtubule interactions (21–24) (Supplementary Material, Fig. S1). This is remarkable, as we previously identified CFEOM3 disease-causing amino acid substitutions at TUBB3 residues E410 and D417 (11).
To estimate the frequency of TUBB2B variants underlying CFEOM, we sequenced TUBB2B in 25 additional families and 80 sporadic individuals with mutation-negative CFEOM or other vertical congenital cranial dysinnervation disorder (CCDD) phenotypes, none of who were known to have PMG, and did not identify any additional disease-associated variants. Together, these data build and expand on a previous case-report that identified a de novo TUBB2B CFEOM mutation in a single individual (14), but also suggest that TUBB2B mutations are a rare cause of CFEOM.
Abnormal intracortical homotopic connectivity is associated with TUBB2B-E421K
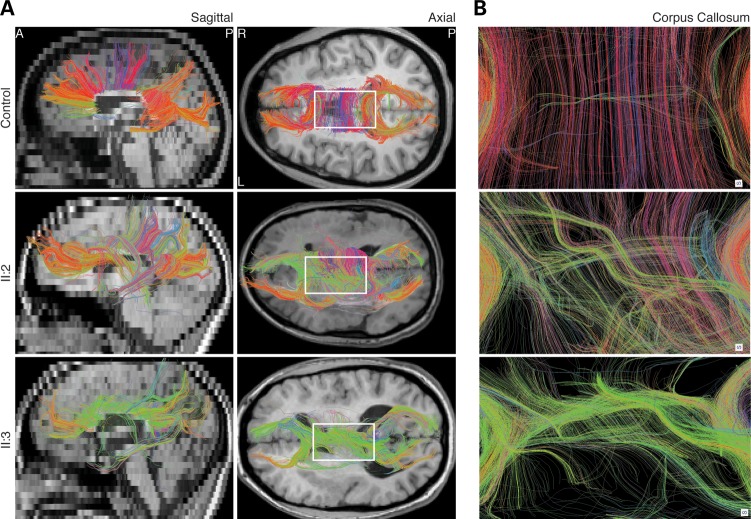
Using diffusion tensor imaging (DTI) data from both daughters, we performed diffusion tractography to interrogate the long-range coherence of the CC, the brain's major interhemispheric/commissural white matter pathway. Consistent with the structural T1 MRI scans, DTI scans showed a mild to moderate paucity of fibers in the CC body, whereas the genu was less affected. Moreover, the directionality of commissural fibers in the CC body was strikingly abnormal. While CC fibers normally display left–right directionality, visualized as red tracks, many commissural fibers in the affected individuals were labeled green, indicating a dominant anterior to posterior directionality from which we can infer a paucity of homotopic connections (Fig. 3A and B). While the overwhelming majority of control CC fibers intersected the cortical midline perpendicularly, fibers in affected individuals traversed through the midline at oblique angles (Fig. 3B). Furthermore, fiber bundles in the control were spaced in regular intervals, whereas fibers in the affected daughters clustered irregularly, sometimes forming thick bundles (Fig. 3B). This pattern of commissural fiber dysinnervation was not seen in nine genetically undefined PMG patients without CFEOM (Supplementary Material, Fig. S2), suggesting that disrupted homotopic intracortical connections associated with TUBB2B-E421K were not secondary to cortical dysplasia, and may be a primary defect.
Figure 3.
Abnormal homotopic connectivity associated with TUBB2B-E421K. Human DTI images segmenting commissural fibers of the CC. (A) Sagittal and axial views show that TUBB2B-E421K patients have a paucity of commissural fibers in the CC body, consistent with structural MRI findings. Furthermore, while many fibers in the healthy control are reconstructed with red color, many commissural fibers in TUBB2B-E421K patients are colored green, indicating a lack of normal homotopic connectivity. (B) Zoom in of the boxed region in (A) confirms that homotopic connectivity is disrupted in patients. CC, corpus callosum. Color coding: red, left–right; green, anterior–posterior; blue, superior–inferior.
TUBB2B-E421K perturbs homotopic connectivity across the midline
To investigate how the TUBB2B-E421K substitution leads to abnormal homotopic connectivity, we introduced the mutant allele into a small number of wild-type callosal projection neurons (CPN) via in utero electroporation of E15.5 mouse cortical progenitors. We co-electroporated constructs to express GFP-alone or GFP with either HA-tagged Tubb2b (Tubb2b-WT-HA) or Tubb2b-E421K (Tubb2b-E421K-HA). As a positive control, we used constructs expressing GFP and either Tubb3-WT-HA or Tubb3-E410K-HA, which causes primary axonal dysinnervation in humans (11) (Supplementary Material, Fig. S3A and B). We verified that all constructs produce functional tubulin that incorporates into neuronal microtubules (Supplementary Material, Fig. S3C).
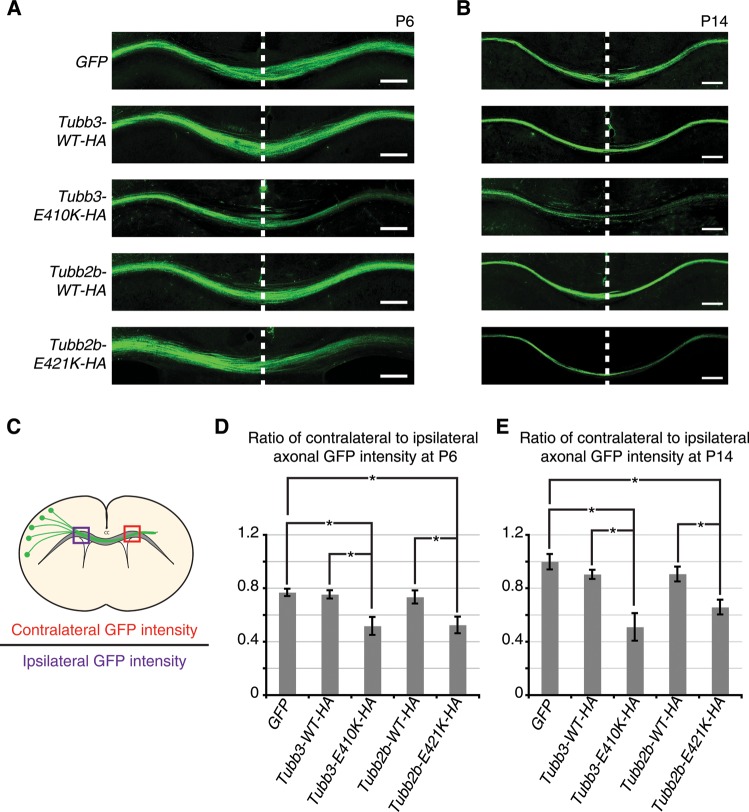
To evaluate homotopic connectivity, we examined coronal sections of electroporated brains at P6 (Fig. 4A) and P14 (Fig. 4B) and compared the ratio of axonal GFP signal intensity in the contralateral CC to that in the ipsilateral CC (Fig. 4C). Since homotopic CPN acquire symmetrical connectivity across the cortical midline, axonal GFP intensity should be roughly equivalent on each side of the CC. As expected, we observed this in brains electroporated with GFP alone, and found no significant change in brains electroporated with Tubb3-WT-HA or Tubb2b-WT-HA. However, there was a significant decrease in the ratio of contralateral to ipsilateral GFP signal in brains expressing Tubb3-E410K-HA or Tubb2b-E421K-HA, compared with their respective controls at both P6 (Fig. 4D) and P14 (Fig. 4E). Since the phenotype did not resolve by P14, it is unlikely that axon outgrowth was simply delayed.
Figure 4.
Tubb2b-E421K-HA perturbs homotopic connectivity at the cortical midline. Fluorescent micrographs and quantification of axonal GFP intensity in the corpus callosum of electroporated mouse brains. (A and B) At P6 and P14, axonal GFP intensity is reduced in the contralateral hemisphere of brains electroporated with Tubb3-E410K-HA and Tubb2b-E421K-HA compared with GFP and WT controls. (C) Schematic describes quantification method. (D and E) Quantification confirms observations of reduced contralateral GFP intensity. (D) At P6, GFP = 0.77 ± 0.05, Tubb3-WT-HA = 0.73 ± 0.04, Tubb3-E410K-HA = 0.52 ± 0.14 (P < 0.05 compared to GFP and Tubb3-WT-HA), Tubb2b-WT-HA = 0.75 ± 0.10, Tubb2b-E421K-HA = 0.52 ± 0.11 (P < 0.05 compared GFP and Tubb2b-WT-HA). (E) At P14, GFP = 1.00 ± 0.08, Tubb3-WT-HA = 0.90 ± 0.06, Tubb3-E410K-HA = 0.51 ± 0.18 (P < 0.01 compared with GFP, and P < 0.05 compared with Tubb3-WT-HA), Tubb2b-WT-HA = 0.90 ± 0.10, Tubb2b-E421K-HA = 0.66 ± 0.08 (P < 0.05 compared with GFP and Tubb2b-WT-HA). For P6 and P14, n = 4,3 (GFP), n = 3,4 (Tubb3-WT-HA), n = 5,4 (Tubb3-E410K-HA), n = 5,3 (Tubb2b-WT-HA), n = 4,4 (Tubb2b-E421K-HA). Each N represents one embryo. One-way ANOVA with a post hoc Tukey t-test was used for multiple comparisons. CC, corpus callosum. Error bars represent SEM. *P < 0.05. Scale bars: 200 µm.
The observed errors in axonal pathfinding occur in the context of a largely normal cortex (Supplementary Material, Fig. S4). Overexpression of Tubb2b-E421K-HA did not affect cell-cycle exit of mitotic neuronal progenitors (Supplementary Material, Fig. S5), and did not produce migratory abnormalities (Supplementary Material, Fig. S6). In addition, neurons expressing Tubb2b-E421K-HA acquired a midline phenotype highly similar to those expressing Tubb3-E410K-HA (Fig. 4). Together, our data demonstrate that Tubb2b-E421K can cause axonal dysinnervation that is not secondary to cortical dysplasia, neuronal production or abnormal neuronal migration. Instead, the observed axonal dysinnervation, which is consistent with findings from human neuroimaging, appears to be a primary and dominant phenotype.
Tubb2b-E421K overexpression inhibits target innervation by commissural projection neurons
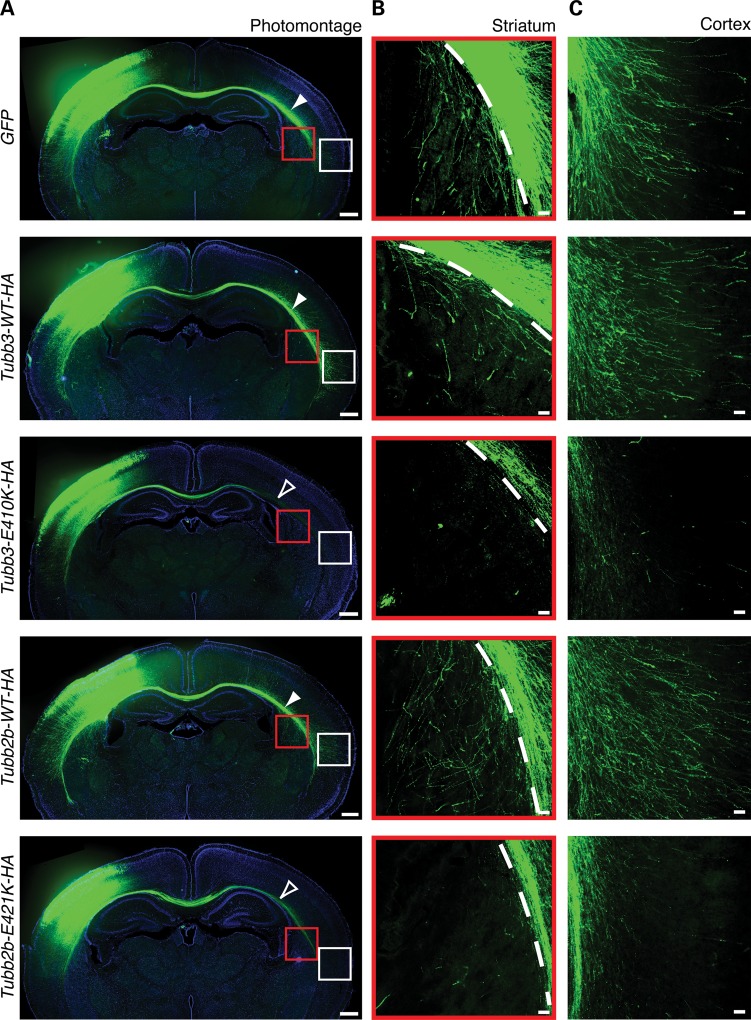
The consistency between human DTI and mouse electroporation results led us to explore other potential axonal deficits caused by the dominant effects of Tubb2b-E421K protein. As expected, CPN axons electroporated with GFP alone at E15.5 had traversed through the entirety of the CC and begun to innervate their most distant contralateral targets in the striatum and lateral neocortex at P6 (25) (Fig. 5). Similar patterns resulted from electroporation with Tubb3-WT-HA or Tubb2b-WT-HA. In contrast, introduction of Tubb3-E410K-HA or Tubb2b-E421K-HA resulted in decreased contralateral innervation of long-distance targets. Though many mutant axons traversed through the CC, they failed to innervate the contralateral striatum and lateral neocortex (Fig. 5B and C). These deficits did not resolve by P14 (Supplementary Material, Fig. S7), indicating that the mutant tubulin alleles prevent target innervation by homotopic projecting neurons. The reduced innervation may be a primary phenotype or may be secondary to abnormal midline crossing and pathfinding of CPN (Fig. 4).
Figure 5.
Tubb2b-E421K-HA perturbs CPN terminal axon extension. Fluorescent micrographs of mouse CPN electroporated at E15.5 and analyzed at P6. (A) Photomontage of coronal sections at P6 shows distal thinning of CPN fibers in brains electroporated with Tubb3-E410K-HA and Tubb2b-E421K-HA (open white arrowheads) compared with GFP and WT controls (solid white arrowheads). Some mutant axons do reach the most lateral region of the CC. (B and C) High magnification microscopy shows that CPN expressing GFP, Tubb3-WT-HA or Tubb2b-WT-HA send interstitial branches into the contralateral striatum (B, red boxes from A) and contralateral neocortex (C, white boxes from A), whereas CPN expressing Tubb3-E410K-HA and Tubb2b-E421K-HA fail to do so. n = 4 (GFP), n = 3 (Tubb3-WT-HA), n = 6 (Tubb3-E410K-HA), n = 5 (Tubb2b-WT-HA), n = 5 (Tubb2b-E421K-HA). Each n represents one embryo. CPN, callosal projection neurons; CC, corpus callosum. Scale bars: 400 µm (A), 50 µm (B and C).
Tubb2b-E421K perturbs the layer-specificity of ipsilateral cortical microcircuitry
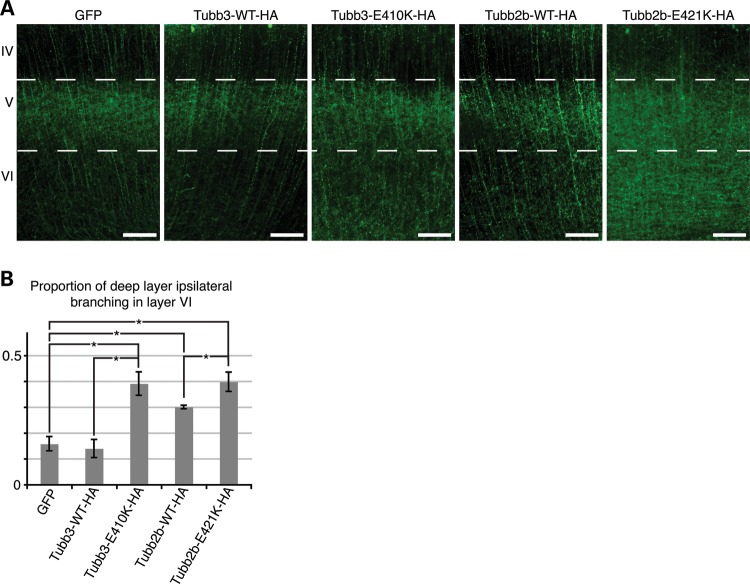
In addition to long-range contralateral connectivity, CPN axons also participate in the construction of local microcircuitry. In agreement with previous studies (26), we observed that at P6, following GFP electroporation at E15.5, the large majority of ipsilateral branching of layer II/III CPN occurred in layer V, whereas little occurred in layer VI. Electroporation of Tubb3-WT-HA produced branching patterns comparable with the GFP-alone condition, whereas electroporation of Tubb2b-WT-HA produced slight, but excessive branching in layer VI. Introduction of Tubb3-E410K-HA or Tubb2b-E421K-HA resulted in more prominent and excessive branching in layer VI, at levels that were visually greater than those of the Tubb2b-WT-HA condition (Fig. 6A). Quantitative analysis of the proportion of fluorescence intensity in layer VI versus layer V supported our observations (Fig. 6B). Together, our electroporation experiments show that overexpression of Tubb2b-E421K perturbs the accuracy of target innervation by both local and long-range projections, processes that normally proceed through the generation of interstitial branches (27,28).
Figure 6.
Tubb2b-E421K-HA alters the layer specificity of local branching. Fluorescent micrographs of CPN electroporated at E15.5 and analyzed at P6. (A) Axons of CPN expressing GFP, Tubb3-WT-HA, or Tubb2b-WT-HA have sent dense interstitial branches into layer V of the ipsilateral cortex, but few branches into the deeper layer VI. In contrast, axons of CPN expressing Tubb3-E410K-HA and Tubb2b-E421K-HA do not show similar specificity as a high density of branching is seen in layer VI, in addition to layer V. (B) Quantitative analysis of the fluorescence intensity in the deep layers (V and VI) reveals that 0.16 ± 0.03 of GFP, 0.14 ± 0.04 of Tubb3-WT-HA and 0.30 ± 0.01 of Tubb2b-WT-HA branches target layer VI. Tubb2b-WT-HA electroporation significantly increases the proportion of layer VI branching (P < 0.01 compared with GFP). There is, however, also a significant increase in the proportion of branching in layer VI in both mutant conditions, with 0.39 ± 0.05 of Tubb3-E410K-HA (P < 0.01 compared to Tubb3-WT-HA control) and 0.40 ± 0.04 of Tubb2b-E421K-HA (P < 0.05 compared with Tubb2b-WT-HA control) branching in layer VI. n = 4 (GFP), n = 3 (Tubb3-WT-HA), n = 3 (Tubb3-E410K-HA), n = 5 (Tubb2b-WT-HA), n = 4 (Tubb2b-E421K-HA). Each n represents one embryo. One-way ANOVA with the post hoc Tukey t-test was used for multiple comparisons. IV, neocortical layer IV; V, neocortical layer V; VI, neocortical layer VI. Error bars represent SEM. *P < 0.05. Scale bars: 100 µm.
TUBB2B-E421K heterodimers co-assemble into microtubule networks
The dominant axonal phenotypes associated with TUBB2B-E421K could occur through various mechanisms. The E421K mutation could block the chaperone-mediated tubulin folding pathway and lead to globally decreased levels of tubulin heterodimer formation, as reported for other TUBB2B mutations (10). Alternatively, folded mutant αβ-heterodimers could incorporate into and alter microtubule polymer function, as reported for TUBB3 mutations that cause CFEOM (11).
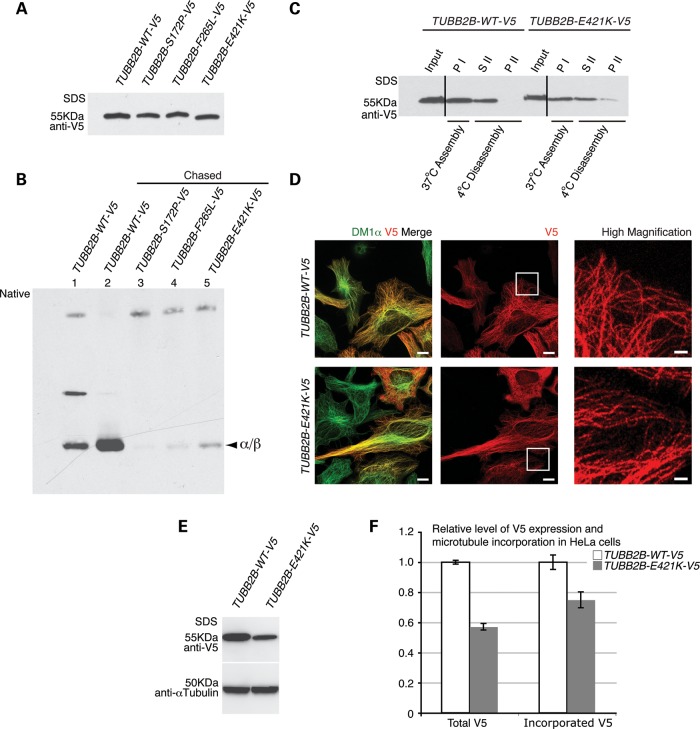
To explore these possibilities, we first tested the efficiency of TUBB2B-E421K αβ-heterodimer formation in vitro. We generated wild-type (TUBB2B-WT-V5) and mutant (TUBB2B-E421K-V5) TUBB2B expression constructs fused to the C-terminal V5 epitope tag and performed in vitro transcription and translation in reticulocyte lysates, a cell-free system containing the chaperones necessary to mediate tubulin folding. Additionally, we expressed two mutants, TUBB2B-S172P-V5 and TUBB2B-F265L-V5, previously shown to obstruct the tubulin folding process (10). TUBB2B-WT-V5, TUBB2B-S172P-V5 and TUBB2B-F265L-V5 cDNAs were transcribed and translated at equivalent levels, while the production of TUBB2B-E421K-V5 was somewhat reduced, even in the presence of protease inhibitors (Supplementary Material, Fig. S8), indicating TUBB2B-E421K is transcribed and/or translated inefficiently. For αβ-heterodimer formation and cycling assays, the production level of TUBB2B-E421K-V5 polypeptide was adjusted by increasing the concentration of template cDNA to match the WT output levels (Fig. 7A). Electrophoresis under native conditions revealed that while production of native TUBB2B-E421K-V5 αβ-heterodimer was decreased compared with that of TUBB2B-WT-V5, it was greater than S172P and F265L αβ-heterodimer production (Fig. 7B).
Figure 7.
The E421K substitution decreases the efficiency of αβ-heterodimer formation, but permits incorporation into microtubule polymers. TUBB2B-E421K-V5 αβ-heterodimer formation and incorporation were assayed by in vitro transcription and translation using rabbit reticulocyte lysates and in vivo by HeLa cell transfection. (A) WT and mutant TUBB2B-V5 plasmids show equivalent polypeptide production in vitro after DNA adjustment. (B) In vitro formation of WT and mutant TUBB2B αβ-heterodimers analyzed by native gel electrophoresis. Chasing the reaction with purified porcine tubulin releases newly synthesized subunits from folding machinery (compare α/β bands from lane 2 to lane 1). The E421K substitution leads to decreased αβ-heterodimer formation (lane 5) compared with WT (lane 2), and greater formation than S172P (lane 3) and F265L (lane 4). (C) In vitro microtubule polymerization of porcine brain tubulin and V5-tagged heterodimers. WT and E421K TUBB2B αβ-heterodimers co-polymerize with brain tubulin at comparable efficiencies. Microtubules containing WT and E421K αβ-heterodimers are cold-labile (compare SII with PI), although some insoluble E421K heterodimer remains after cold disassembly (PII). The efficiency of microtubule incorporation of TUBB2B-E421K-V5 αβ-heterodimers was 90 ± 17% relative to WT (mean ± SEM, n = 3, P > 0.05). PI, pellet following assembly; SII, soluble protein following cold-induced disassembly; PII, insoluble protein following cold-induced disassembly. Vertical lines denote the removal of irrelevant lanes. (D) Exogenously expressed TUBB2B-E421K-V5 (V5, red) co-localizes with α-tubulin (Dm1α, green), and has a filamentous appearance (high magnification, boxed regions), similar to TUBB2B-WT-V5, demonstrating efficient assembly with HeLa cell microtubules. (E) Quantitative western blot of total protein expression demonstrates lower amounts of mutant TUBB2B compared with WT. (F) Fluorescence analysis, after soluble protein extraction, demonstrates roughly equivalent efficiencies of incorporation between WT and mutant proteins, when adjusted for total protein expression in (E). Total TUBB2B-E421K-V5 protein expression is reduced by 43% compared with WT (WT = 1.0 ± 0.01, E421K = 0.57 ± 0.02, P < 0.0001; n = 3, n = one transfected culture), while the amount of incorporated TUBB2B-E421K-V5 protein is reduced by 25% (WT = 1.0 ± 0.05, E421K = 0.75 ± 0.05, P < 0.0005; n = 111 WT and 92 E421K, n = one cell). Scale bars: 10 µm (×63; left and middle panels), 2 µm (×100; high magnification, right panel).
To assess whether folded E421K αβ-heterodimers could assemble into microtubule polymers, the TUBB2B-WT-V5 and TUBB2B-E421K-V5 αβ-heterodimers formed in reticulocyte lysates were co-polymerized with native brain tubulin. The efficiency of TUBB2B-E421K-V5 incorporation into the polymer lattice, scored by one round of microtubule polymerization and depolymerization, was similar, though slightly reduced, when compared with the level of TUBB2B-WT-V5 incorporation (Fig. 7C). We evaluated the co-polymerization of TUBB2B-E421K in vivo by transfecting HeLa cells with TUBB2B-WT-V5 or TUBB2B-E421K-V5 expression constructs, and extracting soluble tubulin with methanol fixation. Consistent with the in vitro polymerization assay, immunostaining against TUBB2B-WT-V5 and TUBB2B-E421K-V5 revealed comparable levels of E421K and WT TUBB2B incorporation into the microtubule lattice, when adjusted for total V5 expression (Fig. 7D). Thus, although the E421K substitution reduces the efficiency of αβ-heterodimer formation, the successfully folded polypeptide assembles into microtubule structures both in vitro and in vivo at levels that are quantitatively similar to those of TUBB2B-WT αβ-heterodimers.
β-Tubulin E421K substitution alters microtubule dynamics in vivo
To investigate how E421K heterodimers alter microtubule function, we utilized budding yeast, which provides several advantages for exploring the functional consequences of human tubulin mutations. First, yeast permits the rapid insertion of site-directed mutations at the endogenous tubulin locus under the native promoter and regulatory elements. Such mutations can then be combined with fluorescently tagged cytoskeletal proteins. This allows for phenotypic examination at a level of experimental control not easily attained in other systems, in which transgenic or over-expression approaches can result in non-physiological paradigms. Second, individual fluorescently tagged yeast microtubules and kinesin motors are easily discerned during time-lapse microscopy, which allows one to quantitatively assess parameters of microtubule dynamics and kinesin localization. Third, CFEOM-causing mutations have now been identified in multiple β-tubulin isotypes, each of which has a different level and pattern of expression. Yeast has a single β-tubulin isotype (Tub2p), which shares high sequence conservation with mammalian β-tubulin isotypes (Fig. 2C). Thus, modeling disease-causing mutations in yeast helps control for these differences and should ultimately help to discriminate between residue-specific and isotype-specific phenotypes.
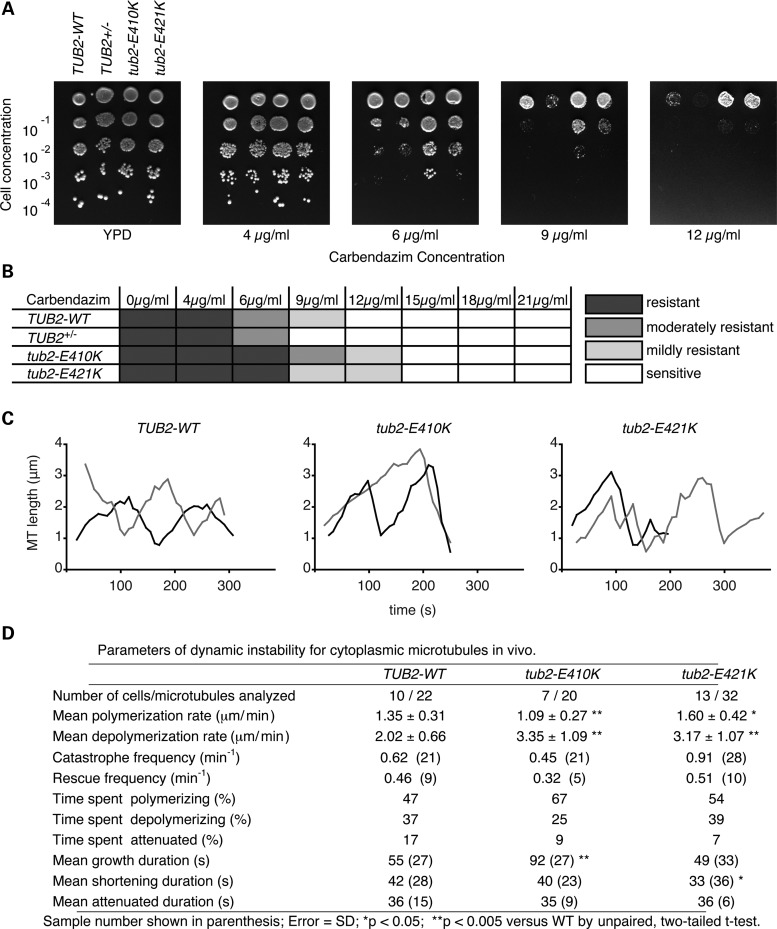
For these reasons, we introduced the E421K corresponding mutation into budding yeast. Heterozygous diploids were recovered at the expected frequency and displayed no growth defects when cultured in nutrient-rich media, while haploid tub2-E410K and tub2-E421K spores were inviable, demonstrating compromised microtubule function (Supplementary Material, Fig. S9). Since CFEOM-causing substitutions in TUBB3 compromise microtubule dynamics (11), we grew diploid tub2-E421K heterozygous cells on media containing increasing concentrations of the microtubule-destabilizing drug carbendazim to assay for changes in microtubule stability. Compared with the WT strain, the tub2-E421K strain showed an increased resistance to carbendazim (Fig. 8A and B). Thus the E421K substitution, similar to E410K and other CFEOM-causing substitutions (11), stabilizes microtubules in vivo. In contrast, Tub2p haploinsufficiency substantially increased carbendazim sensitivity (Fig. 8A and B). Importantly, these results demonstrate that E421K does not result in a loss of function in yeast, and affects microtubule dynamics in a dominant manner.
Figure 8.
The E421K substitution alters microtubule dynamics. (A) A representative carbendazim sensitivity assay. Diploid cells were serially diluted and plated on rich YPD media containing increasing concentrations of carbendazim, a microtubule destabilizing agent. The Tub2p haploinsufficient cells (TUB2+/−) were super-sensitive to carbendazim. In contrast, both heterozygous tub2-E410K and heterozygous tub2-E421K cells displayed increased resistance to carbendazim compared with WT cells. (B) Chart depicting a summary of more than three independent carbendazim assays with concentration ranging from 0 –21 μg/ml. Shaded boxes depict the degree of carbendazim resistance. (C) Life-time history plots of two representative individual microtubules from diploid WT (left), heterozygous tub2-E410K (center) or heterozygous tub2-E421K (right) cells in G1. (D) Parameters of dynamic instability determined for each strain. Notably, tub2-E421K microtubules show increased rates of polymerization, depolymerization, and reduced time spent in attenuation. Error given as standard deviation. Number of events is in parentheses. MT, microtubule. *P < 0.05, **P < 0.005 versus WT by unpaired Student's t-test.
To determine how E421K alters microtubule stability, we generated WT and mutant β-tubulin strains expressing GFP-α-tubulin (GFP-TUB1) and used time-lapse microscopy to observe the dynamic behavior of astral microtubules in G1 cells (Supplementary Material, Movie S1). Astral microtubules in cells harboring the E421K substitution spent less time in the attenuated state and had significantly increased rates of polymerization and depolymerization when compared with microtubules in WT cells. However, the time that individual E421K microtubules spent growing and shortening remained largely unaffected (Fig. 8C and D). Thus, the E421K substitution alters specific aspects of microtubule dynamic instability in a manner that may increase overall polymer stability.
The E421K substitution in β-tubulin can disrupt kinesin–microtubule interactions in yeast
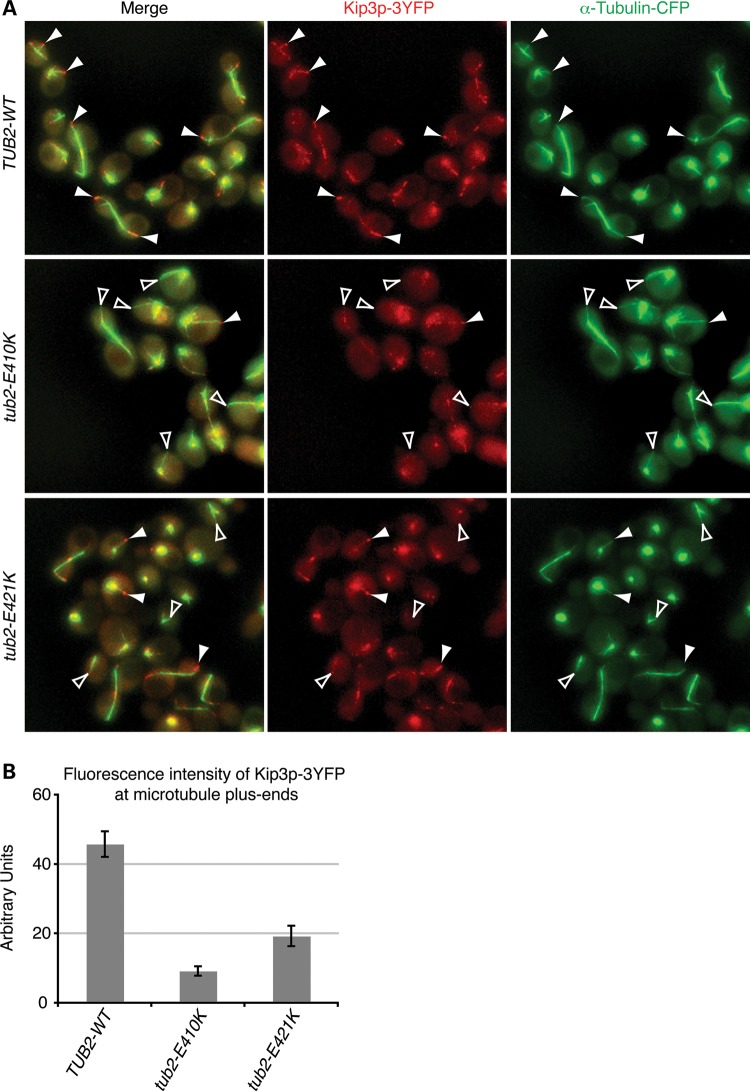
Yeast cells with tub2p-E421K exhibited altered microtubule dynamics, including faster depolymerization rates that are reminiscent of cells lacking Kip3p, a plus-end-directed motor protein that regulates microtubule dynamics (29,30). Furthermore, E421 has previously been shown to be critical for kinesin–microtubule interactions in vitro (23,24). We therefore examined whether the E421K substitution affects Kip3p localization in vivo by generating WT and heterozygous mutant cells that co-express equivalent amounts of Kip3p fused to three tandem copies of YFP (Kip3p-3YFP) (Supplementary Material, Fig. S10). In WT cells, Kip3p-3YFP formed bright foci at the plus ends of growing microtubules and lower intensity spots along the microtubule lattice (Fig. 9A. Compared with WT signal, Kip3p-3YFP signal intensity was significantly reduced on the tips of E421K harboring microtubules, and severely reduced on the tips of E410K harboring microtubules (Fig. 9A). Quantitative analysis of foci intensity supported our observations (Fig. 9B). We next generated WT and heterozygous mutant cells that co-express equivalent amounts of another yeast cytoplasmic kinesin, Kip2p, fused to three tandem copies of YFP (Kip2p-3YFP). We found that Kip2p-3YFP localization was not significantly reduced on microtubule plus-ends harboring the E421K substitution (Supplementary Material, Fig. S11).
Figure 9.
E421K reduces the localization of kinesin Kip3p at microtubule plus-ends. (A) Representative Z-series maximum projections showing fluorescently labeled Kip3p (red) and α-tubulin (green) in live diploid WT, heterozygous tub2-E410K and heterozygous tub2-E421K yeast cells. Kip3p-3YFP forms bright foci at the plus-ends of most WT astral microtubules, but these bright foci are rarely found at the plus-ends of mutant astral microtubules (solid white arrowheads). Similar to microtubules in tub2-E410K cells, most astral microtubule plus-ends in tub2-E421K cells had a significant reduction in Kip3p-3YFP localization (open white arrowheads). Signal intensities were adjusted equally in both channels for all strains. (B) Quantification of Kip3p-3YFP levels at the plus-ends of microtubules in cells containing Tub2, tub2p-E410K and tub2p-E421K. Localization of Kip3p intensity was reduced by 58% in tub2-E421K cells and by 81% in tub2-E410K cells. 60–150 microtubules from three to four clones on two separate days each were imaged for each condition. n≥ 6 for all conditions. n represents the averaged values for each clone from 1 day. Error represented as SEM in graphs. P < 0.001 versus WT by unpaired Student's t-test.
Since Kip3p is a microtubule depolymerase (29,30), it is possible that microtubule stabilization by E421K results in its diminished localization. To address this question, we measured Kip3p localization in cells harboring the C354S substitution, previously shown to directly stabilize microtubules both in vivo and in vitro (31). In contrast to E421K and E410K, the C354S substitution produced a nearly 3-fold increase in Kip3p-3YFP localization at the microtubule tips (Supplementary Material, Fig. S12). Thus, reduced Kip3p-3YFP localization on E421K microtubules is not a result of increased microtubule stability. Together, our data show that E421K can alter kinesin localization and microtubule dynamics in a dominant manner and result in axonal dysinnervation in both central and peripheral axon tracts.
DISCUSSION
We have determined that a heterozygous TUBB2B-E421K amino acid substitution underlies a syndrome characterized by CFEOM and intellectual disability. Using MRI with DTI, we found underlying PMG, asymmetric basal ganglia dysmorphisms and a paucity of homotopic connections. Overexpression of TUBB2B-E421K in mouse CPN prevents normal development of homotopic connectivity and target innervation, without perturbing cell migration. Yeast microtubules harboring the E421K substitution had increased polymerization and depolymerization rates, and reduced localization of Kip3p at their plus ends. Together, these data demonstrate that mutations in TUBB2B can induce axonal dysinnervation as a primary phenotype, and provide further insight into the genetic, molecular and cellular basis underlying the spectrum of tubulin-related neurological phenotypes.
Tubb2b dysfunction and axonal dysinnervation
Two major themes regarding axon development emerge from our studies. First is the apparent primacy of axonal dysinnervation in a TUBB2B disorder. All previously identified TUBB2B mutations result in severe cortical dysplasia (10,12,14), raising the possibility that associated white matter abnormalities are secondary phenotypes. Here, we present several lines of evidence that support TUBB2B-E421K is a cause of primary axonal dysinnervation. First, all three affected family members have CFEOM, of which all dominant forms are known to result from primary axonal dysinnervation (11,32). Second, DTI analysis showed that the affected daughters developed an aberrant pattern of commissural fiber trajectories that is not common for other PMG patients we examined. Third, we demonstrated by electroporation that Tubb2b-E421K can induce axonal dysinnervation with no discernable neuronal production or migratory defects. Finally, introduction of E421K into yeast β-tubulin produced molecular phenotypes that resemble the effects of E410K, a substitution in TUBB3 known to cause primary axonal dysinnervation.
The second emergent theme is the developmental selectivity of Tubb2b-E421K associated phenotypes. Overexpression of Tubb2b-E421K does not inhibit CPN axons from crossing the midline, but does disrupt their development as they traverse past the midline. Moreover, despite relatively normal growth of the primary axon, E421K-expressing neurons fail to generate interstitial branches appropriately; they send promiscuous branches into layer VI of the ipsilateral cortex, whereas their axons fail to innervate contralateral target regions. Both of these processes proceed through the development of interstitial branches (27,28). One explanation for these observed phenotypes is that E421K selectively alters a restricted set of molecular pathways associated with post-midline guidance, such as Wnt5a or calcium-dependent kinase 1α signaling (33–37). Since many neurological disorders are associated with selective phenotypes despite mutations in broadly expressed genes, understanding the relationship between Tubb2b-E421K and the selective vulnerability of certain developmental events and/or cell types is a broadly important and clinically significant area of investigation.
Dominant and potentially haploinsufficient contributions of TUBB2B-E421K
In agreement with previous reports (11,32), our findings support a dominant etiology for CFEOM. Genetically, CFEOM segregates with the E421K substitution, but not with most other reported TUBB2B substitutions. Functionally, yeast expressing the tub2-E421K allele have increased microtubule stability, the phenotypic opposite of yeast haploinsufficient for the TUB2 allele. Furthermore, overexpression of Tubb2b-E421K in wild-type CPN recapitulates human axonal phenotypes.
It remains less clear whether TUBB2B mutations cause PMG through dominant or haploinsufficient effects. Previous functional studies support neuronal TUBB2B haploinsufficiency because mutant proteins fold and incorporate poorly. Furthermore, knockdown of Tubb2b in rat cortical neurons induces a migratory defect (10), providing a strong link between TUBB2B haploinsufficiency and PMG. We show that the E421K substitution reduces TUBB2B αβ-heterodimer production, while it does not significantly alter polymer incorporation. Thus, E421K may cause PMG as a result of TUBB2B haploinsufficiency. TUBB2B is expressed, however, by both post-mitotic neurons and mitotic progenitors, including radial glia (38). Radial glia guide cortical neuron migration (39–45) and their maldevelopment are associated with PMG (46–48). Thus, an alternative possibility is that TUBB2B-related PMG is the result of defective radial glia, in which microtubule function is dominantly altered. Neuropathological analysis of a fetus that harbored the TUBB2B-S172P substitution showed that radial glia were disorganized and lacked apical–basal orientation (10,19). Furthermore, kinesin-binding site substitutions D417N and E421K in TUBB2B, which is expressed in radial glia, cause PMG, while kinesin-binding site substitutions D417N/H and E410K in TUBB3, which is not expressed in radial glia, do not cause PMG (11,12). Thus far, all functional studies of TUBB2B mutations have used electroporation to model the human phenotypes, and radial glial phenotypes are not easily discerned by this approach (49). The generation of knock-in animal models harboring Tubb2b disease mutations will help resolve the cell type and molecular etiology of this phenotype.
Differences between TUBB2B-CFEOM and TUBB3-CFEOM syndromes
There are apparent differences between CFEOM-causing mutations in β-tubulin isotypes TUBB2B and TUBB3. First, the frequency of CFEOM-causing mutations appears to be much higher in TUBB3 than in TUBB2B. We have reported 29 CFEOM probands with TUBB3 mutations (11), but only one with a TUBB2B mutation. Second, the lone TUBB2B mutation in our CCDD cohort associates with PMG, whereas TUBB3 mutations do not cause cortical malformations, but can associate with additional cranial nerve dysfunction, progressive axonal polyneuropathy and central white matter tract abnormalities (11).
These apparent differences may result from distinct spatiotemporal expression patterns of TUBB2B and TUBB3. The expression of TUBB2B, but not TUBB3, in mitotic progenitors, including radial glia (4,38,50), may account for the uniform prevalence of PMG associated with TUBB2B but not TUBB3 mutations. Still, mutations in TUBB3 can sometimes result in cortical malformations, and even PMG, though these mutations do not simultaneously cause CFEOM (13). Thus these mutations may be mechanistically different than CFEOM-causing mutations in TUBB2B and TUBB3. Isotype expression differences may also render TUBB2B mutations to be generally more deleterious than TUBB3 mutations, leading to a higher incidence of embryonic lethality and a paucity of TUBB2B mutations in our CFEOM cohort. In support of this, 6 of the 14 TUBB3 mutations have been familial (11,13), while this is the first of nine reported TUBB2B mutations to be so. Finally, the association of TUBB3-D417N/H and -E410K substitutions with peripheral sensorimotor axonal polyneuropathy may reflect the persistently elevated expression of TUBB3, but not TUBB2B, in the peripheral nervous system (51).
TUBB2B-CFEOM and TUBB3-CFEOM phenotypes may also differ due to intrinsic differences in isotype function. Though β-tubulin isotypes show over 90% conservation in amino acid sequence, key differences exist, especially in the C-terminal tail (3,52) which is known to differentially regulate MAP binding (53–56). Microtubules with different isotype compositions also exhibit different dynamic profiles (57). Interestingly, it was recently reported that a de novo D417N substitution in TUBB2B resulted in symmetric PMG, but not CFEOM (12). This finding might suggest that the conserved D417 residue has divergent functions in distinct isotypes. However, the TUBB3-D417N substitution results in a relatively mild phenotype in which both CFEOM and a progressive axonal neuropathy have variable expressivity (11). Thus, additional individuals with TUBB2B-D417N substitutions will need to be identified in order to determine whether this substitution can also result in CFEOM.
Molecular phenotypes and CFEOM pathogenesis
By defining an allelic series of CFEOM-causing mutations in TUBB3 and now TUBB2B, strong correlations emerge between molecular phenotypes and the disease. All nine CFEOM mutations in our cohort, from two different human isotypes, produce resistance to the microtubule destabilizing drug carbendazim, or its precursor benomyl, and all alter the dynamic growth and shortening of microtubules. This is especially striking since alanine-scanning analysis of yeast Tub1p only produced 6 of 53 mutations that confer any resistance to benomyl, whereas 37 mutations resulted in benomyl sensitivity (58). If microtubule dynamics were not related to CFEOM pathogenesis, it is unlikely that 100% of CFEOM mutations would produce benomyl resistance. Thus, our studies currently support a model in which altered microtubule dynamics underlie cranial nerve misguidance.
Structurally, CFEOM-causing substitutions alter residues predicted to directly interact with MAP and motor proteins, or reside in regions at the interface between αβ-heterodimers (11,14,15). In theory, these substitutions could alter microtubule dynamics by directly interfering with tubulin–tubulin interactions, or by changing the activity of proteins that regulate the growth and shortening of microtubules, such as kinesins (59). Previous analysis of cytoplasmic kinesins in the yeast model system revealed that five of eight CFEOM mutations in TUBB3 perturbed the normal localization of the regulatory kinesin Kip3p, which depolymerizes microtubule plus ends (29,30), whereas Kip2p localization was less severely affected. This trend suggests that some CFEOM mutations alter regulatory molecules, thus leading to pathogenic microtubule dynamics. In this study, we model a CFEOM mutation from a second human tubulin isotype, TUBB2B, and again find that Kip3p localization is more severely affected than that of Kip2p. The striking consistency of molecular phenotypes among CFEOM mutations argues that the emergent biochemical properties are central to the molecular etiology of CFEOM. Future in vitro biophysical and biochemical studies, combined with in vivo modeling in neurons will be necessary to determine the precise molecular cascades that lead to cranial nerve misguidance. These studies will also likely provide important insights into the role of individual β-tubulin isotypes and specific residues during normal development.
MATERIALS AND METHODS
Detailed methods can be found in the Supplementary Methods available online.
Clinical and genetic studies
A family with inherited CFEOM and PMG was enrolled in an ongoing genetic study of CCDD. The study was conducted according to Declaration of Helsinki principles, approved by the Boston Children's Hospital Institutional Review Board, and written informed consent was obtained from each participant and/or his or her guardian. Participants underwent medical examinations and/or provided medical records. Linkage and mutation analysis were performed as previously described (11,17).
Magnetic resonance imaging
Diagnostic structural (I:2, II:2, II:3) and diffusion (II:2, II:3) MRI scans were reviewed. A structural and diffusion MRI of an age-matched female control was acquired at Boston Children's Hospital following appropriate Institutional Review Board approval and informed consent. CC fibers were segmented from DTI data using the Diffusion Toolkit developed at the Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital (Ruopeng Wang, Van J. Wedeen, TrackVis.org; http://trackvis.org). The color coding of fibers is based on a standard RGB code (red: left–right; green: front–back; blue: superior–inferior).
In utero electroporation and histology
All animal experiments were carried out in accordance with protocols approved by the Boston Children's Hospital Institutional Animal Care and Use Committee and with institutional and federal guidelines. Electroporation, histology and immunocytochemistry were performed as previously described (60–62). Briefly, DNA (1 mg/ml for each plasmid) was pressure injected into the lateral ventricle of embryos of timed pregnant CD1 females, and five 50 ms 45 mV pulses at 1 s intervals were applied. Brains were fixed at appropriate ages and cut into 50 µm sections. Primary antibody was applied overnight at 4°C and secondary antibody was applied for 3h at room temperature the next day.
Immunofluorescence analysis
Midline crossing was quantified as the ratio of contralateral to ipsilateral axonal GFP intensity in the CC. Ipsilateral interstitial branching was quantified the ratio of layer VI to layer V branching. Image intensities were acquired using ImageJ software (NIH), and corrected for background intensity.
In vitro heterodimer formation and cycling
The cDNA encoding human TUBB2B was subcloned into pcDNA3.2/V5 DEST (Invitrogen), and disease-associated mutations were introduced using site-directed mutagenesis (Agilent Technologies). 125 ng of WT, S172P and F265L, and 188.5ng of E421K plasmid DNA were incubated with 25 µl of reticulocyte lysate, with or without protease inhibitors (Roche) for heterodimer folding and cycling assays. All subsequent steps were performed as previously described (11). Briefly, following a 90-min incubation at 30°C, samples were chased with purified porcine tubulin, MgCl2 and GTP, and a small aliquot was analyzed by native PAGE. The remaining sample was cycled with porcine tubulin, and analyzed by SDS–PAGE. The relative cycling efficiency of mutant tubulin was quantified using the ImageJ densitometry software and normalized against the WT.
HeLa cell incorporation assay
Transfected HeLa cells were fixed with methanol to extract soluble tubulin, and stained with fluorescent antibodies, as previously described (11). Total V5 expression was determined by collecting total protein lysate and analyzing by standard western blotting procedures, and levels of V5 incorporation were determined by quantitative immunofluorescence of average pixel intensity per cell.
Yeast strains
Mutant yeast strains harboring tub2 mutations corresponding to human TUBB2B and TUBB3 CFEOM mutations, and expressing GFP-Tub1 or CFP-Tub1 and Kip3p-3YFP or Kip2p-3YFP were generated as previously described (11,29,63).
Microtubule stability assay
WT and mutant tub2 yeast strains were grown on increasing concentrations of carbendazim and scored for resistance, as previously described (11).
Yeast in vivo microtubule dynamics
Microtubules were visualized in vivo by time-lapse imaging of WT and mutant tub2 strains expressing GFP-Tub1, as previously described (11).
Kip3p and Kip2p plus-end localization
Localization of Kip3p-3YFP or Kip2p-3YFP to plus ends of astral microtubules was assessed in WT and mutant tub2 strains expressing CFP-Tub1p and Kip3p-3YFP or Kip2p-3YFP using live cell confocal microscopy as previously described (11,29).
SUPPLEMENTARY MATERIAL
FUNDING
Supported by NIH R01EY12498 and IDDRC HD018655 (E.C.E.) and by NIH R01GM094313 and Research Grant No. 5-FY12-80 from the March of Dimes Foundation (M.L.G.). S.C. is funded by the HHMI Medical Fellowship and Harvard Medical School. E.C.E. is a Howard Hughes Medical Institute Investigator.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the study participants for their generosity and time, Kathryn North, Steve Vuvic, Jennifer Lorenzo and Nathalie Pride for providing clinical data, Kiho Im and Michael Paladino for providing MRI data, Matt Gregas for statistical consultation, Carlos Lois for critical reagents and members of the Engle Lab for critical reading of the manuscript.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Little M., Seehaus T. Comparative analysis of tubulin sequences. Comp. Biochem. Physiol. B. 1988;90:655–670. doi: 10.1016/0305-0491(88)90320-3. [DOI] [PubMed] [Google Scholar]
- 2.Luduena R.F. Are tubulin isotypes functionally significant. Mol. Biol. Cell. 1993;4:445–457. doi: 10.1091/mbc.4.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan K.F., Cleveland D.W. Identification of conserved isotype-defining variable region sequences for four vertebrate beta tubulin polypeptide classes. PNAS. 1986;83:4327–4331. doi: 10.1073/pnas.83.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan K.F., Havercroft J.C., Machlin P.S., Cleveland D.W. Sequence and expression of the chicken beta 5- and beta 4-tubulin genes define a pair of divergent beta-tubulins with complementary patterns of expression. Mol. Cell Biol. 1986;6:4409–4418. doi: 10.1128/mcb.6.12.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaltschmidt B., Glatzer K.H., Michiels F., Leiss D., Renkawitz-Pohl R. During Drosophila spermatogenesis beta 1, beta 2 and beta 3 tubulin isotypes are cell-type specifically expressed but have the potential to coassemble into the axoneme of transgenic flies. Eur. J. Cell. Biol. 1991;54:110–120. [PubMed] [Google Scholar]
- 6.Joshi H.C., Cleveland D.W. Differential utilization of beta-tubulin isotypes in differentiating neurites. J. Cell Biol. 1989;109:663–673. doi: 10.1083/jcb.109.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgoyne R.D., Cambray-Deakin M.A., Lewis S.A., Sarkar S., Cowan N.J. Differential distribution of beta-tubulin isotypes in cerebellum. EMBO. J. 1988;7:2311–2319. doi: 10.1002/j.1460-2075.1988.tb03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleveland D.W. The multitubulin hypothesis revisited: what have we learned? J. Cell Biol. 1987;104:381–383. doi: 10.1083/jcb.104.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tischfield M.A., Engle E.C. Distinct alpha- and beta-tubulin isotypes are required for the positioning, differentiation and survival of neurons: new support for the ‘multi-tubulin’ hypothesis. Biosci. Rep. 2010;30:319–330. doi: 10.1042/BSR20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaglin X.H., Poirier K., Saillour Y., Buhler E., Tian G., Bahi-Buisson N., Fallet-Bianco C., Phan-Dinh-Tuy F., Kong X.P., Bomont P., et al. Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nature Genet. 2009;41:746–752. doi: 10.1038/ng.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tischfield M.A., Baris H.N., Wu C., Rudolph G., Van Maldergem L., He W., Chan W.M., Andrews C., Demer J.L., Robertson R.L., et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerrini R., Mei D., Cordelli D.M., Pucatti D., Franzoni E., Parrini E. Symmetric polymicrogyria and pachygyria associated with TUBB2B gene mutations. Eur. J. Hum. Genet. 2012;20:995–998. doi: 10.1038/ejhg.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poirier K., Saillour Y., Bahi-Buisson N., Jaglin X.H., Fallet-Bianco C., Nabbout R., Castelnau-Ptakhine L., Roubertie A., Attie-Bitach T., Desguerre I., et al. Mutations in the neuronal ss-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Hum. Mol. Gen. 2010;19:4462–4473. doi: 10.1093/hmg/ddq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romaniello R., Tonelli A., Arrigoni F., Baschirotto C., Triulzi F., Bresolin N., Bassi M.T., Borgatti R. A novel mutation in the beta-tubulin gene TUBB2B associated with complex malformation of cortical development and deficits in axonal guidance. Dev. Med. Child Neurol. 2012;54:765–769. doi: 10.1111/j.1469-8749.2012.04316.x. [DOI] [PubMed] [Google Scholar]
- 15.Tischfield M.A., Cederquist G.Y., Gupta M.L., Jr, Engle E.C. Phenotypic spectrum of the tubulin-related disorders and functional implications of disease-causing mutations. Curr. Opin. Genet. Dev. 2011;21:286–294. doi: 10.1016/j.gde.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demer J.L., Clark R., Tischfield M.A., Engle E.C. Magnetic Resonance Imaging Evidence Of an Asymmetrical Endophenotype in Congenital Fibrosis of Extraocular Muscles Type 3 Resulting from TUBB3 Mutations. Invest. Ophthalmol. Vis. Sci. 2010;51:4600–4611. doi: 10.1167/iovs.10-5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty E.J., Macy M.E., Wang S.M., Dykeman C.P., Melanson M.T., Engle E.C. CFEOM3: a new extraocular congenital fibrosis syndrome that maps to 16q24.2-q24.3. Invest. Ophthalmol. Vis. Sci. 1999;40:1687–1694. [PubMed] [Google Scholar]
- 18.Mackey D.A., Chan W.M., Chan C., Gillies W.E., Brooks A.M., O'Day J., Engle E.C. Congenital fibrosis of the vertically acting extraocular muscles maps to the FEOM3 locus. Hum. Genet. 2002;110:510–512. doi: 10.1007/s00439-002-0707-5. [DOI] [PubMed] [Google Scholar]
- 19.Jaglin X.H., Chelly J. Tubulin-related cortical dysgeneses: microtubule dysfunction underlying neuronal migration defects. Trends Genet. 2009;25:555–566. doi: 10.1016/j.tig.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Flaherty M.P., Grattan-Smith P., Steinberg A., Jamieson R., Engle E.C. Congenital fibrosis of the extraocular muscles associated with cortical dysplasia and maldevelopment of the basal ganglia. Ophthalmology. 2001;108:1313–1322. doi: 10.1016/s0161-6420(01)00582-6. [DOI] [PubMed] [Google Scholar]
- 21.Li H., DeRosier D.J., Nicholson W.V., Nogales E., Downing K.H. Microtubule structure at 8 A resolution. Structure. 2002;10:1317–1328. doi: 10.1016/s0969-2126(02)00827-4. [DOI] [PubMed] [Google Scholar]
- 22.Lowe J., Li H., Downing K.H., Nogales E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J. Mol. Biol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 23.Uchimura S., Oguchi Y., Hachikubo Y., Ishiwata S., Muto E. Key residues on microtubule responsible for activation of kinesin ATPase. EMBO. J. 2010;29:1167–1175. doi: 10.1038/emboj.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchimura S., Oguchi Y., Katsuki M., Usui T., Osada H., Nikawa J., Ishiwata S., Muto E. Identification of a strong binding site for kinesin on the microtubule using mutant analysis of tubulin. EMBO. J. 2006;25:5932–5941. doi: 10.1038/sj.emboj.7601442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C.L., Zhang L., Zhou Y., Zhou J., Yang X.J., Duan S.M., Xiong Z.Q., Ding Y.Q. Activity-dependent development of callosal projections in the somatosensory cortex. J. Neurosci. 2007;27:11334–11342. doi: 10.1523/JNEUROSCI.3380-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adesnik H., Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature. 2010;464:1155–1160. doi: 10.1038/nature08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalil K., Li L., Hutchins B.I. Signaling mechanisms in cortical axon growth, guidance, and branching. Front. Neuroanat. 2011;5:62. doi: 10.3389/fnana.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halloran M.C., Kalil K. Dynamic behaviors of growth cones extending in the corpus callosum of living cortical brain slices observed with video microscopy. J. Neurosci. 1994;14:2161–2177. doi: 10.1523/JNEUROSCI.14-04-02161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta M.L., Jr, Carvalho P., Roof D.M., Pellman D. Plus end-specific depolymerase activity of Kip3, a kinesin-8 protein, explains its role in positioning the yeast mitotic spindle. Nat. Cell. Biol. 2006;8:913–923. doi: 10.1038/ncb1457. [DOI] [PubMed] [Google Scholar]
- 30.Varga V., Helenius J., Tanaka K., Hyman A.A., Tanaka T.U., Howard J. Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nat. Cell. Biol. 2006;8:957–962. doi: 10.1038/ncb1462. [DOI] [PubMed] [Google Scholar]
- 31.Gupta M.L., Jr, Bode C.J., Thrower D.A., Pearson C.G., Suprenant K.A., Bloom K.S., Himes R.H. beta-Tubulin C354 mutations that severely decrease microtubule dynamics do not prevent nuclear migration in yeast. Mol. Biol. Cell. 2002;13:2919–2932. doi: 10.1091/mbc.E02-01-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada K., Andrews C., Chan W.M., McKeown C.A., Magli A., De Berardinis T., Loewenstein A., Lazar M., O'Keefe M., Letson R., et al. Heterozygous mutations of the kinesin KIF21A in congenital fibrosis of the extraocular muscles type 1 (CFEOM1) Nature Genet. 2003;35:318–321. doi: 10.1038/ng1261. [DOI] [PubMed] [Google Scholar]
- 33.Keeble T.R., Halford M.M., Seaman C., Kee N., Macheda M., Anderson R.B., Stacker S.A., Cooper H.M. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J. Neurosci. 2006;26:5840–5848. doi: 10.1523/JNEUROSCI.1175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutchins B.I., Li L., Kalil K. Wnt/calcium signaling mediates axon growth and guidance in the developing corpus callosum. Dev. Neurobiol. 2011;71:269–283. doi: 10.1002/dneu.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalil K., Dent E.W. Touch and go: guidance cues signal to the growth cone cytoskeleton. Curr. Opin. Neurobiol. 2005;15:521–526. doi: 10.1016/j.conb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Li L., Hutchins B.I., Kalil K. Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J. Neurosci. 2009;29:5873–5883. doi: 10.1523/JNEUROSCI.0183-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ageta-Ishihara N., Takemoto-Kimura S., Nonaka M., Adachi-Morishima A., Suzuki K., Kamijo S., Fujii H., Mano T., Blaeser F., Chatila T.A., et al. Control of cortical axon elongation by a GABA-driven Ca2+/calmodulin-dependent protein kinase cascade. J. Neurosci. 2009;29:13720–13729. doi: 10.1523/JNEUROSCI.3018-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura Y., Yamamoto M., Oda E., Yamamoto A., Kanemura Y., Hara M., Suzuki A., Yamasaki M., Okano H. Expression of tubulin beta II in neural stem/progenitor cells and radial fibers during human fetal brain development. Lab. Invest. 2003;83:479–489. doi: 10.1097/01.lab.0000063930.75913.b3. [DOI] [PubMed] [Google Scholar]
- 39.Rakic P. Principles of neural cell migration. Experientia. 1990;46:882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- 40.Noctor S.C., Flint A.C., Weissman T.A., Dammerman R.S., Kriegstein A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 41.Kriegstein A.R., Noctor S.C. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Noctor S.C., Martinez-Cerdeno V., Ivic L., Kriegstein A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nature Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 43.Anton E.S., Cameron R.S., Rakic P. Role of neuron-glial junctional domain proteins in the maintenance and termination of neuronal migration across the embryonic cerebral wall. J. Neurosci. 1996;16:2283–2293. doi: 10.1523/JNEUROSCI.16-07-02283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anton E.S., Kreidberg J.A., Rakic P. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- 45.Gongidi V., Ring C., Moody M., Brekken R., Sage E.H., Rakic P., Anton E.S. SPARC-like 1 regulates the terminal phase of radial glia-guided migration in the cerebral cortex. Neuron. 2004;41:57–69. doi: 10.1016/s0896-6273(03)00818-3. [DOI] [PubMed] [Google Scholar]
- 46.Guerrini R., Dobyns W.B., Barkovich A.J. Abnormal development of the human cerebral cortex: genetics, functional consequences and treatment options. Trends Neurosci. 2008;31:154–162. doi: 10.1016/j.tins.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Manzini M.C., Walsh C.A. What disorders of cortical development tell us about the cortex: one plus one does not always make two. Curr. Opin. Genet. Dev. 2011;21:333–339. doi: 10.1016/j.gde.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross M.E., Walsh C.A. Human brain malformations and their lessons for neuronal migration. Annu. Rev. Neurosci. 2001;24:1041–1070. doi: 10.1146/annurev.neuro.24.1.1041. [DOI] [PubMed] [Google Scholar]
- 49.Cappello S., Bohringer C.R., Bergami M., Conzelmann K.K., Ghanem A., Tomassy G.S., Arlotta P., Mainardi M., Allegra M., Caleo M., et al. A Radial Glia-Specific Role of RhoA in Double Cortex Formation. Neuron. 2012;73:911–924. doi: 10.1016/j.neuron.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 50.Katsetos C.D., Legido A., Perentes E., Mork S.J. Class III beta-tubulin isotype: a key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology. J. Child. Neurol. 2003;18:851–866. doi: 10.1177/088307380301801205. [DOI] [PubMed] [Google Scholar]
- 51.Jiang Y.Q., Oblinger M.M. Differential regulation of beta III and other tubulin genes during peripheral and central neuron development. J. Cell Sci. 1992;103:643–651. doi: 10.1242/jcs.103.3.643. [DOI] [PubMed] [Google Scholar]
- 52.Wang D., Villasante A., Lewis S.A., Cowan N.J. The mammalian beta-tubulin repertoire: hematopoietic expression of a novel, heterologous beta-tubulin isotype. J. Cell Biol. 1986;103:1903–1910. doi: 10.1083/jcb.103.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cross D., Dominguez J., Maccioni R.B., Avila J. MAP-1 and MAP-2 binding sites at the C-terminus of beta-tubulin. Studies with synthetic tubulin peptides. Biochemistry. 1991;30:4362–4366. doi: 10.1021/bi00231a036. [DOI] [PubMed] [Google Scholar]
- 54.Littauer U.Z., Giveon D., Thierauf M., Ginzburg I., Ponstingl H. Common and distinct tubulin binding sites for microtubule-associated proteins. PNAS. 1986;83:7162–7166. doi: 10.1073/pnas.83.19.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serrano L., Wandosell F., de la Torre J., Avila J. Proteolytic modification of tubulin. Methods Enzymol. 1986;134: 179–190. doi: 10.1016/0076-6879(86)34087-4. [DOI] [PubMed] [Google Scholar]
- 56.Wandosell F., Serrano L., Hernandez M.A., Avila J. Phosphorylation of tubulin by a calmodulin-dependent protein kinase. J. Biol. Chem. 1986;261:10332–10339. [PubMed] [Google Scholar]
- 57.Panda D., Miller H.P., Banerjee A., Luduena R.F., Wilson L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. PNAS. 1994;91:11358–11362. doi: 10.1073/pnas.91.24.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richards K.L., Anders K.R., Nogales E., Schwartz K., Downing K.H., Botstein D. Structure-function relationships in yeast tubulins. Mol. Biol. Cell. 2000;11:1887–1903. doi: 10.1091/mbc.11.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirokawa N., Niwa S., Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 60.Saito T. In vivo electroporation in the embryonic mouse central nervous system. Nat. Protoc. 2006;1:1552–1558. doi: 10.1038/nprot.2006.276. [DOI] [PubMed] [Google Scholar]
- 61.Saito T., Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev. Biol. 2001;240:237–246. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]
- 62.Fricker-Gates R.A., Shin J.J., Tai C.C., Catapano L.A., Macklis J.D. Late-stage immature neocortical neurons reconstruct interhemispheric connections and form synaptic contacts with increased efficiency in adult mouse cortex undergoing targeted neurodegeneration. J. Neurosci. 2002;22:4045–4056. doi: 10.1523/JNEUROSCI.22-10-04045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gupta M.L., Jr, Bode C.J., Dougherty C.A., Marquez R.T., Himes R.H. Mutagenesis of beta-tubulin cysteine residues in Saccharomyces cerevisiae: mutation of cysteine 354 results in cold-stable microtubules. Cell. Motil. Cytoskeleton. 2001;49:67–77. doi: 10.1002/cm.1021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.