Abstract
Background
Epidemiological studies report positive associations between high-temperature cooked meat intake and pancreatic cancer. We assessed associations between dietary intake of heterocyclic amines (HCAs) and benzo(a)pyrene (BaP)—mutagens formed in meat cooked at high temperatures—and incident exocrine pancreatic cancer in a prospective cohort.
Methods
The 62,581 subjects randomized to screening in the Prostate, Lung, Colorectal and Ovarian Screening Trial (PLCO) who completed an initial dietary survey that assessed meat intake, cooking methods, and doneness preferences defined the cohort. Subjects were surveyed annually for incident cancers through 2007. A National Cancer Institute research database (CHARRED) was used to estimate HCA and BaP intake and a Mutagenic Activity Index (MAI) from survey data. Proportional hazard ratios (HRs) for risk of pancreatic cancer were estimated from multivariate Cox regression models by quintile of intake, with the lowest quintile as the referent.
Results
During follow-up (median: 10 yrs), 248 cases of exocrine pancreatic cancer were confirmed. Preferences for well and very well done meat were generally associated with increased risks. Significant elevations in pancreatic cancer risk were found in upper quintiles of MAI, and of exposure to individual mutagens 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (DiMeIQx) and lowest quintile of MAI, the third and fifth quintiles brought HRs of 1.86 (1.22, 2.85) and 1.87 2-Amino-3,8-dimethylimidazo [4,5-f]quinoxaline (MeIQx). Compared to the (1.16, 3.02), respectively. These three exposures exhibited significant (P-trend: 0.01-0.03) positive trends in risk as their levels increased
Conclusion
Consuming well-done meat cooked at high temperatures, which contains high mutagen levels, appears to confer increased risk of pancreatic cancer.
Introduction
Pancreatic cancer is rapidly fatal in most cases. Identifying and modifying risk factors can reduce the morbidity and mortality from this disease; but currently, aside from cigarette smoking and possibly elevated body mass index, few modifiable risk factors for the disease are known. Meat intake has been associated with pancreatic cancer in many epidemiologic studies, but the data are inconsistent (Anderson 2006). This may be due, in part, to analyses that do not account for meat preparation methods and doneness levels - in addition to intake - since these factors greatly affect the levels of carcinogens, such as heterocyclic amines (HCAs) and benzo(a)pyrene (BaP), that form in meat and fish cooked at high temperatures (Knize 1999).
HCAs form on the surface of meat from creatinine or creatine, amino acids and sugars (Knize 1999). Polycyclic aromatic hydrocarbons such as BaP are deposited on the meat as incomplete combustion products from burning fat that drips on the coals when meat is cooked over an open flame (Wogan 2004). Well done barbequed or grilled meats typically contain high levels of HCAs and BaP, while baked, stewed, and microwaved meats do not contain these compounds; HCAs also form in pan-fried meats (Knize 1999; Layton 1995, Sinha 1999; Kazerouni 2001). HCAs and PAHs are known animal carcinogens and are “reasonably anticipated to be human carcinogens” (Weisburger 2000; Report on Carcinogens 2004).
The NCI developed questionnaires and the CHARRED database (Sinha 2005) to estimate intake of several meat carcinogens including BaP and three HCAs: 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) and 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (DiMeIQx). PhIP, MeIQx and DiMeIQx are among the most abundant HCAs in cooked meats of nearly 20 HCAs identified (Report on Carcinogens 2004). An additional metric derived from cooked meat samples and included in the CHARRED database relies on the total mutagenic potency of the meat rather than on the quantity of particular carcinogens. The Mutagenic Activity Index (MAI) (revertants/grams of daily meat intake) is based on mutagenicity in the Salmonella-based Ames Assay (Ames et al, 1975).
Only three epidemiologic studies, two case-control and one large cohort, have collected and analyzed detailed information about cooking methods and doneness preferences, along with meat intake, in relation to pancreatic cancer (Anderson et al, 2002; 2005; Li et al, 2007; Stolzenberg-Solomon et al, 2006). In each, positive associations were reported for various measures of well-done meat intake; but further investigation, including additional prospective studies, is needed before deeming the association as causal. In this report, we used the CHARRED database to estimate exposures to dietary meat carcinogen and mutagenicity levels derived from questionnaires, and then estimated the associations of these exposures with incident confirmed pancreatic cancer occurring during follow up of the PLCO cohort.
Methods
This is a prospective analysis based within the PLCO (Prostate, Lung, Colorectal, and Ovarian) multi-center cancer screening trial. From November 1993 to September 2001, that trial randomized over 150,000 subjects to each of two arms: screening and control. The baseline questionnaire of the PLCO, which was given to all subjects at the time of randomization, provided demographic and medical history information. In addition to the baseline questionnaire, those randomized to screening were given a dietary survey around the time of their PLCO randomization. Approximately 77,500 subjects were sent the dietary survey, which provided details on meat intake, preferred cooking methods, and doneness preferences. The date of cohort entry was defined by the later of: PLCO study randomization, baseline questionnaire completion, or dietary survey completion. The date of cohort exit corresponded to the earlier of: diagnosis with pancreatic cancer, censoring due to death, study dropout, or the NDI cutoff date of December 31, 2007.
A returned survey was considered valid for this analysis if there were no more than seven missing food frequency responses out of approximately 170 questions overall, and the subject had no personal history of pancreatic cancer. Subjects excluded on this basis contributed indirectly to primary analyses by adjustment for nonresponse (see “Statistical Methods”). The responding subjects defined the cohort for this study. Subjects or next of kin were surveyed annually for cancer incidence through 2007 and reported cancers were confirmed through medical records. Topography and histology codes from The WHO International Classification of Diseases for Oncology, 2nd Edition (ICD-O-2) were used to define cases. We included those with topographical codes C250, C251, C252, C253, C257, C258 or C259. If subjects had carcinoma in situ or particular histology codes (8150-8153, 8155, 8156, 8240-8246, 9590-9989) they were not defined as cases and were treated as censored at the date of diagnosis. Additional details of the design of the PLCO trial are reported in Prorok et al (2000), Gohagan et al (2000), and Hayes et al (2000).
HCA, BaP, and mutagenic activity intake
We estimated intake of HCAs, BaP and mutagenic activity using responses from the food frequency questionnaire and the NCI CHARRED database (Sinha et al, 2005). Using frequency and portion size, we estimated grams of consumption of each meat item (steak, hamburger patty, pork chops, bacon, etc.) by cooking technique (fried, grilled/barbequed, oven-broiled, etc.), and doneness level. Possible survey responses for doneness levels for hamburger and steak were: rare, medium rare, medium, medium well done, well done, very well done. Those for bacon were: just until done, well done or crisp, charred. Some levels were aggregated to improve precision; e.g., “rare to medium well” combines rare, medium rare, medium, and medium well. Then we derived intake of total HCAs and BaP by multiplying grams of meat by concentration measured for each cooking technique/doneness level contribution for that meat type. Similarly, the MAI was calculated by replacing “concentration of carcinogen” with “revertants per gram of meat”.
The food composition database used to assign HCA and BaP content values to meat items on the study questionnaire were derived from previous analyses of meat samples as described (Sinha et al, 1998). Briefly, HCA content (PhIP, DiMeIQx, and MeIQ) and BaP were determined in meat samples cooked by various methods to different degrees of doneness by the method of Gross and Gruter (1992) using a solid-phase extraction/high-pressure liquid chromatography method. The MAI of sample extracts was measured using the standard plate incorporation assay with Salmonella typhimurium strain TA98 (Knize et al, 1999; Ames et al, 1975). Agents with mutagenic activity in this assay that are believed to be most relevant to cooked meat include a variety of HCAs and BaP (Layton et al, 1995; Knize et al, 1999; Report on Carcinogens 2004). The relative validity of the meat module at estimating HCA intake was investigated in 165 healthy participants. The correlation coefficients (deattentuated) were 0.60 for MeIQx and 0.36 for PhIP (Cantwell et al, 2004).
Statistical Methods
Proportional-hazards regression was used to estimate the risk of pancreatic cancer as a function of doneness preferences and cooking methods for each of various meat groups. These models included adjustment for baseline covariates found to be significant in previous research on pancreatic cancer: age, sex, cigarette smoking, education, race, diabetes, and total fat intake. In the primary analyses, for each of the five measures of carcinogenic exposure, quintiles were used to define exposure groups. Proportional hazards regression was applied here as well, with adjustment for the same baseline covariates. For each of these derived exposures, the relative hazards among quintiles 2 through 5 was compared to the lowest quintile (referent category). Spearman’s rho statistic formed the basis of a non-parametric trend test, reported as “Spearman’s P” in Results. A semi-parametric test for trend was run as well, based on a single, linear term in the proportional hazards model, relating the cube root of carcinogen exposure to the relative hazard of pancreatic cancer, labeled “linear x^(1/3)” in Results. Additional covariates were considered in separate models for their impact on the primary estimates, but as they had minimal effects, they were not retained; these included: prior history of any cancer, total energy intake, total daily fruit intake, body mass index, and recent use of alcohol, aspirin, ibuprofen and current multi-vitamin use. The proportional hazards assumption was tested for each carcinogenic exposure model. Missing responses for some survey questions, such as smoking, diabetes history and use of multi-vitamins, were treated as indicating “no” or “none” as appropriate. To check for potential changes in diet driven by pre-clinical symptoms of pancreatic cancer, we varied the lag time between dietary survey completion and cohort entry by one-year increments from zero to one, and then two, years after the survey.
The potential for selection bias associated with nonresponse was addressed by a Horvitz-Thompson approach, using logistic regression to estimate probabilities of response as a function of baseline covariates (Horvitz and Thompson, 1952). To facilitate generalization of our results, further re-weighting by age and sex was applied to standardize the results to the U.S. 2000 population (U.S .Census Bureau, 2003). All computations were done using the statistical functions and programming language of “R” (R Development Core Team, 2010).
Results
The baseline dietary survey was sent to approximately 77,500 subjects - all those in the PLCO screening arm. Of these subjects, 62,581 (81%) returned acceptable dietary questionnaires and contribute directly to this analysis. The excluded 19% were primarily due to incomplete questionnaires: seven were outside of the target age range of 55-74 and four had a personal history of pancreatic cancer. Selected demographic and other characteristics obtained at baseline were examined for cases and non-cases (Table 1). Cohort subjects were 91% non-Hispanic Caucasian. A higher proportion of cases than noncases were male (58.1% versus 50.2%, respectively), and cases were slightly older than non-cases at study entry (65.2 and 62.7 years, respectively). Consistent with most of the published epidemiologic data on this cancer, cases were more likely to report a history of diabetes, to be current or former smokers and to have fewer years of post high school education than non-cases. There were not marked differences in mean BMI between cases and non-cases, nor were there marked differences in measures of dietary intake. Estimated mean dietary intake of total meat and red barbequed/grilled meat were slightly lower, while red meat intake was slightly higher, in cases compared to non-cases. Estimated mean dietary intake of total fat, saturated fat, and fruit and vegetable intake were slightly lower in cases than non-cases.
Table 1. Selected Baseline Characteristics of Pancreatic Cancer Cases and Non-Cases within the Analytic Cohort.a.
| Characteristic | Cases | Non-cases |
|---|---|---|
| Overall count (n=62,581) | 248 | 62,333 |
| Age (y), mean (S.D.) | 65.2 (4.9) | 62.7 (5.3) |
| Sex, count (%) | ||
| Female | 104 (41.9) | 31,017 (49.8) |
| Male | 144 (58.1) | 31, 316 (50.2) |
| Race, count (%) | ||
| White, non-hispanic | 220 (88.7) | 56,549 (90.7) |
| Black, non-hispanic | 10 (4.0) | 2,452 (3.9) |
| Other | 18 (7.3) | 3,312 (5.3) |
| Body mass index (kg/meters2), mean (S.D.) | 27.1 (4.7) | 27.3 (4.9) |
| Cigarette smoking status, count (%) | ||
| Never smoked cigarettes | 96 (38.7) | 29, 185 (46.8) |
| Current cigarette smoker | 42 (16.9) | 6, 223 (10.0) |
| Former cigarette smoker | 110 (44.4) | 26, 905 (43.2) |
| Pack-years, mean (S.D.) | 22.9 (31.4) | 14.7 (23.1) |
| Diabetes, count (%) | ||
| No | 201 (81.0) | 55, 723 (89.4) |
| Yes | 38 (15.3) | 4,562 (7.3) |
| Education, count (%) | ||
| Less than high school | 21 (8.5) | 4,249 (6.8) |
| High school | 62 (25.0) | 14, 466 (23.2) |
| Post-high school <4 Years | 80 (32.3) | 21,310 (34.2) |
| Post-high school >=4 Years | 85 (34.3) | 22, 245 (35.7) |
| Meat intake, mean (S.D.) | ||
| Total meat intake (g/day) | 131.1 (124.5) | 131.6 ( 97.8) |
| Total red meat intake (g/day) | 80.4 (106.0) | 78.1 (71.0) |
| Total red BBQ meat intake (g/day) | 13.8 (22.6) | 14.6 (23.7) |
| Dietary fat intake, mean (S.D.) | ||
| Total fat intake (g/day) | 67.5 (43.8) | 68.1 (36.9) |
| Saturated fat intake (g/day) | 22.5 (15.1) | 23.2 (13.5) |
| Fruit and vegetable intake, mean (S.D.) | ||
| Fruit intake (servings/day) | 2.6 (1.7) | 2.7 (1.9) |
| Vegetable intake (servings/day) | 4.1 (2.3) | 4.2 (2.3) |
| Fruit and vegetable intake (servings/day) | 4.1 (2.3) | 4.2 (2.3) |
Some counts fall short of the total due to missing or unknown value
Person-years, unadjusted incidence rates and relative hazards of pancreatic cancer by quintiles of red meat intake, categorized by preferred level of doneness were computed (Table 2). Within the rare to medium well done preference category, increasing levels of meat intake did not show increased relative hazards. In contrast, increasing intake within the well to very well done preference category showed increasing relative hazards; the HR for the fifth versus first quintile was 1.60 (1.01, 2.54) and the p-value for linear trend was 0.039.
Table 2. Pancreatic Cancer Incidence by Quintilea of Red Meat Intake Within Selected Meat Doneness Categories.
| Meat Type by Doneness Preferencesb |
Number of Subjects (Percent) N=62,581 |
Person-Years of Observation |
Number of Cases N=248 |
Cases per 100,000 Person-Years of Observation |
Relative Hazardd (95% CI) |
Linear (x1/3) Trend P-Value |
|---|---|---|---|---|---|---|
| Red meat, rare to medium well | ||||||
| Q1 | 12,383 (19.8) | 121,214 | 53 | 43.7 | 1 (1, 1) | |
| Q2 | 12,643 (20.2) | 124,182 | 57 | 45.9 | 1.11 (0.76, 1.63) | |
| Q3 | 12,500 (20.0) | 124,492 | 43 | 34.5 | 0.81 (0.54, 1.21) | |
| Q4 | 12,538 (20.0) | 125,433 | 50 | 39.9 | 0.91 (0.61, 1.34) | |
| Q5 | 12,517 (20.0) | 123,565 | 45 | 36.4 | 0.84 (0.55, 1.29) | 0.364 |
| Red meat, well to very well | ||||||
| Q1 | 12,502 (20.0) | 126,411 | 39 | 30.9 | 1 (1, 1) | |
| Q2 | 12,524 (20.0) | 125,717 | 58 | 46.1 | 1.52 (1.01, 2.29) | |
| Q3 | 12,521 (20.0) | 124,093 | 47 | 37.9 | 1.25 (0.81, 1.92) | |
| Q4 | 12,517 (20.0) | 122,448 | 49 | 40.0 | 1.37 (0.88, 2.12) | |
| Q5 | 12,517 (20.0) | 120,218 | 55 | 45.8 | 1.60 (1.01, 2.54) | 0.039 |
| Red BBQ meat, rare to medium well | ||||||
| cQ1-4 | 49,937 (79.8) | 494,294 | 209 | 42.3 | 1 (1, 1) | |
| Q5 | 12,644 (20.2) | 124,592 | 39 | 31.3 | 0.79 (0.55, 1.13) | |
| Red BBQ meat, well to very well | ||||||
| Q1-4 | 50,046 (80.0) | 498,245 | 192 | 38.5 | 1 (1, 1) | |
| Q5 | 12,535 (20.0) | 120,641 | 56 | 46.4 | 1.35 (1.00, 1.83) |
Quintiles refer to the daily gram intake of the corresponding red meat at the listed doneness level.
Possible doneness level responses for red meat were: rare, medium rare, medium, medium well done, well done, very well done.
For BBQ meat, quintiles 1-4 were grouped as one because of the majority of grams per day of derived intake were zero or near zero
Relative hazard estimates adjusted for age, sex, education, race, diabetes, dietary fat intake, and cigarette smoking history (pack years and years since stopping).
A similar pattern was observed for red barbequed meat. Among those who preferred rare or medium rare meat, there was no significant elevation in risk for pancreatic cancer among fifth quintile of intake relative to the lowest four quintiles. However, the same intake comparison (fifth quintile of intake relative to the lowest four quintiles) among those who prefer well or very well done red barbequed meat yielded an HR of 1.35 (1.00, 1.83).
A closer examination of the association of cooking methods (Table 3 A) and doneness preferences (Table 3 B and 4) with pancreatic cancer was conducted within individual meat groups. Unadjusted incidence rates as well as multivariate-adjusted hazard ratios were computed. There were not consistent patterns of elevated risk by preparation method, or within meat type by doneness preference. A proportional hazards regression model, adjusted for baseline covariates, estimated the HRs for pancreatic cancer in those preferring very well done meat compared to those reporting all other levels of doneness preferences plus those who indicated no consumption of the three specified meats (Table 4). Although the relative hazards were increased by 21% and 43% for subjects preferring very well done steak or hamburger, respectively, these were not significantly different from the null.
Table 3. Pancreatic Cancer Incidence by Meat Type Within Categories of Cooking Methods (A) and Doneness Preferences (B).a.
| (A) Meat Type by Cooking Methods | Number of Subjects (Percent) N=62,581 |
Person- Years of Observation |
Number of Cases N=248 |
Cases per 100,000 Person- Years of Observation |
Relative Hazardb (95% CI) |
|---|---|---|---|---|---|
| Chicken | |||||
| Don't eat OR Stewed or boiled | 5,732 (9.3) | 56,226 | 28 | 49.8 | 1 (1, 1) |
| Roasted or baked | 36,261 (59.0) | 360,259 | 136 | 37.8 | 0.82 (0.54, 1.23) |
| Oven broiled | 5,415 ( 8.8) | 54,440 | 26 | 47.8 | 1.02 (0.59, 1.74) |
| Eat only fried chicken | 2,913 (4.7) | 28,090 | 13 | 46.3 | 0.86 (0.44, 1.66) |
| Grilled or barbequed | 11,117 (18.1) | 108,044 | 41 | 37.9 | 0.91 (0.56, 1.49) |
| Pork chops | |||||
| Don't eat pork chops | 6,784 (11.0) | 68,699 | 19 | 27.7 | 1 (1, 1) |
| Baked | 18,800 (30.4) | 187,010 | 67 | 35.8 | 1.44 (0.86, 2.40) |
| Oven broiled | 6,567 (10.6) | 65,136 | 31 | 47.6 | 1.78 (1.00, 3.17) |
| Fried | 19,115 (30.9) | 187,724 | 86 | 45.8 | 1.74 (1.05, 2.90) |
| Grilled or barbequed | 10,509 (17.0) | 102,055 | 42 | 41.2 | 1.80 (1.04, 3.13) |
| Hamburger | |||||
| Don't eat | 3,360 (5.5) | 33,871 | 11 | 32.5 | 1 (1, 1) |
| Oven broiled | 6,014 (9.8) | 60,300 | 23 | 38.1 | 1.11 (0.54, 2.30) |
| Pan fried | 18,113 (29.6) | 178,963 | 75 | 41.9 | 1.32 (0.69, 2.51) |
| Grilled or barbequed | 33,638 (55.0) | 331,061 | 133 | 40.2 | 1.43 (0.77, 2.67) |
| Steak | |||||
| Don't eat | 4,877 (7.9) | 48,589 | 20 | 41.2 | 1 (1, 1) |
| Oven broiled | 15,385 (24.9) | 153,547 | 76 | 49.5 | 1.15 (0.70, 1.89) |
| Pan fried | 6,611 (10.7) | 64,886 | 32 | 49.3 | 1.10 (0.62, 1.94) |
| Grilled or barbequed | 34,925 (56.5) | 343,999 | 119 | 34.6 | 0.93 (0.57, 1.50) |
| (B) Meat Type by Doneness Preferences |
Number of Subjects (Percent) N=62,581 |
Person- Years of Observation |
Number of Cases N=248 |
Cases per 100,000 Person- Years of Observation |
Relative Hazardb (95% CI) |
|---|---|---|---|---|---|
| Bacon/Sausage | |||||
| Don't eat | 6,884 (11.0) | 70,255 | 25 | 35.6 | 1 (1, 1) |
| Just until done | 6,050 (9.7) | 59,133 | 28 | 47.4 | 1.44 (0.83, 2.51) |
| Well done or crisp OR Charred | 49,432 (79.3) | 487,423 | 194 | 39.8 | 1.19 (0.78, 1.82) |
| Hamburger | |||||
| Don't eat | 2,932 (4.7) | 28,900 | 10 | 34.6 | 1 (1, 1) |
| Rare OR Medium rare | 4,300 (6.9) | 45,590 | 26 | 57.0 | 1.40 (0.67, 2.93) |
| Medium | 11,836 (19.0) | 120,286 | 38 | 31.6 | 0.88 (0.43, 1.78) |
| Medium well done | 16,712 (26.8) | 165,919 | 60 | 36.2 | 1.04 (0.53, 2.06) |
| Well done | 23,235 (37.3) | 223,772 | 99 | 44.2 | 1.32 (0.68, 2.55) |
| Very well done | 3,284 (5.3) | 31,708 | 15 | 47.3 | 1.39 (0.62, 3.11) |
| Steak | |||||
| Don't eat | 4,059 (6.5) | 39,238 | 13 | 33.1 | 1 (1, 1) |
| Rare OR Medium rare | 15,392 (24.7) | 151,366 | 72 | 47.6 | 1.43 (0.79, 2.61) |
| Medium | 16,754 (26.8) | 166,962 | 55 | 32.9 | 0.99 (0.54, 1.83) |
| Medium well done | 15,808 (25.3) | 156,905 | 61 | 38.9 | 1.16 (0.64, 2.13) |
| Well done | 8,345 (13.4) | 82,609 | 35 | 42.4 | 1.19 (0.62, 2.26) |
| Very well done | 2,079 (3.3) | 20,317 | 12 | 59.1 | 1.68 (0.76, 3.70) |
Total subject numbers may vary within categories due to missing values.
Relative hazard estimates adjusted for age, sex, education, race, diabetes, dietary fat intake, and cigarette smoking history (pack years and yearssince stopping).
Table 4. Relative Hazard Estimates and 95% CI for Pancreatic Cancer by Selected Meat Types and Doneness Preferences.
| Meat Type and Doneness Preference | Na | Relative Hazard Estimate (95% CI)b |
|---|---|---|
| Bacon and sausage:Well done or crisp or charred (vs. referentc) |
62,275 | 0.99 (0.73, 1.35) |
| Hamburger: Very well done (vs. referent) | 62,207 | 1.21 (0.71, 2.04) |
| Steak: Very well done (vs. referent) | 62,346 | 1.43 (0.80, 2.56) |
Out of 62,581 originally; nonresponders to these survey questions are excluded.
All estimates adjusted for age, sex, education, race, diabetes, dietary fat intake, cigarette smoking history (pack years and years since stopping) and daily intake of the listed meat.
The referent category includes those who reported the meat was not consumed as well as cooked to a lesser degree of doneness; these subgroups had relatively few events, and their hazards were not significantly different.
For the mutagen/carcinogen exposures derived from the dietary data, DiMeIQx, MeIQx, PhIP, BaP and MAI, Spearman’s correlations were calculated and ranged from 0.41 to 0.85 (Table 5). Each of the three HCAs is a more potent mutagen than BaP and not surprisingly, is more highly correlated with the MAI than BaP (Sugimura et al, 2000).
Table 5. Spearman Correlation Coeffieicnts of BaP, Individual HCAs and the Mutagenic Activity Index (MAI).a.
| BaP | DiMeIQx | MeIQx | PhIP | |
|---|---|---|---|---|
| MAI | 0.55 | 0.79 | 0.85 | 0.80 |
| BaP | 0.41 | 0.42 | 0.61 | |
| DiMeIQx | 0.74 | 0.59 | ||
| MeIQx | 0.56 |
Based on 62,343 subjects in the cohort. All p-values for the pairwise associations are < 0.0001.
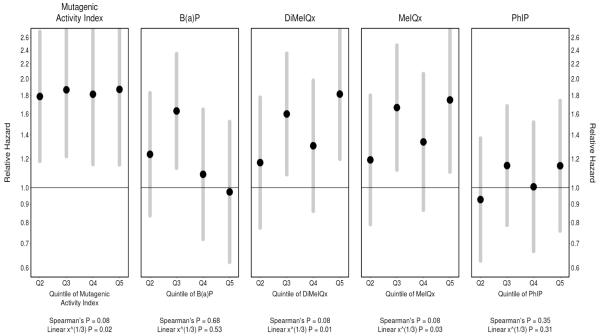
The proportional hazards regression results indicate that the MAI is associated with the most consistent increases in the hazard of pancreatic cancer, with elevated hazards for quintiles 2 through 5 being significantly different from the null (Figure 1 and Table 6). For both DiMeIQx and MeIQx, quintiles 2 and 5 have significantly higher hazards than quintile 1. Furthermore, the overall upward trend is significant, based on the test for linear trend, and nearly so by the non-parametric, rank-based Spearman test of the five point estimates. PhIP shows upward, though non-significant, trends. BaP did not show a consistent trend of increasing intake and increasing risk. Quintiles 2-4 were elevated compared to quintile 1, but not significantly so and the HR for quintile 5 was close to null. Tests of the proportional hazards assumption were satisfied for all five carcinogen-related measures. In sum, except for BaP, there are increased hazards associated with the higher quintiles of intake of each of these measures.
Figure 1. Pancreatic Cancer Risk by Quintiles of Derived Carcinogen Exposures.
For each of the five exposure metrics, proportional hazard estimates (dots) and 95% confidence intervals (vertical segments) for the second through the fifth quintiles, relative to the first quintile (referent category). Adjusted for non-response within PLCO, also adjusted for age, sex, race, education, diabetes, total dietary fat intake and cigarette smoking history (pack years, and years since stopping). Also standardized to the U.S. 2000 population. “Spearman’s P” refers to the p-value based on Spearman’s rho statistic as a non-parametric test of association—applied here as a test for trend. A corresponding parametric trend test is identified by “Linear x^(1/3)”, where a single coefficient was added to relate the cube root of exposure to the relative hazard in the proportional hazards regression model.
Table 6. Proportional Hazard Estimates and 95% Confidence Intervals, by Quintile of Exposure to Each Carcinogen.a.
| Quintile of Exposurea: | Q2 | Q3 | Q4 | Q5 |
|---|---|---|---|---|
| MAI | 1.79 (1.18, 2.70) | 1.86 (1.22, 2.85) | 1.81 (1.16, 2.83) | 1.87 (1.16, 3.02) |
| BaP | 1.24 (0.84, 1.83) | 1.63 (1.13, 2.34) | 1.09 (0.72, 1.64) | 0.97 (0.62, 1.52) |
| DiMeIQx | 1.17 (0.77, 1.78) | 1.60 (1.09, 2.35) | 1.31 (0.86, 1.98) | 1.81 (1.20, 2.74) |
| MeIQx | 1.19 (0.79, 1.80) | 1.66 (1.12, 2.47) | 1.34 (0.87, 2.06) | 1.75 (1.11, 2.76) |
| PhIP | 0.93 (0.63, 1.37) | 1.15 (0.79, 1.68) | 1.01 (0.67, 1.52) | 1.15 (0.76, 1.74) |
Referent is the lowest quintile of exposure, Q1.
To account for possible dietary changes that could have occurred just prior to disease diagnosis, we varied the time from dietary survey completion to the defined cohort entry from zero to one, and then two, years. This resulted in attenuation of some estimated relative hazards toward unity, but did not produce substantively different results from the zero-year definition. For example, the estimated relative hazards (and 95% CIs) with exposure to the fifth quintile of MAI and a one- and two-year lag were 1.76 (1.08, 2.88) and 1.77 (1.06, 2.96), respectively. None of the other baseline covariates (described in Statistical Methods) qualitatively changed the relative hazard estimates or their precision, so they were left out of the final model.
Discussion
Associations between dietary intake of meat-borne carcinogens and pancreatic cancer were assessed in this large, multi-center, prospective cohort study of subjects randomized to screening in the PLCO trial. The analyses suggest that intake of meat-derived HCAs confers a higher risk of pancreatic cancer, but are less suggestive for BaP. Upward trends in risk of pancreas cancer were observed with increasing quintiles of dietary intake of DiMeIQx and MeIQx (P for linear trend: 0.01 and 0.03, respectively). There were increased hazards associated with higher quintiles of intake of each HCA measure and statistically significantly increased HRs were found for the MAI. Increasing BaP intake was associated with elevation in risk in some quintiles, but these were not statistically significant and there was not a dose response pattern. There were increased hazards among individuals reporting preferences for well-done meat intake versus meat cooked to lower degrees of doneness, particularly when both doneness preference and intake level were considered. Analyses of cooking method alone did not reveal consistent patterns of elevated risk for categories of high temperature cooked meats. It is not surprising that measures with less information about carcinogen exposure, such as meat cooking methods, doneness preferences or type, yield less consistent patterns of risk than summary measures. The most striking and consistent patterns of increased risk with increasing intake were found with the integrated measures of HCA carcinogen intake and the MAI. These data may help explain the inconsistent results from previous studies that analyzed limited information on meat preparation and intake.
Approximately 30 epidemiologic studies have examined meat intake in relation to pancreatic cancer and overall, and those data point to an increased risk with increasing intake of meat, but there are many inconsistencies (Anderson 2006; WCRF 2009). There have only been three studies that examined cooking methods and doneness preferences in detail and all report increases in the hazard associated with various measures of well-done meat intake and meat-derived carcinogens. These previous studies include a population-based case-control study in the upper Midwest (Anderson, et al., 2002; 2005); a hospital-based case-control study in Texas (Li D et al., 2007) and the NIH-AARP prospective study in retired men and women (Stolzenberg-Solomon et al., 2006).
The study based in the upper Midwest (Anderson, et al 2005) found elevated odds ratios and 95% confidence intervals (CI) in comparisons of fifth to first quintiles of intake for PhIP 1.8 (1.0-3.1); DiMeIQx 2.0 (1.2-3.5), MeIQx 1.5 (0.9-2.7), BaP 2.2 (1.2-4.0) and the MAI 2.4 (1.3-4.3).
In the Texas study (Li D et al., 2007), daily intake of MeIQx, DiMeIQx, BaP and the MAI were all statistically significant predictors of pancreatic cancer when comparisons were based on the upper 40% of intake versus lower 60%. Odds ratios (95%, CI) were 1.48 (1.12-1.95) for MeIQx; 1.39(1.05-1.83) for DiMeIQx; 1.12 (0.85-1.48) for PhIP; 1.51 (1.15-1.98) for BaP and 1.41 (1.07-1.85) for the MAI.
The AARP cohort study (Stolzenberg-Solomon et al., 2006) was the only other prospective study to have reported on meat-derived carcinogens and risk of pancreatic cancer. The strongest associations were for meat-derived MAI in men (OR = 2.11, 95% CI 1.39-3.91) for the fifth versus first quintile of intake. High versus low intake of high-temperature cooked meat also increased risk in men. The findings in women were generally null, which may have resulted from lower power due to a smaller number of cases as well as lower absolute intake of meat and meat-derived carcinogens.
In general, the results in the current study are similar to those reported in the previous studies cited. The differences among these studies may reflect, in part, differences in methods of dietary assessment of meat doneness preferences and intake. The studies in Minnesota and Texas administered questionnaires with colored photographs of meat cooked to varying degrees of doneness and in the former, three-dimensional food models were used to help subjects accurately estimate portion sizes.
The AARP study and this PLCO study relied on data from dietary surveys that assessed meat doneness preferences with written descriptions; subjects were instructed to report intake based in relation to specified serving sizes. This approach may result in less accurate assessment of doneness preferences and intake resulting in more misclassification and attenuated HRs.
The prospective nature of this analysis within the PLCO cohort is a strength. The dietary information collected at baseline, reduces the potential for both recall bias and recall error present in retrospective observational studies. Another strength of this study is the use of multiple levels of aggregation in exposure assessment, so designed to better approximate the total intake of the targeted carcinogens. Rather than relying on either meat doneness preferences or cooking methods alone, both dimensions, along with meat intake, were surveyed and incorporated. Furthermore, by using the CHARRED database of estimated mutagen content associated with these doneness preferences and cooking methods, net measures of carcinogens are obtained across the most commonly consumed meats. Finally, the MAI provides a measure of the total mutagenic potential across all of the meats; accounting for mutagens - both known and unknown. These aggregational features are likely responsible for the relative significance of increased MAI among the results.
This study does have limitations. Despite our findings implicating meat-derived carcinogens and pancreas cancer, it is difficult to implicate specific meat-derived carcinogens and cancer risk in any population study (Report on Carcinogens 2004). The HCAs and polycyclic aromatic hydrocarbons in cooked meat are correlated with each other and with other potentially carcinogenic constituents. Both the relative and absolute amounts of these carcinogens vary in any meal or diet, and cannot be entirely captured by surveys. However, their cumulative effect as measured by the MAI, and their concentration in meats that are prepared as described merits understanding.
This was not a randomized study of these exposures and the possibility remains that these diet-related effects are confounded with some unmeasured, but more directly causal, characteristic. In addition, the PLCO includes subjects that may be healthier overall, than the general population. Since healthier subjects are more likely to accept screening, and possibly less likely to consume meat, the resulting estimates of relative hazard among higher quintiles of exposure would then be lower than in the population at large.
In sum, the results of this prospective study support the hypothesis that consuming meat cooked at high temperatures, and to a high degree of doneness, with the resulting production of mutagenic compounds, confers a higher risk of pancreatic cancer. This association, if causal, identifies means to reduce risk of pancreatic cancer by reducing meat consumption or, by adopting simple changes in meat preparation techniques that reduce or eliminate production of these compounds.
Acknowledgements
The authors wish to thank the PLCO centers and staff for making this study possible. In addition, we thank Tom Hickey, IMS, for suggestions regarding this analysis. We are also grateful to the men and women in PLCO study for their participation.
This research was funded, in part, by contract number N01-CN-25513 from the U.S. National Cancer Institute, National Institutes of Health, Department of Health and Human Services and by National Institutes of Health grant DA-13333 to the University of Minnesota.
References
- Ames BN, McCann J, Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/Mammalian-Microsomal Mutagenicity Test. Mutat Res. 1975;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Anderson KE, Kadlubar FF, Kulldorff M, et al. Dietary intake of heterocyclic amines and benzo(a)pyrene: Associations with pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2261–2265. doi: 10.1158/1055-9965.EPI-04-0514. [DOI] [PubMed] [Google Scholar]
- Anderson KE, Mack T, Silverman D. Cancer of the pancreas. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 3rd ed Oxford University Press; New York, NY: 2006. pp. 721–762. [Google Scholar]
- Anderson KE, Sinha R, Kulldorff M, et al. Meat intake and cooking techniques: Associations with pancreatic cancer. Mutat Res. 2002;506-507:225–231. doi: 10.1016/s0027-5107(02)00169-0. [DOI] [PubMed] [Google Scholar]
- Cantwell M, Mittl B, Curtin J, et al. Relative validity of a food frequency questionnaire with a meat-cooking and heterocyclic amine module. Cancer Epidemiol Biomarkers Prev. 2004;13:293–298. doi: 10.1158/1055-9965.epi-270-2. [DOI] [PubMed] [Google Scholar]
- Gohagan JK, Prorok PC, Hayes RB, Kramer BS, PLCO Project Team The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: History, Organization, and Status. Control Clin Trials. 2000;21:251S–272S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Gross GA, Gruter A. Quantitation of mutagenic/carcinogenic heterocyclic aromatic amines in food products. J Chromatogr. 1992;592:271–278. doi: 10.1016/0021-9673(92)85095-b. [DOI] [PubMed] [Google Scholar]
- Hayes RB, Reding D, Kopp W, et al. Etiologic and early marker studies in the prostate, lung, colorectal and ovarian (PLCO) Cancer Screening Trial. Controlled Clin Trials. 2000;21:349S–355S. doi: 10.1016/s0197-2456(00)00101-x. [DOI] [PubMed] [Google Scholar]
- Horvitz DG, Thompson DJ. A generalization of sampling without replacement from a finite universe. J Am Stat Assoc. 1952;47:663–685. [Google Scholar]
- Kazerouni N, Sinha R, Hsu CH, Greenberg A, Rothman N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol. 2001;39:423–436. doi: 10.1016/s0278-6915(00)00158-7. [DOI] [PubMed] [Google Scholar]
- Knize MG, Salmon CP, Pais P, et al. Food heating and the formation of heterocyclic aromatic amine and polycyclic aromatic hydrocarbon mutagens/carcinogens. Adv Exp Med Biol. 1999;459:179–193. doi: 10.1007/978-1-4615-4853-9_12. [DOI] [PubMed] [Google Scholar]
- Layton DW, Bogen KT, Knize MG, Hatch FT, Johnson VM, Felton JS. Cancer risk of heterocyclic amines in cooked foods: An analysis and implications for research. Carcinogenesis. 1995;16:39–52. doi: 10.1093/carcin/16.1.39. [DOI] [PubMed] [Google Scholar]
- Li D, Day RS, Bondy ML, et al. Dietary mutagen exposure and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(4):655–661. doi: 10.1158/1055-9965.EPI-06-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prorok PC, Andriole GL, Bresalier RS, et al. Design of the prostate, lung, colorectal and ovarian (PLCO) Cancer Screening Trial. Controlled Clin Trials. 2000;21:273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- R Development Core Team: A Language and Environment for Statistical Computing . R Foundation for Statistical Computing. Vienna, Austria: ISBN 3-900051-07-0. 2010. Available from: http://www.R-project.org. [Google Scholar]
- Report on Carcinogens, Eleventh Edition, U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program. Substance Profiles: Selected Heterocyclic Amines; Polycyclic Aromatic Hydrocarbons). 2004. Available from: http://ntp.niehs.nih.gov.floyd.lib.umn.edu.
- Sinha R, Cross A, Curtin J, et al. Development of a food frequency questionnaire module and databases for compounds in cooked and processed meats. Mol Nutr Food Res. 2005;49:648–655. doi: 10.1002/mnfr.200500018. [DOI] [PubMed] [Google Scholar]
- Sinha R, Rothman N, Salmon CP, et al. Heterocyclic amine content in beef cooked by different methods to varying degrees of doneness and gravy made from meat drippings. Food Chem Toxicol. 1998;36:279–287. doi: 10.1016/s0278-6915(97)00162-2. [DOI] [PubMed] [Google Scholar]
- Stolzenberg-Solomon RZ, Cross AJ, Silverman DT, et al. Meat and meat-mutagen intake and pancreatic cancer risk in the NIH-AARP cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2664–2675. doi: 10.1158/1055-9965.EPI-07-0378. [DOI] [PubMed] [Google Scholar]
- Sugimura T, Nagao M, Wakabayashi K. How we should deal with unavoidable exposure of man to environmental mutagens: cooked food mutagen discovery, facts and lessons for cancer prevention. Mutat Research. 2000;447:15–25. doi: 10.1016/s0027-5107(99)00196-7. [DOI] [PubMed] [Google Scholar]
- US Census Bureau Monthly Population Estimates 2000 to 2003 (for 1 July 2000) http://www.census.gov/popest/national/asrh/2003_nat_detail.html; (24 Sep 2009)
- Weisburger JH. Eat to live, not live to eat. Nutrition. 2000;16:767–773. doi: 10.1016/s0899-9007(00)00400-7. [DOI] [PubMed] [Google Scholar]
- Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA. Environmental and chemical carcinogenesis. Semin Cancer Biol. 2004;14:473–486. doi: 10.1016/j.semcancer.2004.06.010. [DOI] [PubMed] [Google Scholar]
- WCRF/AICR Second Expert Report, Food, Nutrition, and the Prevention of Cancer: a Global Perspective . World Cancer Research Fund/American Institute for Cancer Research; Washington, D.C.: 2009. [Google Scholar]