Abstract
Telomere synthesis in cancer cells and stem cells involves trafficking of telomerase to Cajal bodies, and telomerase is thought to be recruited to telomeres through interactions with telomere-binding proteins. Here, we show that the OB-fold domain of the telomere-binding protein TPP1 recruits telomerase to telomeres through an association with the telomerase reverse transcriptase, TERT. When tethered away from telomeres and other telomere-binding proteins, the TPP1 OB-fold domain is sufficient to recruit telomerase to a heterologous chromatin locus. A minimal TPP1 OB-fold serves to inhibit telomere maintenance by blocking access of telomerase to its cognate binding site at telomeres. We identify specific loop residues within the TPP1 OB-fold necessary for association with telomerase, and critical residues in TERT, including those mutated in a subset of pulmonary fibrosis patients. These data define a potential interface for telomerase-TPP1 interaction required for telomere maintenance and implicate defective telomerase recruitment in telomerase-related disease.
Introduction
The addition of telomere repeats to chromosome ends by the enzyme telomerase is essential to counter the incomplete replication of telomeres that occurs with cell division in stem cells and in cancer cells (Cech, 2004; Palm and de Lange, 2008; Artandi and DePinho, 2010; O’Sullivan and Karlseder, 2010). Disruption of this process by mutations in telomerase components causes stem cell dysfunction and results in a number of diseases in humans, including dyskeratosis congenita, aplastic anemia, pulmonary fibrosis and multiple types of cancer (Savage and Alter, 2008; Calado and Young, 2009). Human telomerase consists of a minimal catalytic core including the reverse transcriptase subunit, TERT, and the telomerase RNA component, TERC, which are assembled into a mature enzyme along with additional holoenzyme proteins (Collins, 2008). To elongate telomeres, telomerase is thought to be recruited to chromosome ends through interactions with telomere binding proteins, but the precise mechanisms of telomerase recruitment remain incompletely understood.
Telomerase undergoes a highly orchestrated process of assembly and trafficking within the nucleus of human cells. TERC encodes the template for the reverse transcription reaction in telomere addition, but also serves as the central scaffold for assembly of the telomerase RNP (Cech, 2004; Zappulla and Cech, 2006; Egan and Collins, 2012). A newly transcribed TERC RNA molecule is bound and stabilized by the dyskerin core complex, which includes dyskerin, NHP2 and NOP10 (Darzacq et al., 2006). Loading of TERT into telomerase complexes generates an enzymatically active RNP, but this complex is unable to act on telomeres without completing additional trafficking and assembly steps in vivo. In human cancer cells and embryonic stem cells, telomerase localizes within Cajal bodies, nuclear sites of RNP modification and assembly (Gall, 2000; Jady et al., 2004; Zhu et al., 2004; Batista et al., 2011). RNA FISH studies using probes specific for TERC revealed that telomerase-containing Cajal bodies associated with a subset of telomeres specifically in S-phase of the cell cycle (Jady et al., 2004; Zhu et al., 2004; Tomlinson et al., 2008). Concentration of telomerase within Cajal bodies depends upon an interaction between the CAB box motif within TERC and TCAB1, a WD40 repeat protein that is part of the active telomerase holoenzyme (Cristofari et al., 2007; Tycowski et al., 2009; Venteicher et al., 2009). TCAB1 is required for telomere maintenance and is mutated in an autosomal recessive form of dyskeratosis congenita (Venteicher et al., 2009; Zhong et al., 2011). Loss of TCAB1 function causes mislocalization of telomerase from Cajal bodies to nucleoli, cripples the ability of telomerase to maintain telomeres and impairs recruitment of telomerase to chromosome ends (Venteicher et al., 2009; Batista et al., 2011; Zhong et al., 2011; Stern et al., 2012). Depletion of the Cajal body scaffold coilin also blunts the ability of telomerase RNA to associate with telomeres, suggesting that Cajal bodies may be important for recruiting telomerase to telomeres (Stern et al., 2012).
In yeast, the telomere binding protein Cdc13p positively regulates telomerase recruitment through an interaction with the telomerase component Est1p (Pennock et al., 2001; Taggart et al., 2002; Chan et al., 2008). In human cells, telomere-binding proteins exert both positive and negative effects on telomerase function. Human telomeres consist of long tracks of double-stranded repeats ending in a single-stranded overhang, which together are bound by the six-protein shelterin complex (Smogorzewska and de Lange, 2004; de Lange, 2005; Verdun and Karlseder, 2007; Xin et al., 2008; O’Sullivan and Karlseder, 2010). TRF1 and TRF2, factors that bind double stranded telomere repeats, inhibit telomerase function presumably by transducing telomere length information to the chromosome terminus (Smogorzewska et al., 2000). The single stranded overhang is bound by a subcomplex of shelterin components in which POT1 directly contacts DNA and TPP1 bridges POT1 to TIN2, which connects to the TRF1-TRF2 double stranded DNA binding complex. Depletion of POT1 or TPP1, or overexpression of a POT1 variant with a deletion in the DNA binding domain (POT1ΔOB), each leads to telomere elongation by telomerase, indicating that POT1 and TPP1 prevent telomerase action at telomeres (Loayza and De Lange, 2003; Ye and de Lange, 2004; Ye et al., 2004). In contrast to these genetic findings in cultured cells, biochemical studies in vitro have established that recombinant TPP1 and POT1 enhance processivity of telomerase on oligonucleotide substrates, suggesting that TPP1 and POT1 act as positive co-factors in telomerase catalysis (Wang et al., 2007; Zaug et al., 2010). These dual functions of the TPP1-POT1 complex in regulating telomerase function remain to be resolved.
Experiments designed to address telomerase recruitment in human cells have exploited ‘super-telomerase’ cells, cancer cells in which both TERT and TERC are overexpressed to enable telomerase detection at telomeres (Cristofari and Lingner, 2006; Cristofari et al., 2007; Abreu et al., 2010). Loss of function studies showed that TPP1 and TIN2 were required for efficient recruitment of telomerase to telomeres in super-telomerase cells (Abreu et al., 2010). TERT has been shown to interact with the OB-fold of TPP1 (Xin et al., 2007) and this same domain of TPP1 was implicated in recruiting telomerase to telomeres in super-telomerase cells (Abreu et al., 2010). However, TPP1 serves to tether POT1 to telomeres, therefore inhibition of TPP1 leads to loss of the TPP1-POT1 complex from telomeres and the induction of a DNA damage response at telomeres (Houghtaling et al., 2004; Liu et al., 2004; Ye et al., 2004; Kibe et al., 2010; Tejera et al., 2010; Takai et al., 2011). In addition, loss of TPP1 in vivo is accompanied by reduced levels of TIN2 (Rai et al., 2011), suggesting that TPP1 might serve a structural role in the shelterin complex. Therefore, separating the putative recruitment function of TPP1 from its end-protection function is inherently challenging.
In this study, we investigate mechanisms of telomerase recruitment to telomeres in human cancer cells. We find that the OB-fold domain of TPP1 recruits telomerase to telomeres and that this is an essential step in telomere maintenance. We identify a putative interaction surface governing this interaction and show that this binding region is mutated in a subset of patients with diseases caused by telomerase mutations.
Results
Neo-Cajal bodies form at telomeres in super-telomerase cells
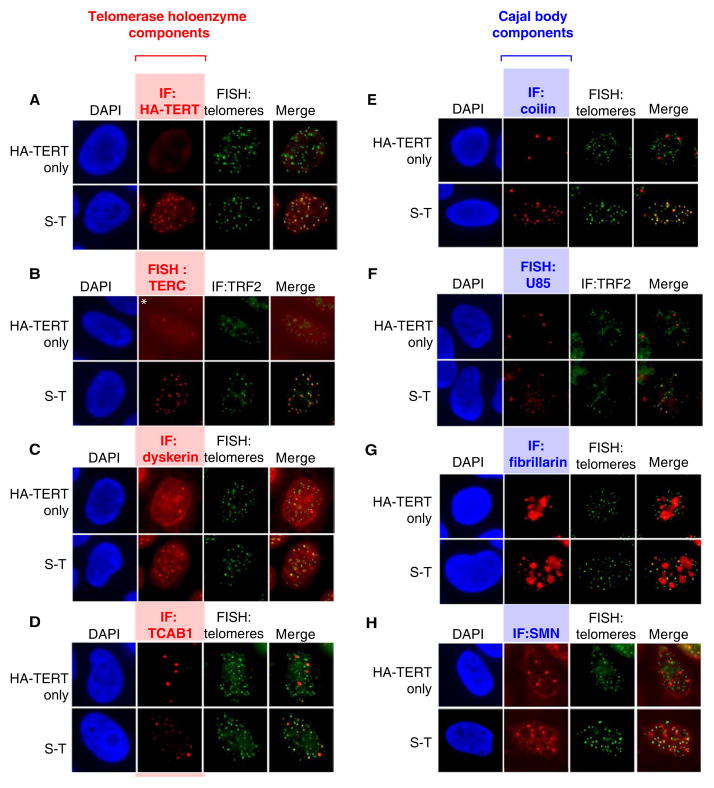
To investigate telomerase recruitment, we employed a modified “super telomerase” assay that uses transient, plasmid-based expression to overcome inherent limitations in expressing TERC from a retrovirus (Figure S1A–C). Elevated expression of TERC together with HA-tagged TERT resulted in uniform colocalization of telomerase with telomeres by RNA FISH and by immunostaining with an anti-HA antibody, respectively (Figure 1A, B). In the absence of co-expressed TERC, HA-TERT was detected by immunofluorescence in a nucleoplasmic pattern. When transfected alone in cells lacking overexpressed TERT, transient TERC was found in Cajal bodies and rarely colocalized with telomeres (Figure S1D). To investigate whether other telomerase holoenzyme components were also found at telomeres, we performed immunofluorescence for dyskerin and TCAB1. Dyskerin and TCAB1 efficiently colocalized with telomeres in S-T cells, but showed minimal overlap with telomeres in cells expressing HA-TERT alone, where they accumulated in their typical nuclear compartments, nucleoli and Cajal bodies, respectively (Figure 1C,D). Taken together, these results indicate that telomerase foci at telomeres in super-telomerase (S-T) cells contain the entire holoenzyme.
Figure 1. Telomerase holoenzyme components at telomeres and formation of neo-Cajal bodies in S-T cells.
HeLa cells transduced with an HA-TERT retrovirus were transfected with a TERC expression plasmid (S-T, super-telomerase) or empty vector (HA-TERT only).
(A–D) Immunofluorescence or FISH for telomerase components and telomeres using: (A) anti-HA antibody (red), DNA FISH for telomeres (green); (B) RNA FISH for TERC (red), anti-TRF2 antibody (green); (C) anti-dyskerin antibody (red), DNA FISH for telomeres (green); (D) anti-TCAB1 antibodies (red), DNA FISH for telomeres (green).
(E–H) Immunofluorescence or FISH for Cajal body components and telomeres using: (E) anti-coilin antibody (red), DNA FISH for telomeres (green); (F) RNA FISH for U85 (red), anti-TRF2 antibody (green); (G) anti-fibrillarin antibody (red), DNA FISH for telomeres (green); (H) anti-SMN antibodies (red), DNA FISH for telomeres (green).
See also Figure S1.
The striking localization of telomerase holoenzyme components to telomeric foci in S-T cells suggested the possibility that Cajal bodies were forming de novo at telomeres. To test this idea, we stained for telomeric DNA and for coilin, a classical marker of Cajal bodies. Remarkably, we found colocalization of coilin at most telomeres in S-T cells, whereas in control cells coilin was detected in classical Cajal bodies that typically did not co-localize with telomeres (Figure 1E). The number of total Cajal bodies in S-T cells (17.5±5.5 per nucleus, n=75) far exceeded that of cells expressing HA-TERT alone (3.0±1.5 per nucleus, n=150, p<0.001 by Fisher’s Exact Test) (Figure 1E), suggesting that telomerase overexpression results in ‘neo-Cajal bodies’ at telomeres and that these new foci may contain other Cajal body components. To test this hypothesis, we stained S-T cells for other well-characterized Cajal body components, including the scaRNA U85, fibrillarin and SMN, which do not participate in telomerase function. In control cells expressing HA-TERT alone, scaRNA U85 was detected exclusively in 3–5 strong nuclear foci by RNA FISH, consistent with its Cajal body localization (Figure 1F, top); fibrillarin was found in both Cajal bodies and the nucleolus consistent with its role in modification of splicing RNAs and rRNAs (Figure 1G, top) and SMN protein was detected primarily in 3–10 foci per nucleus consistent with its localization in both nuclear gems and Cajal bodies (Figure 1H, top). In contrast, scaRNA U85, fibrillarin and SMN were each readily detected at telomeres in S-T cells (Figure 1F, G, H, bottom), indicating that the foci at telomeres in S-T cells resemble bona fide Cajal bodies and that overexpression of telomerase forms neo-Cajal bodies at telomeres.
Depletion of TIN2 or TPP1 stalls telomerase recruitment in conventional Cajal bodies
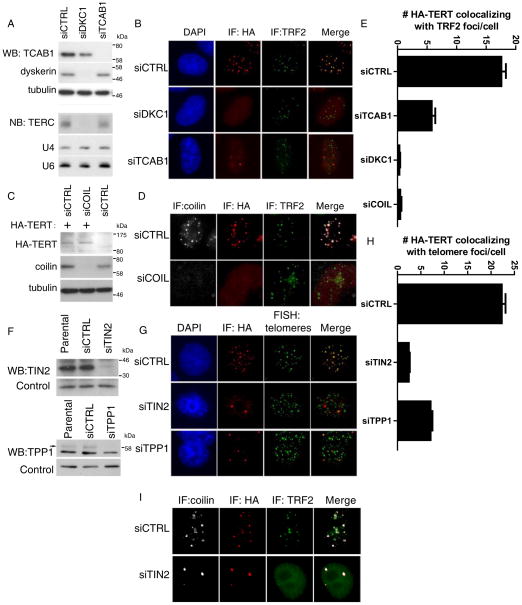
To understand the requirements for formation of telomerase foci at telomeres, we depleted proteins implicated in telomerase recruitment to telomeres with siRNAs. Dyskerin depletion led to a loss of TERC (Figure 2A) and eliminated TERT foci at telomeres in S-T cells consistent with a requirement for TERC in telomerase recruitment to telomeres (Figure 2B, middle). Depletion of TCAB1 efficiently diminished the number of telomerase foci at telomeres and caused HA-TERT to mislocalize to nucleoli (Figure 2A–B, bottom). Some HA-TERT foci persisted at telomeres in siTCAB1-treated cells (Figure 2G, 17.6 ± 0.8 in control siRNA treated vs 5.8 ± 0.6 siTCAB1 treated cells, p<0.0001 by Fisher’s exact test), likely due to incomplete depletion of the protein (data not shown). These results indicate that TCAB1 is needed for efficient recruitment to telomeres, consistent with previous studies showing a requirement for TCAB1 in localization of endogenous TERC to telomeres (Figure S2A) (Venteicher et al., 2009; Stern et al., 2012). To determine whether Cajal bodies themselves are required for telomerase recruitment, we treated cells with coilin siRNA, which efficiently depleted coilin protein by Western blot (Figure 2C) and resulted in loss of coilin-positive Cajal bodies in both HeLa cells and S-T cells (Figure 2D, E). Coilin depletion eliminated HA-TERT foci at telomeres in S-T cells (Figure 2D), without affecting HA-TERT protein levels or telomerase activity (Figure 2C, Figure S2B, C). These data show that coilin, which serves as a scaffold for assembly of Cajal bodies, is required for formation of telomerase foci at telomeres in S-T cells.
Figure 2. Depletion of TIN2 or TPP1 stalls telomerase recruitment in conventional Cajal bodies.
(A) Western blot for TCAB1 and dyskerin in S-T HeLa cells treated with siRNAs indicated (top). Northern blot for TERC (bottom). U4 and U6 RNAs, loading controls.
(B) S-T HeLa cells were transfected with siRNAs indicated, followed by immunofluorescence using anti-HA antibody (red) and anti-TRF2 antibody (green).
(C) Western blot for HA-TERT and coilin in S-T cells treated with siRNAs indicated. Tubulin, loading control.
(D) S-T HeLa cells were transfected with coilin siRNAs, followed by Immunofluorescence using antibodies against coilin (white), HA-TERT (red) and TRF2 (green).
(E) Quantification of (B) and (D), >100 nuclei were scored for number of HA-TERT foci at telomeres. Error bars, S.E.M. p<0.005 by Student’s T test.
(F) Western blot for TIN2 and TPP1 in S-T HeLa cells treated with indicated siRNAs. Nonspecific bands, loading controls.
(G) S-T HeLa cells were transfected with TIN2 or TPP1 siRNAs, followed by Immunofluorescence using antibodies against HA-TERT (red) and DNA-FISH with a telomere probe (green).
(H) Quantification of (G), >100 nuclei were scored for the number of HA-TERT foci at telomeres. Error bars, S.E.M. p<0.005 by Student’s T test.
(I) S-T HeLa cells were transfected with TIN2 siRNAs, followed by Immunofluorescence using antibodies against coilin (white), HA-TERT (red) and TRF2 (green).
See also Figure S2, S3.
To understand the determinants at telomeres that control formation of telomerase foci, we used RNA interference to deplete TIN2 or TPP1, each of which had been implicated in telomerase recruitment (Abreu et al., 2010). Treatment of S-T cells with siRNAs against TIN2 or TPP1 efficiently depleted each protein (Figure 2F), which compromised telomere end-protection, resulting in 53BP1-positive DNA damage foci at telomeres (Takai et al., 2003)(Figure S3). Loss of TIN2, but not TPP1, led to reduced TRF2 protein at telomeres, consistent with the role of TIN2 as a core shelterin component (Figure 2I) (de Lange, 2005; Takai et al., 2011). In agreement with previous work, depletion of either TIN2 or TPP1 resulted in a loss of HA-TERT foci colocalizing with telomeres (Figure 2G–H)(Abreu et al., 2010). Interestingly, instead of colocalizing with telomeres, HA-TERT was detected in a small number of bright foci that also stained positive for coilin in cells treated with siRNA against either TIN2 or TPP1 (2.5±0.2, 7.0±0.4 per nucleus for siTIN2 and siTPP1, respectively, vs. 22.4±0.8 in control treated cells) (Figure 2G–H). The number and morphology of these foci were indistinguishable from Cajal bodies in control HeLa cells (data not shown), indicating that loss of TIN2 or TPP1 arrests telomerase in Cajal bodies.
A tethered TPP1 OB-fold domain recruits telomerase to a non-telomeric chromatin locus
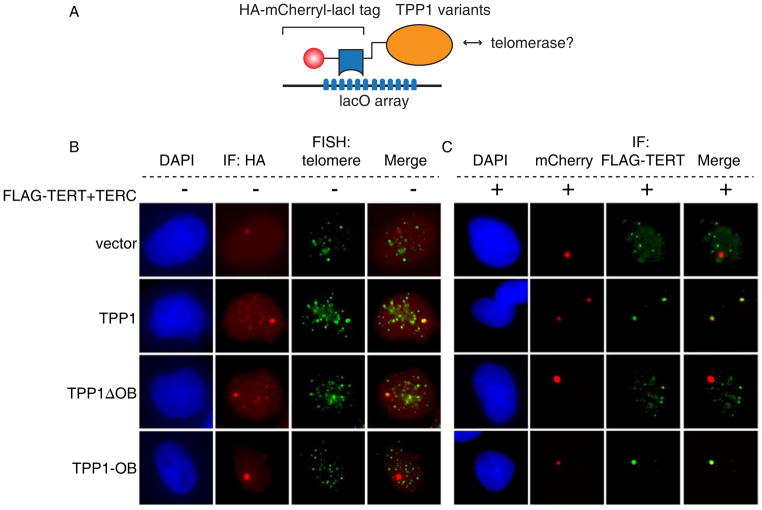
Studying telomerase recruitment using loss-of-function approaches is limited by: (1) the interdependence of many shelterin components for optimal accumulation (Rai et al., 2011; Takai et al., 2011) and by (2) the fact that perturbing shelterin proteins induces a DNA damage response at telomeres, which could in turn affect recruitment of telomerase. To develop an assay that would facilitate testing potential interactions between telomerase and candidate interacting partners outside the context of both the shelterin complex and telomeric DNA, we employed a tethering strategy that allows the expression of a lacI fusion protein ‘bait’ to be visualized as a strong single nuclear focus at a multimerized lacO array stably integrated into a single genomic locus in U2OS2-6-3 cells (Janicki et al., 2004)(Figure 3A). We used this approach to study potential interactions between telomerase and TPP1 at a heterologous chromatin site and in isolation from the effects of DNA damage responses at uncapped telomeres. Whereas HA-lacI-mCherry localized only in a single lacO array focus in the nucleus, the HA-lacI-mCherry-TPP1 fusion protein localized both to the lacO array and to telomeres, indicating that the TPP1 fusion protein retains the ability to be incorporated into the shelterin complex at telomeres (Figure 3B, first and second row). In addition, telomere signals were readily detected within the HA-lacI-mCherry-TPP1 focus at the lacO array using a telomere FISH probe, indicating that the immobilized TPP1 fusion protein recruits telomeres to the lacO focus. In U2OS2-6-3 cells cotransfected with the HA-lacI-mCherry tag, Flag-TERT and TERC, HA-lacI-mCherry remained in a single lacO array focus and did not interfere with the ability of telomerase to localize to telomeres (Figure 3C, first row and data not shown). In contrast, in cells expressing HA-lacI-mCherry-TPP1, FLAG-TERT localization to telomeres was diminished, and instead, FLAG-TERT was detected together with HA-lacI-mCherry-TPP1 in the lacO array focus (Figure 3C, second row). In this setting, HA-lacI-mCherry-lacI-TPP1 acted as a sink to preferentially recruit telomerase to the lacO array, effectively competing for telomerase binding sites at telomeres.
Figure 3. A tethered TPP1 OB-fold domain recruits telomerase to a non-telomeric locus.
(A). Tethering system uses TPP1 variants fused with an HA-mCherry-lacI tag transfected in cells containing an integrated lacO array.
(B). U2OS2-6-3 cells transfected with HA-mCherry-lacI tagged TPP1 variants were stained for HA (red) followed by telomere-FISH (green).
(C). U2OS2-6-3 cells transfected with FLAG-TERT, TERC and indicated HA-mCherry-lacI tagged TPP1 variants were analyzed for TPP1 by mCherry epifluorescence (red) and for TERT by immunofluorescence with anti-FLAG (green).
To determine whether the OB-fold of TPP1, previously implicated in binding TERT (Xin et al., 2007; Abreu et al., 2010), mediated recruitment of telomerase to the lacO array, we constructed a fusion protein lacking the OB-fold (HA-lacI-mCherry-TPP1ΔOB) and a minimal fusion protein comprising only the OB-fold of TPP1 (HA-lacI-mCherry-TPP1OB). HA-lacI-mCherry-TPP1ΔOB localized to the lacO array and to telomeres (Figure 3B, third row), but was unable to recruit telomerase to the lacO array and could no longer compete telomerase away from telomeres (Figure 3C, third row). Conversely, HA-lacI-mCherry-TPP1OB, which localized only to the lacO array (Figure 3B, bottom row), effectively recruited FLAG-TERT to the lacO array and blocked telomerase binding to telomeres (Figure 3C, bottom row). Importantly, telomeres were not detected at the lacO focus in cells expressing HA-lacI-mCherry-TPP1OB (Figure 3B, bottom row), indicating that HA-lacI-mCherry-TPP1OB likely recruited telomerase in the absence of other shelterin components. Taken together, these results show that the OB-fold domain of TPP1, when isolated from telomeric DNA and other shelterin components, is necessary and sufficient to recruit telomerase to a heterologous chromatin locus.
Specific loop residues within the TPP1 OB-fold mediate recruitment of telomerase to telomeres
To further understand the interaction between telomerase and the TPP1 OB-fold domain, we tested whether overexpressed TPP1 OB-fold could effectively compete with endogenous TPP1 for telomerase binding. Because TPP1-OB lacks the ability to be incorporated into the shelterin complex at telomeres, we reasoned that an isolated and un-tethered TPP1-OB would sequester telomerase away from telomeres in a dominant negative manner. To investigate this hypothesis, we developed a ‘competitive sequestration’ assay. Specifically, mCherry-tagged TPP1 OB-fold was cotransfected along with GFP-TERT and TERC in HeLa cells (Figure 4A–C). Similar to super-telomerase cells described above, GFP-TERT localized to telomeres in the presence of TERC (Figure 4C, top row). In contrast to the mCherry vector, expression of mCherry-TPP1 OB (mCherry-OB) abolished localization of GFP-TERT to telomeres and caused GFP-TERT to be sequestered within conventional Cajal bodies (Figure 4C, middle row), results reminiscent of TPP1 depletion in S-T HeLa cells (Figure 2G). Unexpectedly, mCherry-OB itself strongly accumulated within Cajal bodies together with GFP-TERT, indicating that a telomerase-mCherry-OB complex was sequestered in Cajal bodies. Localization of mCherry-OB in Cajal bodies was not observed without coexpressed TERT and TERC. Taken together, we conclude that overexpressed TPP1 OB-fold acts as a competitive inhibitor of telomerase recruitment, presumably by blocking an interacting surface on telomerase that is engaged by endogenous TPP1 during normal telomerase action at telomeres. These results further support the necessity and sufficiency of TPP1-OB in recruiting telomerase from Cajal bodies to telomeres.
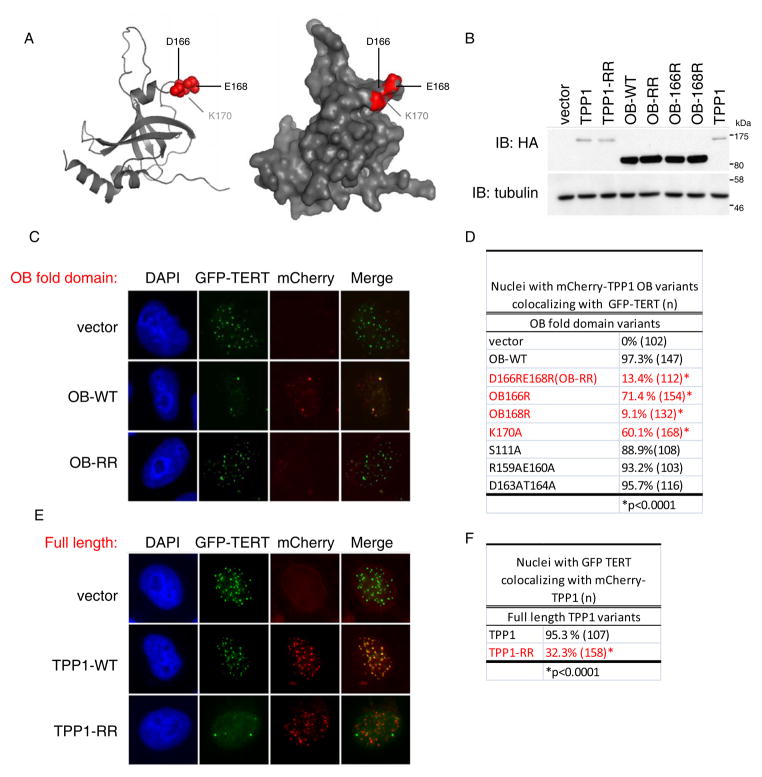
Figure 4. Loop residues in TPP1-OB are required for telomerase recruitment.
(A) Structural representation of TPP1-OB domain (PDB 2i46). Residues required for telomerase interaction shown in red.
(B) Western blot for TPP1 variants transfected in HeLa cells, assayed using anti-HA antibody.
(C) Competitive sequestration assay in HeLa cells transfected with GFP-TERT, and TERC together with mCherry tagged TPP1-OB, TPP1-OB-D166RE168R (OB-RR) or empty vector. Epifluorescence for GFP-TERT (green) and mCherry-OB (red).
(D) Quantification of colocalization between mCherry-TPP1-OB mutants and GFP-TERT from assay in (C). More than 100 nuclei scored. P value, Fisher’s Exact Test.
(E) Competitive sequestration assay in HeLa cells transfected with GFP-TERT, TERC and mCherry-tagged full-length TPP1 or TPP1-D166RE168R (TPP1-RR). Epifluorescence for GFP-TERT (green) and mCherry-OB (red).
(F) Quantification of (E). More than 100 nuclei scored. P value, Fisher’s Exact Test.
See also Figure S4, S5.
The structure of the TPP1-OB-fold domain is closely related to the structure of certain OB-folds in telomere-associated or telomerase-associated proteins from other species (Wang et al., 2007; Xin et al., 2007). In Saccharomyces cerevisiae and Candida albicans, Est3 is a telomerase-associated co-factor whose OB-fold shows structural similarity to TPP1-OB-fold when modeled using structure prediction algorithms. Sequences within Est3 responsible for binding yeast telomerase have been identified using functional and biochemical assays (Lee et al., 2008; Yu et al., 2008). Using structure-guided mutagenesis, we sought to identify specific amino acids in the TPP1-OB-fold domain required for association with human telomerase. We chose residues that were solvent-exposed based on the TPP1-OB-fold crystal structure, including those that were conserved in mammals, present in loop regions connecting β-strands, and near the analogous Est3-yeast telomerase association site (Figure S5). Using the ‘competitive sequestration’ assay described above, we tested each TPP1-OB variant for its ability to inhibit telomerase localization to telomeres and sequester telomerase within Cajal bodies (Figure S4).
Many mutations in TPP1-OB had no effect on the efficiency of mCherry-OB in blocking localization of GFP-TERT to telomeres (Figure 4D, Figure S4), indicating that these residues are dispensable for TPP1-OB association with TERT. These included double mutants R159A;E160A and D163A;T164A, both of which reside in a short alpha-helix (helix αβ) (Figure 4D and S4A–D). Mutation of a conserved serine in loop LA1 (S111A) similarly had no effect on the activity of mCherry-OB (Figure 4D and S4A–D). In marked contrast, a double charge swap mutation – D166R;E168R, hereafter referred to as OB-RR – in conserved residues in loop L34 completely eliminated the activity of mCherry-OB (Figure 4A, C). mCherry-OB-RR was expressed at similar levels compared to wild-type mCherry-OB, but failed to inhibit localization of GFP-TERT to telomeres, and as a result was not detected in Cajal bodies (Figure 4B, C, bottom row). Deconvolution of this double mutant revealed that E168R was more severely impaired in its ability to sequester telomerase away from telomeres than D166R (Figure 4C and S4A–D), suggesting that the inactivity of the OB-RR mutant is largely due to mutation of E168. Introduction of K170A at a nearby residue in the same loop caused a modest reduction in the activity of mCherry-OB in this assay (Figure 4A,D and S4A–D). Introduction of the RR mutations into full length TPP1 revealed that both wild-type mCherry-TPP1 and mCherry-TPP1-RR localized to telomeres in HeLa cells. Although wild-type mCherry-TPP1 did not interfere with the localization of GFP-TERT at telomeres, mCherry-TPP1-RR effectively blocked the ability of GFP-TERT to localize to telomeres, resulting in localization of GFP-TERT to Cajal bodies (Figure 4E,F). In this case, mCherry-TPP1-RR inhibited telomerase recruitment to telomeres presumably by competing away endogenous TPP1 from the shelterin complex, replacing it with a mutant defective in the ability to associate with telomerase. Taken together, these data show that residues D166, E168 and K170 are required for telomerase association and may define a critical surface required for interaction between TPP1 and telomerase.
Telomerase-TPP1-OB fold association is essential for telomere maintenance
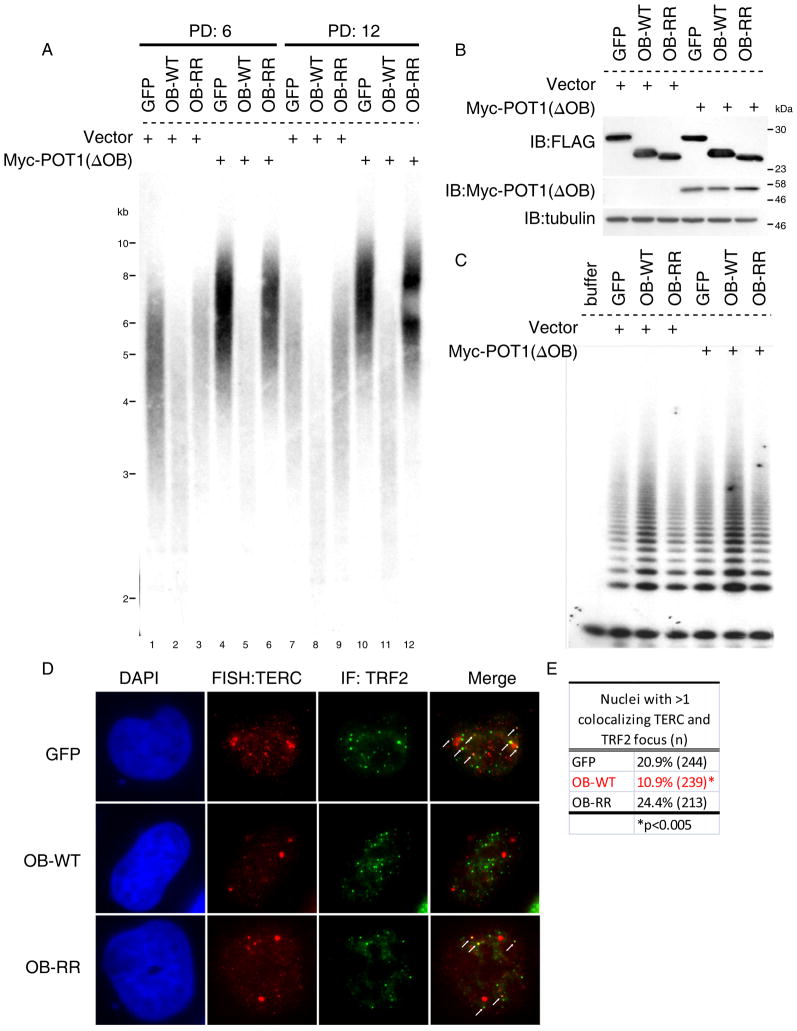
To test the functional significance of the telomerase-TPP1 OB interaction in telomere length maintenance, we tested whether isolated TPP1-OB fold domain could prevent telomerase from elongating telomeres. We used retroviral transduction to express wild-type TPP1-OB-fold domain, TPP1-OB-RR or GFP as a negative control in HTC75 cells, a telomerase-positive fibrosarcoma cell line widely used to study telomere maintenance (Smogorzewska et al., 2000). After selection, each culture was transduced either with an empty vector or with a retrovirus expressing Myc-POT1(ΔOB), a POT1 variant that lacks the N-terminal OB-fold domain. Myc-POT1(ΔOB) causes rapid telomere elongation by telomerase, presumably by relieving inherent negative regulation at the chromosome terminus (Loayza and De Lange, 2003). As expected, telomeres significantly elongated in cells expressing GFP and Myc-Pot1(ΔOB) through successive population doublings (Figure 5A, Lane 1 vs 4 and 7 vs 10). In comparison, telomere elongation by Myc-Pot1(ΔOB) was abrogated by prior expression of wild-type TPP1-OB (Figure 5A, Lane 2 vs 5 and 8 vs 11). This inhibitory effect of TPP1-OB was dependent on its association with telomerase, because expression of TPP1-OB-RR exerted no inhibitory effect on telomere elongation by telomerase in Myc-Pot1(ΔOB) cells. Furthermore, telomeres in cells expressing wild-type TPP1-OB (without Myc-POT1(ΔOB)) showed rapid telomere shortening as compared to GFP-expressing cells in which telomere lengths were maintained with passage. By population doubling 12, the mean telomere length in cells expressing TPP1-OB was 1.5kb shorter than cells expressing GFP (Figure 5A, Lane 7 vs 8). In contrast, TPP1-OB-RR showed no effect on telomere maintenance (Figure 5A, Lane 1 vs 3 and 7 vs 9), despite similar expression compared to wild-type TPP1-OB protein (Figure 5B). The strong inhibitory effect of TPP1-OB was not due to a reduction in telomerase catalytic function; catalytic assays performed on extracts from these cells showed no inhibition of enzymatic activity by expression of TPP1-OB (Figure 5C). In addition, TPP1-OB did not interfere with cell growth (Figure S6A, B) or telomere protection, as there was no increase in DNA damage foci at telomeres (Figure S6 C,D). These findings demonstrate the TPP1 OB-fold inhibits telomere length maintenance by telomerase both at the basal level and in the context of rapid telomere elongation induced by POT1(ΔOB).
Figure 5. TPP1-OB inhibits telomere length maintenance by telomerase and blocks endogenous telomerase recruitment.
(A) Telomere lengths by Southern blot in HTC75 cells transduced first with retroviruses expressing FLAG-GFP, FLAG-OB-WT or FLAG-OB-RR, followed by transduction with either empty vector or Myc-POT1(ΔOB). Genomic DNA harvested at population doubling (PD) 6 and 12 for Southern.
(B) Western blots for expression of OB variants and Myc-POT1(ΔOB) in cells used in (A).
(C) TRAP assays for telomerase activity in cells used in (A).
(D) RNA FISH for endogenous TERC colocalization (red) with TRF2 (green) by immunofluorescence using anti-TRF2 antibody in HeLa cells transduced with retroviruses expressing GFP, TPP1-OB or TPP1-OB-RR. Arrows, TERC foci colocalizing with telomeres.
(E) Quantification of TERC and TRF2 colocalization in (D). More than 200 nuclei scored; P value, Fisher’s Exact Test.
See also Figure S6.
To understand how expression of TPP1-OB variants affected recruitment of endogenous telomerase to telomeres, we performed RNA FISH for endogenous TERC in HeLa cells stably transduced with TPP1-OB or TPP1-RR. The frequency of cells harboring TERC foci that overlapped with TRF2 was significantly diminished by overexpression of TPP1-OB, but not by TPP1-OB-RR (Figure 5D, E, Figure S6E). These findings corroborate our observations from our ‘competitive sequestration’ assay (Figure 4) at the endogenous level and provide functional evidence that association between the OB-fold domain of TPP1 and telomerase is essential for telomerase to be efficiently recruited to telomeres and to synthesize telomere repeats.
IPF mutations in TERT block recruitment and show diminished association with TPP1 OB-fold
Based on these findings indicating a functionally important association between TPP1 and telomerase, we hypothesized that specific amino acid residues within TERT are required for its recruitment to telomeres. To address this question, we carried out domain mapping studies on TERT by generating a panel of TERT deletion mutants (Figure S7A). HA-tagged TERT mutants and TERC were co-expressed in HeLa cells and assayed for their localization by triple immunofluorescence staining for HA-TERT, TRF2 and coilin. We found three distinct classes of localization patterns for these deletion mutants (Figure S7A). Whereas wild-type TERT showed robust colocalization with TRF2, the N-terminal truncations of TERT were detected in a nucleoplasmic pattern (Figure 6B; ΔTEN). C-terminal deletions within TERT abrogated telomere localization, but instead showed strong accumulation in coilin-positive Cajal bodies (Figure 6B; ΔCTE). Based on these results, we conclude that Cajal body-localization and telomere association are each governed by distinct structural domains of TERT. The N-terminus of TERT, including the TEN and TRBD domains, is essential for Cajal body localization, while the CTE is required for recruitment to telomeres.
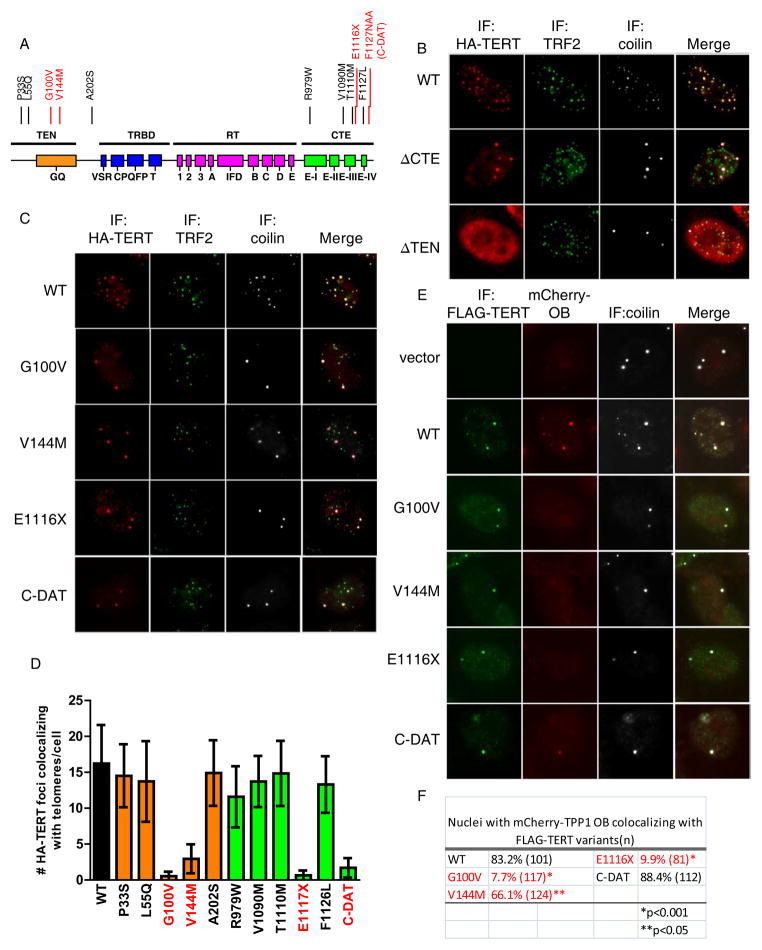
Figure 6. Subset of TERT mutations in IPF arrest telomerase in Cajal bodies due to impaired interaction with TPP1-OB.
(A). Summary of TERT functional domains, adapted from (Podlevsky and Chen, 2012). Point mutations in the TEN and CTE domains indicated; recruitment-defective mutants V144M, G100V, E1117X and C-DAT highlighted in red.
(B). Localization of HA-tagged TERT deletion variants in HeLa cells also transfected with TERC. Immunofluorescence using antibodies against HA (red), TRF2 (green) and coilin (white).
(C). Immunofluorescence for HA-TERT (red), TRF2 (green) and coilin (white) in HeLa cells transfected with TERT point mutants in the TEN and CTE domains, along with TERC.
(D). Quantification TERT-mutant colocalization with telomeres from (C). At least 100 nuclei scored. Error bars, S.E.M. p<0.0001 for TERT mutants in red, by two-tailed Student’s t-test.
(E). Competitive sequestration assay in HeLa cells transfected with FLAG-TERT mutants, and TERC together mCherry-TPP1-OB. Immunofluorescence using anti-FLAG antibody (green), and anti-coilin antibody (white), together with mCherry epifluorescence to detect mCherry-TPP1-OB (red).
(F). Quantification of (E). At least 100 nuclei scored for colocalization of mCherry-OB and FLAG-TERT. P values, Fisher’s Exact Test.
See also Figure S7.
Upon establishing the roles of the TEN and CTE domains in telomerase trafficking, we examined a panel of disease-associated or engineered TERT point mutants in the TEN and CTE domain (Figure 6A). Four mutants - G100V, V144M, E1117X and F1127NAA (C-DAT) - were found to be defective in localizing to telomeres when coexpressed with TERC (Figure 6C, D) and strongly accumulated in Cajal bodies. This pattern was reminiscent of the relocalization of TERT into Cajal bodies upon depletion of TPP1 or upon expression of the OB-fold of TPP1 (Figures 2 and 4). To determine whether these mutants failed to be recruited to telomeres because of a defect in association with TPP1, we coexpressed each mutant with mCherry-TPP1-OB. Three of the four mutants - G100V, V144M, E1117X - were significantly impaired in capturing mCherry-TPP1-OB into Cajal bodies, consistent with a defect in association between these mutant TERT proteins and the OB-fold of TPP1. The C-DAT mutant retained the ability to colocalize within mCherry-OB in Cajal bodies, although mCherry fluorescence intensity was reduced in Cajal bodies as compared to wild-type TERT, evidence for weaker association with mCherry-OB (Figure 6E, F). These data indicate that the mutations in the TEN and CTE domains of TERT block association with TPP1 OB-fold and this defect explains their inability to be recruited to telomeres.
Importantly, the ability of the examined TERT variants to localize to telomeres was unrelated to their catalytic activity measured in vitro (Figure S7B). The V144M and E1117fsX mutants derive from patients with idiopathic pulmonary fibrosis (Yamaguchi et al., 2005; Armanios et al., 2007; Tsakiri et al., 2007). Although E1117fsX has diminished catalytic activity, V144M retained wild-type activity in in vitro assays (Tsakiri et al., 2007; Tsang et al., 2012). Our results suggest that defective trafficking from Cajal bodies to telomeres underlies the telomerase dysfunction in patients with the V144M mutation. G100V is an engineered mutation in the TEN domain and has been shown to be essential for the enhancement of telomerase processivity mediated by recombinant TPP1-POT1 (Zaug et al., 2010). Thus, an impaired interaction between TERT-G100V and TPP1-OB-fold may explain both the absence of processivity enhancement in this mutant and the defect in telomerase recruitment. F1127NAA (C-DAT) is an engineered mutation that “dissociates activities of telomerase” (DAT) by preserving enzymatic function while interfering with the ability of telomerase to immortalize primary human cells (Banik et al., 2002; Armbruster et al., 2003). It is worth noting that all the recruitment-disrupting mutations lie within or close to the previously characterized DAT domains in the TEN and CTE domains, raising the possibility that the DAT domains represent structural motifs directly involved in recruitment. Taken together, these data show that specific residues within TERT govern recruitment to telomeres via interaction with the TPP1-OB-fold domain and that this recruitment step is impaired in a subset of patients with pulmonary fibrosis.
Discussion
A step-wise model for telomerase recruitment to telomeres
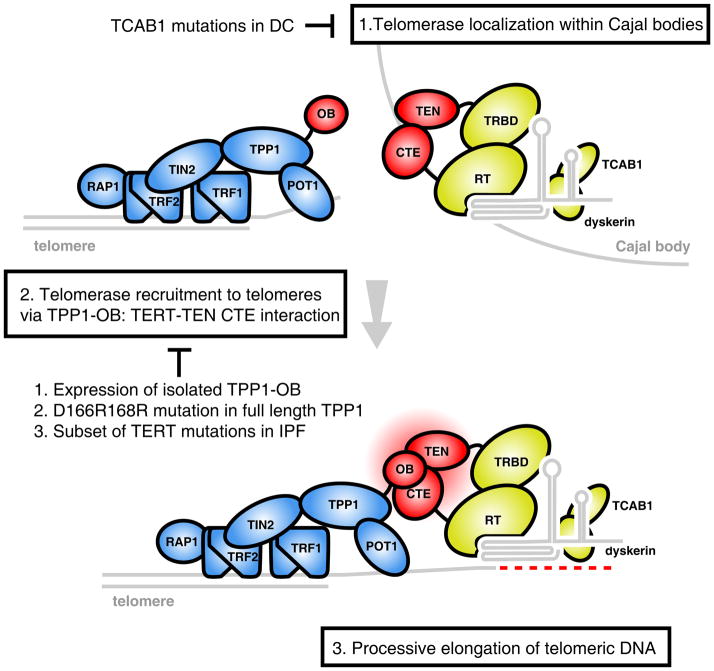
Recruitment of large protein complexes to their sites of action on chromatin is a critical rate-limiting step for many biological processes, such as the formation of kinetochores at centromeres and the origin-of-replication complexes on DNA. Similarly, telomerase must be recruited to telomeric chromatin to synthesize telomere repeats, although this process has been difficult to dissect in human cells. In this study, we identified an obligatory interaction between the telomerase RNP and the OB-fold domain of the shelterin component TPP1 that recruits telomerase from a Cajal body reservoir to telomeric chromatin. Blocking the telomerase-TPP1 OB-fold interaction inhibited telomerase recruitment to telomeres and abrogated telomere synthesis by telomerase in vivo.
Our findings suggest a stepwise mechanism for recruitment of the telomerase RNP to telomeres (Figure 7). In the first step, telomerase localizes to Cajal bodies by virtue of the interaction between the TERC CAB-box sequence and the Cajal body-enriched telomerase holoenzyme component, TCAB1 (Step 1, Figure 7, top). The importance of the Cajal body is suggested by observations showing that mislocalization of telomerase to the nucleolus, either by TCAB1 depletion (Figure 2) or by TERC CAB-box mutations inhibits telomerase recruitment and telomere synthesis by telomerase (Cristofari et al., 2007; Venteicher et al., 2009; Zhong et al., 2011). Enhanced loading of telomerase on telomeres in super-telomerase cells leads to formation of neo-Cajal bodies at telomeres, and furthermore, depletion of the Cajal body protein coilin can reduce telomerase foci at telomeres (Figure 2) (Stern et al., 2012). Together, these findings establish the Cajal body as an important reservoir for telomerase, but it remains uncertain precisely what function is served by Cajal bodies and whether the bodies themselves are required for telomere synthesis by telomerase.
Figure 7. Model for a stepwise mechanism of telomerase to telomeres.
Telomerase accumulates in Cajal bodies, a step disrupted in patients with mutations in TCAB1 (1). Association between the TPP1 OB-fold and the TEN and CTE domains of TERT (colored red) mediates recruitment to telomeres, a step dependent on specific OB-fold loop residues and sequences in TERT mutated in IPF patients (2). Once recruited to telomeres, TPP1-telomerase association may support processive elongation of telomeres (3).
TPP1-OB fold: a docking platform for telomerase recruitment and an enzymatic cofactor for processivity
In the second step (Figure 7, Step 2), telomerase RNP is recruited to telomeres via an obligatory ‘docking’ step, whereby the TERT TEN and CTE domains interact with the OB-fold domain of TPP1 (Step 2, Figure 7). The importance of this interaction is supported by a number of observations. First, removal of TPP1 from telomeres by using short interfering RNAs against TPP1 or TIN2 abrogated the ability of overexpressed telomerase to localize to telomeres and led to strong accumulation in Cajal bodies (Figure 2)(Abreu et al., 2010). Second, tethered TPP1-OB fold alone was able to recruit telomerase to a heterologous non-telomeric locus (Figure 3). Third, the expression of TPP1-OB fold competitively sequestered overexpressed telomerase away from telomeres into Cajal bodies. Furthermore, the TPP1-OB domain itself was captured by telomerase in this context, localizing in Cajal bodies (Figure 4). Fourth, mutations in TPP1 OB-fold, such as OB-RR, or TERT mutations in the TEN and CTE domain (Figure 6) abrogated this interaction and inhibited telomerase recruitment, again arresting telomerase in its pre-recruitment, Cajal body-localized state.
In the third step (Figure 7, Step 3), telomerase engages with telomeric DNA substrates and processively synthesizes telomeric repeats. Previously, the TPP1-POT1 heterodimer has been shown to aid telomerase catalysis by enhancing its processivity on oligonucleotide substrates in vitro. A single amino acid mutation in TERT(G100V) abrogated the processivity enhancement by TPP1-POT1 (Zaug et al., 2010). We found that the same mutation severely impairs TERT-TPP1 OB interaction and telomerase recruitment. Based on these observations, we postulate that the processive elongation of telomeric DNA and telomerase recruitment rely on the same TERT TEN-CTE: TPP1-OB interaction, which persists after the docking step and throughout the entire duration of telomerase catalysis (Step 7, bottom). In other words, TERT TEN-CTE: TPP1 OB interaction not only recruits telomerase to telomeres, but also allows telomerase to be tethered to the shelterin bound telomeric DNA substrate, preventing premature release of the telomere substrate and/or aiding telomerase translocation on telomeric tracts (Latrick and Cech, 2010). It is possible that the residues which we have identified in the TPP1 OB-fold – D166, E168 and K170 – may contribute to the actual interface at which TPP1 and telomerase interact. These residues in the L34 loop are solvent-exposed and form a ridge along a groove running across the TPP1 OB-fold structure. The development of small molecules targeting this region of the OB-fold could act as telomerase inhibitors to block telomere synthesis, in an analogous fashion to the effects of overexpressed OB-fold which we describe here.
Dual function of the shelterin complex in telomerase regulation
The shelterin complex has consistently been found to inhibit telomerase in its ability to lengthen telomeres. This role of the shelterin complex was supported by both loss-of-function and gain-of-function genetic experiments, which demonstrated that telomeres lengthen upon shRNA-mediated depletion of the shelterin components TIN2, POT1 and TPP1 (Loayza and De Lange, 2003; Houghtaling et al., 2004; Liu et al., 2004; Ye and de Lange, 2004; Ye et al., 2004), or by overexpression of TRF1 and TRF2 (Smogorzewska et al., 2000). However, it has been challenging to study the function of a single shelterin protein using these approaches due to the interdependence among these proteins at telomeres (Rai et al., 2011; Takai et al., 2011). Furthermore, disrupting the stoichiometry of the shelterin complex causes a DNA damage response at telomeres, which could affect telomerase recruitment. By using a minimal TPP1-OB fold domain to recruit TERT to a heterologous chromatin locus and by specifically interfering with telomerase-shelterin interaction through expression of the isolated OB-fold domain, we were able to separate the capping function of TPP1 from its interaction with telomerase.
Our results are consistent with a model in which the TPP1-POT1 module serves a dual function at telomeres, restricting telomerase access to the chromosome terminus through POT1 in order to prevent unscheduled telomere elongation, while recruiting telomerase to telomeres via the TPP1-OB fold. POT1 binds single stranded telomeric DNA with high affinity and is a potent inhibitor of telomere extension both in vivo and in vitro (Loayza and De Lange, 2003; Lei et al., 2004; Kelleher et al., 2005). Once telomerase has been recruited to telomeres it may compete with POT1 for the terminal single-stranded overhang for productive elongation. Telomere lengthening upon the depletion of TIN2 or TPP1 may occur because of concomitant decrease in POT1 occupancy at telomeres, favoring telomerase-telomere binding following recruitment by residual TPP1. Alternatively, additional contacts between telomerase and shelterin components could facilitate recruitment in the context of depletion of TIN2 or TPP1.
Telomerase trafficking and disease
Germline mutations in telomerase or in TIN2 result in very short telomeres, which in turn precipitate several disease states including dyskeratosis congenita, aplastic anemia, cancer, liver fibrosis and pulmonary fibrosis (Calado and Young, 2009). Certain telomerase mutations can cause disease without an apparent change in telomerase enzymatic activity. Mutations in TCAB1 cause dyskeratosis congenita by disrupting telomerase trafficking to the Cajal body, while leaving telomerase enzymatic activity intact (Batista et al., 2011; Zhong et al., 2011). Our results with the TERT mutations V144M and E1116fsX from IPF patients reveal that disease mutations can arrest telomerase trafficking in Cajal bodies, rendering telomerase unable to be recruited to telomeres. These findings expand our understanding of the contribution of telomerase trafficking defects in disease: TCAB1 mutations can force mislocalization of telomerase to nucleoli, whereas certain TERT mutations can prevent recruitment and trap telomerase in Cajal bodies. Telomerase activation confers replicative immortalization to primary human cells and is a hallmark of cancer (Hanahan and Weinberg, 2011). Our data demonstrate that telomerase-TPP1 OB interaction is rate limiting for telomere length maintenance in human cancer cells. These findings highlight a potential vulnerability in the telomerase pathway that could be exploited through the development of targeted therapeutics.
Experimental Procedures
Small-Scale Cell Culture, cDNA and siRNA Transfections, and Retroviral Transductions
HeLa, 293T, HTC75 (a gift from T. de Lange) and U2OS2-6-3 cells (a gift from S. Janicki) were grown in DMEM/10% fetal bovine serum /1% penicillin-streptomycin. Lipofectamine 2000 (Life Technologies) was used for all cDNA transfection experiments with and without siRNAs. For transfection with siRNA alone, Dharmafect 4 (Dharmacon) was used. All siRNAs were purchased from Dharmacon as siGENOME pools. Cells were reseeded 24hrs post transfection and assayed 24–48hrs later. For transient overexpression, TERT and TPP1 coding sequences were cloned into pCDNA3.1 (Invitrogen) with indicated amino terminal tags. To generate cells by retroviral gene transfer, 293T cells were first transfected with RSV Gag-pol and VSV-g packaging vectors together with retroviral plasmids. Viral supernatant was collected and 24, 48 and 72hrs post transfection and concentrated using Retro-X concentrator (Clontech). Infected cells were selected in antibiotic containing media up to 1 week. All TERT and TPP1 point mutants were generated using site-directed mutagenesis (QuikChange II, Agilent). See Table S1 for primer sequences.
Immunofluorescence and in situ hybridization
All immunofluorescence was carried out as previously described (Zhong et al., 2011) on cells seeded on coverslips. RNA FISH was carried out using Quasar 570 labeled oligonucleotide probes (Biosearch). See Table S2 for probe sequences. Telomere DNA FISH using PNA probes was carried out as described (Kibe et al., 2010). For combined immunofluorescence (IF) and DNA/RNA FISH, IF was carried out first and cells re-fixed with 1mM DSP in 1xPBS for 5 mins. Images were subsequently acquired with a Leica wide-field fluorescence microscope. LAF AS Lite suite (Leica) and ImageJ were used for image analyses.
Telomere Repeat Amplification Protocol (TRAP) and Telomere Restriction Fragment analysis (TRF)
TRAP was carried out using Trapeze kit according to the manufacturer’s protocol (Milipore) with minor modifications. Cells were lysed in NP40 buffer (25 mM HEPES-KOH, 400 mM NaCl, 1.5 mM MgCl2, 10% glycerol, 0.5% NP40, and 1 mM DTT [pH 7.5] supplemented with protease inhibitors). Each reaction was programmed with 0.5ug to 2ug of protein lysate. To measure telomere lengths during extended culture, HTC75 cells were grown in 6 well plates and reseeded every 3 days. Harvested cells were pelleted and digested with Proteinase K at 6 μg/ml overnight. DNA was extracted using the standard phenol-chloroform based method and digested overnight with HinfI and RsaI before electrophoresis and Southern blotting with an end-labeled (CCATTT)4 oligonucleotide probe.
Supplementary Material
Enforced expression of telomerase forms neo-Cajal bodies at telomeres
TPP1-OB-fold domain recruits telomerase to a heterologous chromatin locus
TPP1-OB alone sequesters telomerase within Cajal bodies and causes telomere shortening
OB-fold mutations and some disease mutations in TERT block telomerase recruitment
Acknowledgments
We thank Amy Li for technical assistance and members of the Artandi lab for helpful discussion. F.Z. was supported by a fellowship from the Agency for Science, Technology, and Research (A*STAR), Singapore. L.F.Z.B. was supported by a fellowship from the California Institute of Regenerative Medicine. A.F. was supported by NIH 5T32 CA09302. M.F.P. is the recipient of an NSF graduate research fellowship. This work was supported by NIH grants AG033747, CA125453, and CA111691, by a SCOR grant from the Leukemia and Lymphoma Society and by the Glenn Foundation for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu E, Aritonovska E, Reichenbach P, Cristofari G, Culp B, Terns RM, Lingner J, Terns MP. TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo. Mol Cell Biol. 2010;30:2971–2982. doi: 10.1128/MCB.00240-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Etheridge KT, Broccoli D, Counter CM. Putative telomere-recruiting domain in the catalytic subunit of human telomerase. Mol Cell Biol. 2003;23:3237–3246. doi: 10.1128/MCB.23.9.3237-3246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik SS, Guo C, Smith AC, Margolis SS, Richardson DA, Tirado CA, Counter CM. C-terminal regions of the human telomerase catalytic subunit essential for in vivo enzyme activity. Mol Cell Biol. 2002;22:6234–6246. doi: 10.1128/MCB.22.17.6234-6246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista LF, Pech MF, Zhong FL, Nguyen HN, Xie KT, Zaug AJ, Crary SM, Choi J, Sebastiano V, Cherry A, et al. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature. 2011;474:399–402. doi: 10.1038/nature10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech TR. Beginning to understand the end of the chromosome. Cell. 2004;116:273–279. doi: 10.1016/s0092-8674(04)00038-8. [DOI] [PubMed] [Google Scholar]
- Chan A, Boule JB, Zakian VA. Two pathways recruit telomerase to Saccharomyces cerevisiae telomeres. PLoS Genet. 2008;4:e1000236. doi: 10.1371/journal.pgen.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K. Physiological assembly and activity of human telomerase complexes. Mech Ageing Dev. 2008;129:91–98. doi: 10.1016/j.mad.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofari G, Adolf E, Reichenbach P, Sikora K, Terns RM, Terns MP, Lingner J. Human telomerase RNA accumulation in Cajal bodies facilitates telomerase recruitment to telomeres and telomere elongation. Mol Cell. 2007;27:882–889. doi: 10.1016/j.molcel.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Cristofari G, Lingner J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 2006;25:565–574. doi: 10.1038/sj.emboj.7600952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzacq X, Kittur N, Roy S, Shav-Tal Y, Singer RH, Meier UT. Stepwise RNP assembly at the site of H/ACA RNA transcription in human cells. J Cell Biol. 2006;173:207–218. doi: 10.1083/jcb.200601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- Egan ED, Collins K. An Enhanced H/ACA RNP Assembly Mechanism for Human Telomerase RNA. Mol Cell Biol. 2012;32:2428–2439. doi: 10.1128/MCB.00286-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG. Cajal bodies: the first 100 years. Annu Rev Cell Dev Biol. 2000;16:273–300. doi: 10.1146/annurev.cellbio.16.1.273. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Houghtaling BR, Cuttonaro L, Chang W, Smith S. A dynamic molecular link between the telomere length regulator TRF1 and the chromosome end protector TRF2. Curr Biol. 2004;14:1621–1631. doi: 10.1016/j.cub.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Jady BE, Bertrand E, Kiss T. Human telomerase RNA and box H/ACA scaRNAs share a common Cajal body-specific localization signal. J Cell Biol. 2004;164:647–652. doi: 10.1083/jcb.200310138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki SM, Tsukamoto T, Salghetti SE, Tansey WP, Sachidanandam R, Prasanth KV, Ried T, Shav-Tal Y, Bertrand E, Singer RH, et al. From silencing to gene expression: real-time analysis in single cells. Cell. 2004;116:683–698. doi: 10.1016/s0092-8674(04)00171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher C, Kurth I, Lingner J. Human protection of telomeres 1 (POT1) is a negative regulator of telomerase activity in vitro. Mol Cell Biol. 2005;25:808–818. doi: 10.1128/MCB.25.2.808-818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibe T, Osawa GA, Keegan CE, de Lange T. Telomere protection by TPP1 is mediated by POT1a and POT1b. Mol Cell Biol. 2010;30:1059–1066. doi: 10.1128/MCB.01498-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latrick CM, Cech TR. POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation. EMBO J. 2010;29:924–933. doi: 10.1038/emboj.2009.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Mandell EK, Tucey TM, Morris DK, Lundblad V. The Est3 protein associates with yeast telomerase through an OB-fold domain. Nat Struct Mol Biol. 2008;15:990–997. doi: 10.1038/nsmb.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Podell ER, Cech TR. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat Struct Mol Biol. 2004;11:1223–1229. doi: 10.1038/nsmb867. [DOI] [PubMed] [Google Scholar]
- Liu D, Safari A, O’Connor MS, Chan DW, Laegeler A, Qin J, Songyang Z. PTOP interacts with POT1 and regulates its localization to telomeres. Nat Cell Biol. 2004;6:673–680. doi: 10.1038/ncb1142. [DOI] [PubMed] [Google Scholar]
- Loayza D, De Lange T. POT1 as a terminal transducer of TRF1 telomere length control. Nature. 2003;423:1013–1018. doi: 10.1038/nature01688. [DOI] [PubMed] [Google Scholar]
- O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- Pennock E, Buckley K, Lundblad V. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell. 2001;104:387–396. doi: 10.1016/s0092-8674(01)00226-4. [DOI] [PubMed] [Google Scholar]
- Podlevsky JD, Chen JJ. It all comes together at the ends: Telomerase structure, function, and biogenesis. Mutat Res. 2012;730:3–11. doi: 10.1016/j.mrfmmm.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R, Li JM, Zheng H, Lok GT, Deng Y, Huen MS, Chen J, Jin J, Chang S. The E3 ubiquitin ligase Rnf8 stabilizes Tpp1 to promote telomere end protection. Nat Struct Mol Biol. 2011;18:1400–1407. doi: 10.1038/nsmb.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA, Alter BP. The role of telomere biology in bone marrow failure and other disorders. Mech Ageing Dev. 2008;129:35–47. doi: 10.1016/j.mad.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Annu Rev Biochem. 2004;73:177–208. doi: 10.1146/annurev.biochem.73.071403.160049. [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, de Lange T. Control of human telomere length by TRF1 and TRF2. Mol Cell Biol. 2000;20:1659–1668. doi: 10.1128/mcb.20.5.1659-1668.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JL, Zyner KG, Pickett HA, Cohen SB, Bryan TM. Telomerase Recruitment Requires both TCAB1 and Cajal Bodies Independently. Mol Cell Biol. 2012;32:2384–2395. doi: 10.1128/MCB.00379-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart AK, Teng SC, Zakian VA. Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science. 2002;297:1023–1026. doi: 10.1126/science.1074968. [DOI] [PubMed] [Google Scholar]
- Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- Takai KK, Kibe T, Donigian JR, Frescas D, de Lange T. Telomere protection by TPP1/POT1 requires tethering to TIN2. Mol Cell. 2011;44:647–659. doi: 10.1016/j.molcel.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejera AM, Stagno d’Alcontres M, Thanasoula M, Marion RM, Martinez P, Liao C, Flores JM, Tarsounas M, Blasco MA. TPP1 is required for TERT recruitment, telomere elongation during nuclear reprogramming, and normal skin development in mice. Dev Cell. 2010;18:775–789. doi: 10.1016/j.devcel.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson RL, Abreu EB, Ziegler T, Ly H, Counter CM, Terns RM, Terns MP. Telomerase reverse transcriptase is required for the localization of telomerase RNA to cajal bodies and telomeres in human cancer cells. Mol Biol Cell. 2008;19:3793–3800. doi: 10.1091/mbc.E08-02-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang AR, Wyatt HD, Ting NS, Beattie TL. hTERT mutations associated with idiopathic pulmonary fibrosis affect telomerase activity, telomere length, and cell growth by distinct mechanisms. Aging Cell. 2012 doi: 10.1111/j.1474-9726.2012.00810.x. [DOI] [PubMed] [Google Scholar]
- Tycowski KT, Shu MD, Kukoyi A, Steitz JA. A conserved WD40 protein binds the Cajal body localization signal of scaRNP particles. Mol Cell. 2009;34:47–57. doi: 10.1016/j.molcel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venteicher AS, Abreu EB, Meng Z, McCann KE, Terns RM, Veenstra TD, Terns MP, Artandi SE. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science. 2009;323:644–648. doi: 10.1126/science.1165357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdun RE, Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–931. doi: 10.1038/nature05976. [DOI] [PubMed] [Google Scholar]
- Wang F, Podell ER, Zaug AJ, Yang Y, Baciu P, Cech TR, Lei M. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature. 2007;445:506–510. doi: 10.1038/nature05454. [DOI] [PubMed] [Google Scholar]
- Xin H, Liu D, Songyang Z. The telosome/shelterin complex and its functions. Genome Biol. 2008;9:232. doi: 10.1186/gb-2008-9-9-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Liu D, Wan M, Safari A, Kim H, Sun W, O’Connor MS, Songyang Z. TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase. Nature. 2007;445:559–562. doi: 10.1038/nature05469. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, Young NS. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- Ye JZ, de Lange T. TIN2 is a tankyrase 1 PARP modulator in the TRF1 telomere length control complex. Nat Genet. 2004;36:618–623. doi: 10.1038/ng1360. [DOI] [PubMed] [Google Scholar]
- Ye JZ, Hockemeyer D, Krutchinsky AN, Loayza D, Hooper SM, Chait BT, de Lange T. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 2004;18:1649–1654. doi: 10.1101/gad.1215404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu EY, Wang F, Lei M, Lue NF. A proposed OB-fold with a protein-interaction surface in Candida albicans telomerase protein Est3. Nat Struct Mol Biol. 2008;15:985–989. doi: 10.1038/nsmb.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappulla DC, Cech TR. RNA as a flexible scaffold for proteins: yeast telomerase and beyond. Cold Spring Harb Symp Quant Biol. 2006;71:217–224. doi: 10.1101/sqb.2006.71.011. [DOI] [PubMed] [Google Scholar]
- Zaug AJ, Podell ER, Nandakumar J, Cech TR. Functional interaction between telomere protein TPP1 and telomerase. Genes Dev. 2010;24:613–622. doi: 10.1101/gad.1881810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong F, Savage SA, Shkreli M, Giri N, Jessop L, Myers T, Chen R, Alter BP, Artandi SE. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev. 2011;25:11–16. doi: 10.1101/gad.2006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tomlinson RL, Lukowiak AA, Terns RM, Terns MP. Telomerase RNA accumulates in Cajal bodies in human cancer cells. Mol Biol Cell. 2004;15:81–90. doi: 10.1091/mbc.E03-07-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.