Abstract
Postural displacements in response to emotional activation have recently been proposed as a direct and objective index of approach–avoidance behavior in humans. Here, we present the results of an experiment designed to assess spontaneous postural responses to discrete affective pictures, briefly presented in random order of valence. Our findings question the interpretation of phasic postural responses to emotional stimuli as approach–avoidance behavior. Further, we identify a robust dynamical pattern, characterized by specific features indicating that attentional processes may play a role in human postural responses to emotional stimuli.
Descriptors: Postural responses, Emotion, Approach-avoidance, Orienting response, Center of pressure
The approach–avoidance distinction is central to the understanding of both human and animal motivated behavior (Elliot, 2008). Whereas in animal investigations the actual movement toward a reward or away from an aversive stimulus has been and still is a relevant objective measure to probe the psychological and related neurophysiological mechanisms of motivated behavior (Blanchard & Blanchard, 1969; Fonio, Benjamini, & Golani, 2009), in human research, an objective index of physical approach or avoidance remains elusive.
A series of empirical studies has examined the pulling or pushing of levers as a possible motor index of approach–avoidance behavior. In this framework, a faster pull of the lever (arm flexion) in the presence of a positive compared to a negative stimulus is assumed to indicate approach, whereas a faster push of the lever (arm extension) in the presence of a negative compared to a positive stimulus is believed to represent avoidance (Rutherford & Lindell, 2011). Experimental evidence, however, has shown that the matching of arm flexion or extension to approach or avoidance behavior, respectively, depends on the experimental instructions, which may influence the affective coding of specific motor actions on a representational level that interferes with response selection (Eder & Rothermund, 2008).
A recent study has suggested an alternative experimental methodology to assess approach–avoidance responses to emotional activation in controlled laboratory settings (Hillman, Rosengren, & Smith, 2004). Postural sway was measured while subjects stood on a force platform viewing pictures from three affective categories: pleasant, unpleasant, and neutral. It was hypothesized that positively evaluated pictures would evoke body movement toward the stimulus (approach behavior) and, correspondingly, negatively evaluated pictures would produce body movement away from the stimulus (avoidance behavior). Because forward movement decreases the actual distance between the subject and the affective stimulus, whereas backward leaning increases the subject–stimulus distance, it is reasonable to argue that postural sway can be used as an objective measure of approach–voidance behavior that is not influenced by experimental instructions. In this study, women exhibited a posterior displacement in response to all pictorial stimuli, which was more pronounced, however, for the unpleasant pictures. Although this posterior displacement to unpleasant pictures could be interpreted as avoidance behavior, the observation that pleasant stimuli also triggered a posterior movement in women, whereas in the same study men exhibited less backward displacement to unpleasant pictures than to pleasant and neutral ones, renders the approach–avoidance interpretation problematic. Furthermore, in that experiment, pictures from the same affective category were presented together in blocks. Thus, the authors aimed at exploring postural behavior induced by sustained emotional states, rather than assessing phasic responses to discrete emotional stimuli.
A second study examined postural sway in response to emotional stimuli as a possible index of approach–avoidance behavior utilizing pictures from three affective categories (pleasant, unpleasant, and neutral) presented in a random order. This experimental design is more appropriate for the assessment of spontaneous phasic responses to individual emotional stimuli. The results, however, did not reveal any significant effect of picture viewing on anteroposterior postural movement (Stins & Beek, 2007).
Here, we investigated the dynamical pattern of postural responses to affective picture viewing by performing a fine-grained computation of the effects of each affective category on posturographic data at high temporal resolution. We also recorded and analyzed simultaneous heart rate stimulus-related responses in order to validate the effectiveness of our experimental design in inducing emotional activation and to investigate potential correlations with posturographic data.
Method
Subjects and Stimuli
Thirty-eight undergraduate and graduate students (19 women, 19 men) aged 19–32 years (mean = 23.6 ± 2.9) from the University of Granada in Spain volunteered to participate in this study for course credit. Stimuli were 81 pictures (27 pleasant, 27 unpleasant, 27 neutral) drawn from the International Affective Picture System (IAPS). Pleasant pictures depicted erotic scenes, unpleasant pictures included scenes of mutilated bodies, and neutral pictures were images of household objects.1
Procedure
Subjects stood on the force platform with stocking feet, adapting a comfortable natural stance with their arms relaxed along the trunk and their feet slightly separated (approximately 5 cm apart). Stimuli were displayed on a computer LCD 19″ monitor placed in front of the subject at a viewing distance of 50 cm (45.6° viewing angle) and a height adjusted in order to align its center with the subject’s center of gaze. Subjects viewed pictures in three blocks with every block including 9 pictures from each affective category (total of 27 pictures per block). Each single picture was presented for 6 sec followed by a variable delay of 5–7 sec. Pictures within each block were randomly ordered in a way that avoided the consecutive presentation of pictures from the same category, and block order was counterbalanced between subjects. After each block, subjects were allowed to sit for 2 min in a comfortable chair inside the experimental room to reduce fatigue from prolonged standing. Upon completion of the three experimental blocks, participants were asked to evaluate the valence and arousal of the displayed pictures using the Self-Assessment Manikin (SAM; Bradley & Lang, 1994).
Data Recording
Posturography
Posturographic data were recorded at 50 Hz by an AMTI AccuSway (Advanced Mechanical Technology, Inc., Watertown, MA) force platform consisting in a square aluminum plate (width 50 × 50 cm; weight 11.4 kg; height: 14.4 cm). The point projection of the vertical reaction forces recorded by the platform was decomposed by the AMTI Balance Clinic software into two center of pressure (COP) signals, one for the anteroposterior (A-P) and one for the mediolateral (M-L) axis of movement, which were subsequently exported to Matlab (MathWorks Inc., MA) for further analysis. One subject (male) was excluded from COP analysis because of recording artifacts.
Electrocardiogram
Electrocardiographic (ECG) data were recorded at 1000 Hz by a Biopac data acquisition system (MP150, Biopac Systems Inc., Santa Barbara, CA) and exported to Matlab for further processing. R-wave detection and artifact correction were performed with ecglab (Carvalho, da Rocha, Nascimento, Souza Neto, & Junqueira, 2002). KARDIA (Perakakis, Joffily, Taylor, Guerra, & Vila, 2010), a Matlab software designed for cardiac interbeat interval analysis, was used to obtain heart rate (HR) time series sampled at 5 Hz. Three subjects (two women, one man) were excluded from HR analysis because of recording artifacts.
Data Analysis
Continuous COP and HR signals were segmented into 11 sec epochs, including 1 sec of prestimulus baseline and 10 sec post-stimulus response for each picture presentation trial. Phasic COP and HR responses were subsequently expressed as differential values from baseline activity by subtracting the mean baseline value of each epoch from all subsequent time samples. Group average COP and HR responses were obtained by averaging across trials the values at each sample. A nonparametric permutations test based on 200 surrogate data sets obtained by permutating the data points at each time sample from all individual trials of each subject was subsequently carried out to detect statistical differences between picture categories. To control for false positive statistical error the false discovery rate correction method for multiple comparisons was used. A two-way analysis of variance (ANOVA; Gender × Category) was then performed using as dependent variable the mean COP and HR values for the time samples where significant picture category differences were detected by the non-parametric test. Subjective picture ratings were analyzed by means of a two-way ANOVA (Gender × Category) performed both for valence and arousal dimensions. Bonferroni tests were used for paired comparisons.
Results
COP and HR Responses
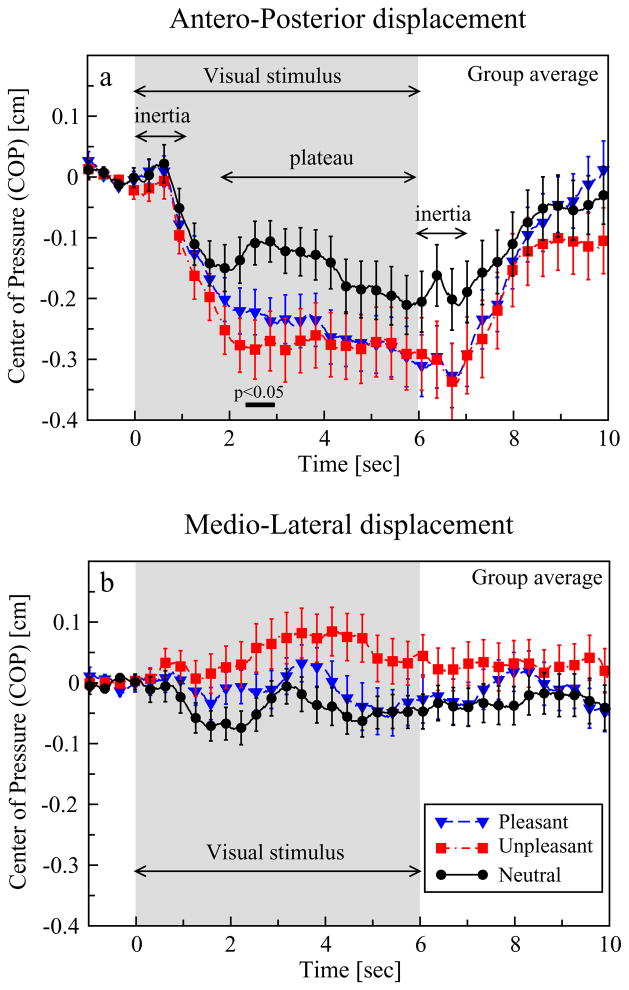
Group average COP responses as a function of picture category are presented in Figure 1a,b. Subjects exhibit a marked negative COP deviation from baseline in the A-P axis (backward movement) during the presentation of pictures pertaining to all three affective categories (pleasant, unpleasant, and neutral). This displacement, however, is more pronounced when subjects view pleasant and unpleasant pictures. A significant effect of picture category (p < .05) was found at a range of latencies extending from 2.34 sec to 2.96 sec after stimulus onset (horizontal black bar in the graph). The ANOVA on the mean COP value at this latency range revealed a significant effect of Category, F36 = 6.86, p < .01, and a significant Gender × Category interaction, F36 = 3.18, p < .05, which is further explored in the following section on gender differences. Post hoc multiple comparison tests revealed a significant difference between unpleasant and neutral pictures (p < .01), whereas no significant difference was detected between unpleasant and pleasant or pleasant and neutral pictures. As regards the M-L axis, statistical comparisons failed to detect any significant effects. Affective picture viewing produced a HR deceleration only when subjects were attending at pleasant and unpleasant pictures, replicating previous findings of cardiac deceleration during the presentation of emotional compared to neutral visual stimuli. The nonparametric permutations test applied to HR data revealed a significant effect of picture category ranging from 1.4 sec to 10 sec. The ANOVA applied to the mean HR value at this latency range only revealed a significant effect of Category, F34 = 30.42, p < .01. Post hoc multiple comparisons detected significant differences only between unpleasant and neutral pictures (p < .01). No significant correlations were found between HR and COP data.
Figure 1.
Group average plots of COP responses during affective picture-viewing. a: A negative COP response in the anteroposterior axis indicates a posterior displacement that is more pronounced for pleasant and unpleasant pictures. Double arrows indicate the plateau region and the inertia effect, consistently observed in pictures from all three affective categories. The shaded area represents stimulus duration and the black bar the latency range where significant differences between picture categories were found. b: No significant deviations from baseline were observed in the mediolateral axis. Error bars represent the standard error. For visual purposes, every 15th data point in each data set is shown.
Gender Effects
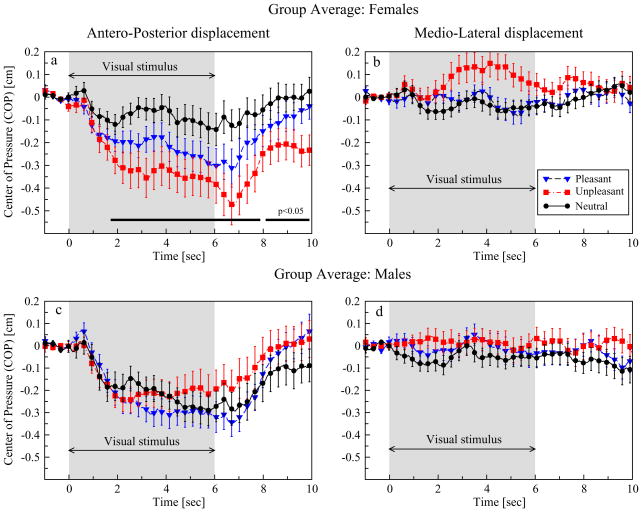
Figure 2a shows that in women, unpleasant pictures produced the most pronounced backward displacement, followed by pleasant pictures with an intermediate displacement and finally neutral pictures with the smallest deviation from baseline equilibrium. On average, men responded with a similar backward displacement to pictures from all three affective categories (Figure 2c).
Figure 2.
Group average plots of COP responses for female and male subjects separately. a: In female subjects, unpleasant pictures evoked a pronounced posterior displacement that continued to accumulate during the entire stimulus presentation period. Pleasant pictures elicited an intermediate displacement, whereas neutral pictures followed with a moderate deviation from postural baseline equilibrium. The shaded area represents stimulus duration and the black bar the latency range where significant differences between picture categories were found. c: In men, pictures from all three categories elicited a similar posterior COP displacement. b,d: Picture viewing did not produce any observable postural deviation in the mediolateral axis of movement. Error bars represent the standard error. For visual purposes, every 15th data point in each data set is shown.
The black bar in the A-P graph of female subjects represents a significant effect of picture category from 1.72 sec to 7.88 sec and from 8.1 sec to 10 sec. Post hoc comparisons detected statistical differences only between unpleasant and neutral pictures (p < .01). Statistical comparisons did not reveal any significant effects in men.
Subjective Evaluation of Pictures
The ANOVA on SAM ratings revealed a significant effect of Category both for valence and arousal dimensions (p < .001). Post hoc comparisons showed that valence ratings for pleasant pictures were significantly higher than those for neutral pictures (mean values: 6.98 vs. 5.09; p < .001), whereas valence ratings for unpleasant pictures were significantly lower than those for neutral ones (mean values: 2.32 vs. 5.09; p < .001). Unpleasant pictures were rated as more arousing than both neutral pictures (mean values: 5.77 vs. 1.44; p < .001) and pleasant pictures (mean values: 5.77 vs. 4.15; p < .001). Pleasant pictures were also more arousing than neutral (mean values: 4.15 vs. 1.44; p < .001).
Discussion
The primary objective of this study was to explore the intriguing hypothesis that humans respond to visual emotional stimuli with spontaneous postural displacements that can be clearly interpreted as approach–avoidance behavior. Interestingly, our findings do not provide evidence in favor of this hypothesis. The intuitive assumption that negative emotion induced by unpleasant pictures would be accompanied by a posterior postural displacement indicating physical withdrawal from the stimulus whereas positive emotion, activated by pleasant pictures, would be associated with an anterior displacement indicating approximation of the stimulus is not supported by the data. Our results do not show evidence of a selective anterior displacement to pleasant pictures that could be interpreted as approach behavior in any of the subjects. Instead, we observe a marked posterior deviation from baseline induced by pictures from all three affective categories (Figure 1a). Notably, visual affective stimuli did not evoke any significant deviation from postural baseline equilibrium in the mediolateral axis of movement (Figures 1b and 2b,d).
Further, we identified a previously unrecognized robust postural response pattern to all visual stimuli regardless of their affective value (Figure 1a). Specifically, we observed that, on average, subjects react to passive picture viewing with an abrupt, high-gradient posterior deviation from baseline that continues until approximately 2 sec after stimulus onset. At ≈ 2 sec after stimulus presentation, this negative displacement reaches a plateau, although in some cases it continues to accumulate with a lower gradient, reaching a maximum deviation from baseline approximately 1 sec after stimulus offset. Interestingly, this inertia effect is also consistently observed at stimulus onset, because the postural deviation from baseline equilibrium initiates approximately 1 sec after the stimulus is displayed on the screen (Figures 1a and 2a,c).
Regarding these distinctive features of the dynamical response pattern—inertia and plateau region—we can hypothesize that they relate to concrete underlining physiological processes. Specifically, the inertia effect, which is observed after any change in visual stimulation regardless of the emotional content of the stimulus, may tap into elemental properties of the transformation of perceptual information into specific motor actions and could have interesting implications for computational models of visuomotor integration. Further research may discover stimulus parameters, individual differences or neurological conditions, that modulate the duration of the inertia before and after a marked change in postural equilibrium in response to visual stimulation. The plateau region, on the other hand, indicates a persisting deviation from postural equilibrium throughout picture presentation. This could reflect that the observed backward displacement is related to an attentional phase of prolonged observation and information extraction that lasts for as long as the visual cue is present and even after the initial automatic assessment of the potential significance of the stimulus. An interpretation of this backward displacement as a postural adaptation that facilitates enhanced cognitive performance is congruous with research showing that body locomotion, and specifically stepping backward, improves cognitive control in attentionally demanding tasks (Koch, Holland, Hengstler, & van Knippenberg, 2009).
Athough our analysis indicates that the postural response pattern is common for all visual stimuli, in female subjects the amplitude of the backward displacement is more pronounced for unpleasant pictures, followed by pleasant pictures, which are characterized by an intermediate response amplitude, and finally neutral pictures, which show a moderate deviation from baseline postural equilibrium (Figure 2a). This finding is consistent with evidence from other physiological variables showing increased reactivity to aversive pictures in women (Bradley, Codispoti, Sabatinelli, & Lang, 2001). However, the posterior displacement in response to unpleasant pictures cannot be interpreted as avoidance behavior, as a similar backward movement is observed also for pleasant and even for neutral pictures.
Here we propose an alternative hypothesis that postural responses to emotional stimuli may be related to attentional processes. This hypothesis is supported by the following findings: (a) A similar dynamical pattern of COP responses is evoked by all visual stimuli regardless their valence (Figures 1a and 2a,c); (b) the amplitude of the COP negative (posterior) displacement in women is potentiated for pleasant and unpleasant stimuli, which are more attentionally demanding compared to neutral stimuli (Figure 2a); and (c) the dynamical pattern of COP responses reveals a characteristic plateau region that persists during the visual stimulation period and terminates after the stimulus offset (Figures 1a and 2a,c). The presence of such a prolonged (up to 5 sec) plateau may indicate a sustained period of perceptual processing requiring increased allocation of attentional resources. (d) Simultaneous heart rate recordings reveal a cardiac deceleration in response to emotional pictures that replicates previous findings, where such decelerations were characterized as an attentional bradycardia indicative of an orienting response (Bradley, 2009).
In summary, in this brief report we uncover a dynamical pattern of spontaneous human postural responses to discrete, briefly presented affective pictures. Our findings question the interpretation of postural responses to emotional stimuli as approach–avoidance behavior and suggest a previously unrecognized involvement of attentional processes.
Acknowledgments
This research was supported by the Spanish Ministry of Science and Innovation (Grants PSI2008-04372, JCI-2010-06790), The Brigham and Women’s Hospital Biomedical Research Institute Fund, The Office of Naval Research (ONR Grant 000141010078), and The National Institutes of Health Grant 1RO1-HL098437.
Footnotes
The following pictures from the IAPS were used: pleasant: 4606, 4607, 4608, 4609, 4611, 4641, 4651, 4652, 4653, 4656, 4658, 4659, 4660, 4664, 4666, 4669, 4670, 4672, 4676, 4680, 4681, 4683, 4687, 4689, 4690, 4800, 4810; neutral: 7000, 7002, 7004, 7006, 7009, 7010, 7020, 7025, 7030, 7031, 7035, 7040, 7050, 7060, 7080, 7090, 7100, 7110, 7150, 7170, 7175, 7217, 7224, 7233, 7235, 7705, 7950; unpleasant: 3000, 3010, 3030, 3051, 3053, 3060, 3061, 3062, 3063, 3064, 3068, 3069, 3080, 3100, 3102, 3110, 3120, 3130, 3140, 3150, 3168, 3170, 3261, 3400, 9252, 9253, 9405.
References
- Blanchard R, Blanchard D. Passive and active reactions to fear-eliciting stimuli. Journal of Comparative and Physiological Psychology. 1969;68:129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- Bradley M. Natural selective attention: Orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M, Codispoti M, Sabatinelli D, Lang P. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1:300–319. doi: 10.1037/1528-3542.1.3.300. [DOI] [PubMed] [Google Scholar]
- Bradley M, Lang P. Measuring emotion: The Self-Assessment Manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94) 90063-9. [DOI] [PubMed] [Google Scholar]
- Carvalho J, da Rocha A, Nascimento F, Souza Neto J, Junqueira L., Jr . Development of a Matlab software for analysis of heart rate variability. In: Yuan B, Tang X, editors. 6th International Conference on Signal Processing. Vol. 2. Beijing, China: Institute of Electrical and Electronics Engineers, Inc; 2002. pp. 1488–1492. [DOI] [Google Scholar]
- Eder A, Rothermund K. When do motor behaviors (mis)match affective stimuli? An evaluative coding view of approach and avoidance reactions. Journal of Experimental Psychology: General. 2008;137:262–281. doi: 10.1037/0096-3445.137.2.262. [DOI] [PubMed] [Google Scholar]
- Elliot A. Handbook of approach and avoidance motivation. New York: Psychology Press; 2008. [Google Scholar]
- Fonio E, Benjamini Y, Golani I. Freedom of movement and the stability of its unfolding in free exploration of mice. Proceedings of the National Academy of Sciences, USA. 2009;106:21335–21340. doi: 10.1073/pnas.0812513106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman C, Rosengren K, Smith D. Emotion and motivated behavior: Postural adjustments to affective picture viewing. Biological Psychology. 2004;66:51–62. doi: 10.1016/j.biopsycho.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Koch S, Holland R, Hengstler M, van Knippenberg A. Body locomotion as regulatory process. Psychological Science. 2009;20:549–550. doi: 10.1111/j.1467-9280.2009.02342.x. [DOI] [PubMed] [Google Scholar]
- Perakakis P, Joffily M, Taylor M, Guerra P, Vila J. KARDIA: A Matlab software for the analysis of cardiac interbeat intervals. Computer Methods and Programs in Biomedicine. 2010;98:83–89. doi: 10.1016/j.cmpb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Rutherford H, Lindell A. Thriving and surviving: Approach and avoidance motivation and lateralization. Emotion Review. 2011;3:333–343. doi: 10.1177/1754073911402392. [DOI] [Google Scholar]
- Stins J, Beek P. Effects of affective picture viewing on postural control. BMD Neuroscience. 2007;8:83. doi: 10.1186/1471-2202-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]