Abstract
Ovarian hormones modulate the pharmacological effects of psychostimulants and may enhance vulnerability to drug addiction. Female rats have more midbrain dopamine neurons than males and greater dopamine uptake and release rates. Cocaine stimulates motor behavior and dopamine efflux more in female than male rats, but the mediating mechanisms are unknown. This study investigated individual differences in anatomic, neurochemical, and behavioral measures in female rats to understand how ovarian hormones affect the relatedness of these endpoints.
Ovarian hormone effects were assessed by comparing individual responses in ovariectomized (OVX) and sham adult female rats. Locomotion was determined before and following 10 mg/kg cocaine. Electrically-stimulated dopamine efflux was assessed using fast cyclic voltammetry in vivo. Dopamine neuron number and density in substantia nigra (SN) and ventral tegmental area (VTA) were determined in the same animals using tyrosine-hydroxylase immunohistochemistry and unbiased stereology. Locomotor behavior and dopamine efflux did not differ at baseline but were greater in sham than OVX following cocaine. Cocaine increased dopamine release rates in both groups but uptake inhibition (Km) was greater in sham than OVX. Dopamine neuron number and density in SN and VTA were greater in shams. Sham females with the largest uterine weights exhibited the highest density of dopamine neurons in the SN, and the most cocaine-stimulated behavior and dopamine efflux. Ovariectomy eliminated these relationships. We postulate that SN density could link ovarian hormones and high-psychostimulant responses in females. Similar mechanisms may be involved in individual differences in the addiction vulnerability of women.
Keywords: Individual differences, Ovarian hormones, Cocaine, Voltammetry, Sex differences, Dopamine
1. Introduction
Women start using cocaine earlier in life, are more sensitive than men to some cocaine effects and progress to dependence more rapidly (Becker and Hu, 2008; Brady and Randall, 1999; Lynch et al., 2002). These female-specific vulnerabilities reflect in part underlying biologic differences (Becker and Hu, 2008; Lynch, 2006). Sex and age are among the most important biologic factors that influence individual differences in drug abuse vulnerability (Carroll and Anker, 2010; Lynch, 2006; Schramm-Sapyta et al., 2009).
Animal models have been useful in identifying these biologic differences. Like humans, female rats exhibit greater sensitivity to psychostimulants compared to males. They demonstrate greater cocaine-stimulated locomotion, establish place preference for cocaine at lower doses, acquire cocaine self-administration faster and work harder for cocaine reinforcement (Becker et al., 1982; Harrod et al., 2005; Kantak et al., 2007; Lynch and Carroll, 1999; Lynch and Taylor, 2004; Roth and Carroll, 2004; Russo et al., 2003; Savageau and Beatty, 1981; Walker et al., 2001). Many studies show that enhanced reactivity of dopamine neurons to psychostimulants probably contributes to this enhanced behavioral response. For example, females show more cocaine-stimulated dopamine efflux (Walker et al., 2006).
The biologic substrates of these sex differences have not been established definitely, but estradiol and progesterone are proposed to contribute significantly. Estradiol increases psychostimulant-induced locomotion, enhances cocaine self-administration and also augments multiple aspects of dopamine function including cocaine- and amphetamine-stimulated dopamine release, and expression of dopamine-specific proteins including the dopamine transporter and dopamine receptors (Becker, 1999; Carroll et al., 2004; Festa and Quinones-Jenab, 2004; Hu et al., 2004; Miller et al.,1998; Morissette et al., 2008; Sanchez et al., 2010). In contrast, progesterone attenuates dopamine release, suppresses the subjective effects of cocaine and decreases cocaine-induced locomotion and self-administration (Carroll and Anker, 2010; Evans and Foltin, 2010; Feltenstein and See, 2007; Jackson et al., 2006; Quinones-Jenab and Jenab, 2010; Sofuoglu et al., 2004).
Our laboratory and others have shown that estradiol also plays a critical role in maintaining dopamine neuron number in the SN and VTA (Gillies and McArthur, 2010; Johnson et al., 2010b; Morissette et al., 2008). We postulate that gonadal steroids produce sex dimorphisms in the anatomy of dopamine systems that support the observed ovarian steroid effects on cocaine action. In the present study, cocaine-stimulated locomotor behavior and dopamine efflux were determined in individual animals and correlated with anatomic variables (uterine weight and dopamine neuron density) to determine relationships among these parameters. This work is one of only a few studies examining individual differences in psychostimulant responses in female rodents and it appears to be the first to investigate an anatomic basis of individual differences in rodents of either sex.
2. Methods
2.1. Subjects
Female Sprague-Dawley rats (CD) were acquired from Charles River Laboratories (Raleigh, NC) and housed in self-ventilated cages. Rats were sham-OVX or OVX by the supplier on postnatal day 55 and then shipped. Sham surgery included all steps except removal the ovaries. Animals were housed in a vivarium with a 12 h light: dark cycle and given ad libitum access to food and water for 30 days before use. All experiments were approved by the Duke University Institutional Animal Care and Use Committee.
2.2. Experimental procedure
The goals of this study were two-fold. First, we characterized effects of ovariectomy on cocaine-stimulated motor behavior and dopamine efflux and maintenance of dopamine cell numbers. Second, we related changes in each parameter to each other in individual rats. To accomplish these aims, one animal was tested daily. A rat was placed in a novel open-field at about 0800 for 1 h, then injected with 10 mg/kg cocaine and behavior was recorded for 1 h. Two to three hours after cocaine injection the rat was anesthetized for voltammetry surgery and data collection. The rationale for this sequence was that the effects of the first cocaine injection would be completed by the time voltammetry data collection began and no sensitization was expected because of the low dose of cocaine used and the brief interval between injections. After the dopamine measurements, rats were perfused while still deeply anesthetized, about 1.5 h after the second cocaine injection. The brain was removed and processed for stereologic neuron counting.
2.3. Locomotor activity
Motor activity was determined in an open-field photocell device (Kinder Scientific, Inc., Poway, CA) as previously described (Parylak et al., 2008). The device was comprised of a Plexiglas arena (40 cm for each dimension) with corn cob bedding on the floor. Computer software supplied by the manufacturer recorded interruptions of photobeams spaced one inch apart. Horizontal ambulations were defined as relocations of the entire body of the rat. Fine movements were non-ambulatory horizontal beam breaks. Habituation test sessions began when rats were placed in the open arena without injection. After this 1 h session all rats were injected with 10 mg/kg cocaine i.p. and data recording was started immediately and continued for 1 h.
2.4. Dopamine release by voltammetry
2.4.1. In vivo methods
These experiments employed our previously described methods (Walker and Kuhn, 2008; Walker et al., 2006). Rats were anesthetized with urethane (1.5 g/kg i.p.) and positioned in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Body temperature was maintained at 37 °C with a Deltaphase Isothermal Pad (Braintree Scientific, Braintree, MA). A bipolar stimulating electrode (Plastics One Inc., Roanoke, VA)was positioned in the medial forebrain bundle (MFB) and biphasic stimulation parameters were 300 µA, 2 ms each phase. The stereotactic coordinates (in mm) anteroposterior (AP) and mediolateral (ML) from bregma and dorsoventral (DV) from dura that follow are based on a brain atlas. The stimulating electrode was placed at: −4.6 AP, +1.4 ML, −7.5 to −9.0 DV. The carbon-fiber microelectrode was directed at the center of the caudate (+1.2 AP, 2.0 ML, −4.5 to −5.6 DV).
The locations of the stimulating and working electrodes were optimized to give maximal dopamine responses. Extracellular dopamine concentrations resulting from sixty pulse stimulation trains at frequencies from 10, 20, 30, 40, 50, and 60 Hz were recorded. Immediately after the final baseline data collection, the rat was administered 10 mg/kg cocaine. The time course of cocaine effects on extracellular dopamine was monitored at 20 Hz because the effect of uptake inhibition is frequency-dependent and most robust at this frequency (Wightman and Zimmerman, 1990). Twenty-hertz stimulations were started immediately after drug injection (about 1 min) and were repeated at 2.5, 5, 7.5, 10, 15 and 30 min post-cocaine. Drug responses to stimulations at the other frequencies were recorded between 20 and 40 min following drug administration. The maximal current following electrical stimulations was recorded and later converted to concentration to yield the maximal dopamine elicited by each stimulation frequency.
2.4.2. Electrochemistry
Fast-scan cyclic voltammetry was conducted with an EI-400 potentiostat (Ensman Instrumentation, Bloomington, IN, U.S.A.). The potential at carbon-fiber electrodes was held at −400 mV, ramped to 1 V and back to −400 mV at 300 V/sec. Cyclic voltammograms were recorded at 10 Hz. Carbon-fiber microcylinder electrodes, prepared from 7 µm diameter T-300 fibers with about 50–100 µm exposed carbon-fiber (Amoco, Greenville, SC, U.S.A.) were used in the in vivo experiments along with a Ag/AgCl reference wire.
Changes in extracellular dopamine were determined by monitoring the current over a 100 mV window at the peak oxidation potential for dopamine. The electroactive substance was identified as dopamine by comparing background subtracted cyclic voltammograms from the in vivo stimulations to those collected at the same electrode in vitro after the experiment. Oxidation currents in vivo were converted to dopamine concentrations by calibrating the electrodes with 1 and 2 µM dopamine standard solutions in a flow injection system following experimental use. The composition of the buffer for post calibration was 20 mM HEPES, 155 mM NaCl, pH = 7.4.
2.5. Dopamine neuron and terminal counting
After dopamine efflux was determined, each rat was transcardially perfused with 10% formalin and the brain processed for quantitation of dopamine neuron number using a previously validated method (Johnson et al., 2010a, 2010b). The brain was removed and postfixed overnight at 4 °C and cryoprotected with a 30% sucrose solution. Serial coronal sections (30 µm thick) were cut on a cryostat and every 3rd section was thaw-mounted onto slides. Sections were allowed to dry overnight at 37 °C. Heat mediated antigen retrieval was performed to increase tyrosine-hydroxylase (TH) immunoreactivity of the tissue. Sections were pressure cooked at high pressure for 1.5 min in citrate buffer (pH = 6.0). Sections were rinsed in PBS and incubated in 0.3% hydrogen peroxide–methanol for 30 min to quench endogenous peroxidase. Sections were rinsed and blocked in 0.5% BSA + 0.3% Triton X-100 for 15 min at room temperature. After blocking, sections were incubated in primary antibody diluted in blocking buffer (1:10000, mouse anti-TH (Immunostar, Inc.) overnight at 4 °C. The next day, sections were rinsed and incubated in a biotinylated horse anti-mouse secondary antibody (1:1000, Vector Labs) for 1 h at room temperature and then in avidin–biotin complex for 1 h at room temperature. The sections were rinsed and stained with diaminobenzidine (DAB, Vector Labs). Sections were then counterstained with cresyl violet, mounted and coverslipped.
Tyrosine-hydroxylase-immunoreactive (TH-IR) cell number in the SN pars compacta and VTA was estimated using the optical fractionator method. Every 6th section through the extent of both hemispheres of these midbrain regions was analyzed. Starting sections were selected at random, and all brains were coded and analyzed blindly. Midbrain regions were manually traced at low magnification (4×). Individual cells were visualized for counting with a 100× oil immersion objective (numerical aperture = 1.3). Sections were systematically sampled with counting frames measuring 40 × 40 µm (1600 µm2 area) spaced randomly 100 µm apart along the x and y axes (sampling grid area = 10,000 µm2). Some tissue shrinkage occurred during histologic preparation, resulting in a mounted thickness of 14 µm. Therefore, a dissector height of 8 µm was used, with top and bottom guard zones of 3 µm. Only cells that came into focus at the fixed height were counted. Using these parameters, we counted enough cells to result in a coefficient of error for each estimate that ranged between 0.05 and 0.10.
TH-IR terminal density in the dorsal striatum was also estimated using the optical fractionator method. Every 12th section was counted through the extent of the dorsal striatum, and the left hemisphere of every 24th section was analyzed (3 sections total). The entire dorsal striatum was traced at 4× magnification before a sampling grid was superimposed on the traced region by the software. After shrinkage, final thickness of the sections used averaged 10.7 µm. For stereology, a 40 × 40 µm counting frame with a dissector height of 6 µm was used. Each counting frame was randomly spaced 650 × 650 µm apart and guard zones of 2 µm from the top and bottom of the section were used. Terminals were visualized with a 100× oil immersion lens (numerical aperture = 1.3). Terminals that appeared as dark TH-IR spots on a pale background were counted. Enough terminals were counted to achieve a coefficient of error that was ≤0.15. “Patch areas” were not counted as terminal numbers were very low (<10% of that occurring in matrix). The stereologer was blinded to animal identity and surgical group during all counting.
2.6. Drugs and chemicals
Cocaine HCl (Sigma-Aldrich, St. Louis, MO) solutions (10 mg/mL) were made fresh in saline and injected intraperitoneally (i.p.) at 1 mL/kg. Urethane and all other chemicals were purchased from Sigma-Aldrich.
2.7. Data analysis
Group averages are expressed as the mean ± SEM and N is the number of rats. Effects of surgery (sham vs. OVX) and time during habituation or after cocaine injection on motor behavior were determined using mixed model two-way ANOVA with surgery as the between subjects factor and time as the repeated, within-subject, factor. Group averages of maximal dopamine concentrations in sham and OVX rats before and after cocaine administration following a range of stimulation frequencies were calculated and displayed. Values for the peak dopamine concentrations were log-transformed to stabilize variances prior to ANOVA. The effects of surgery and cocaine on dopamine efflux were analyzed by mixed model three-way ANOVA with surgery as the between subjects factor and frequency and drug (baseline, then cocaine) as within-subjects factors. As significant main effects and interactions were identified, baseline and cocaine data were analyzed separately with a 2-way ANOVA with frequency as the repeated measure. Cocaine-induced changes in dopamine efflux were expressed as percent of the baseline at each frequency in each rat and also analyzed by 2-way ANOVA. When significant main effects were found post-hoc analysis was employed using Newman–Keul’s multiple-comparison test in order to determine differences between groups. Differences between sham and OVX rats for the dopamine kinetic and the anatomic measures were determined by t-test. Statistical analysis used NCSS 2007 software (NCSS, Kaysville, UT). Linear regression analysis was used to determine correlation coefficients (r) and significance levels (Prism 5.3, Graphpad Software, San Diego, CA). Differences were considered to be significant when p < 0.05.
Voltammetry overflow curves were analyzed to resolve dopamine release and uptake parameters. The changes in extracellular dopamine are described by: d[DA]/dt = f[DA]p − Vmax/((Km/[DA]) + 1) where f is the frequency of stimulation, DA is dopamine, [DA]p is the concentration of dopamine released per stimulus pulse, Vmax and Km are Michaelis–Menten parameters for the maximal uptake rate and the affinity of the transporter, respectively. Kinetic analysis was performed similar to our prior reports (Walker and Kuhn, 2008; Walker et al., 2006, 2000) using a combination of nonlinear regression and single curve analysis (Wu et al., 2001). The nonlinear regression method used a simplex algorithm to calculate Vmax, Km, and [DA]p using data at each frequency from 10 to 60 Hz simultaneously (Wu et al., 2001). All three parameters were typically allowed to float in this analysis although occasionally Vmax was fixed to the value determined from the slope of the clearance phase of 60 Hz overflow curves.
3. Results
The effect of ovariectomy on motor behavior and stimulated dopamine efflux was assessed at baseline and following cocaine administration. Differences in these measures and ovariectomy effects on dopamine neuron number and density in SN and VTA will be discussed first. Then we will describe relationships among these three measures in individual sham and OVX rats.
3.1. Motor behavior
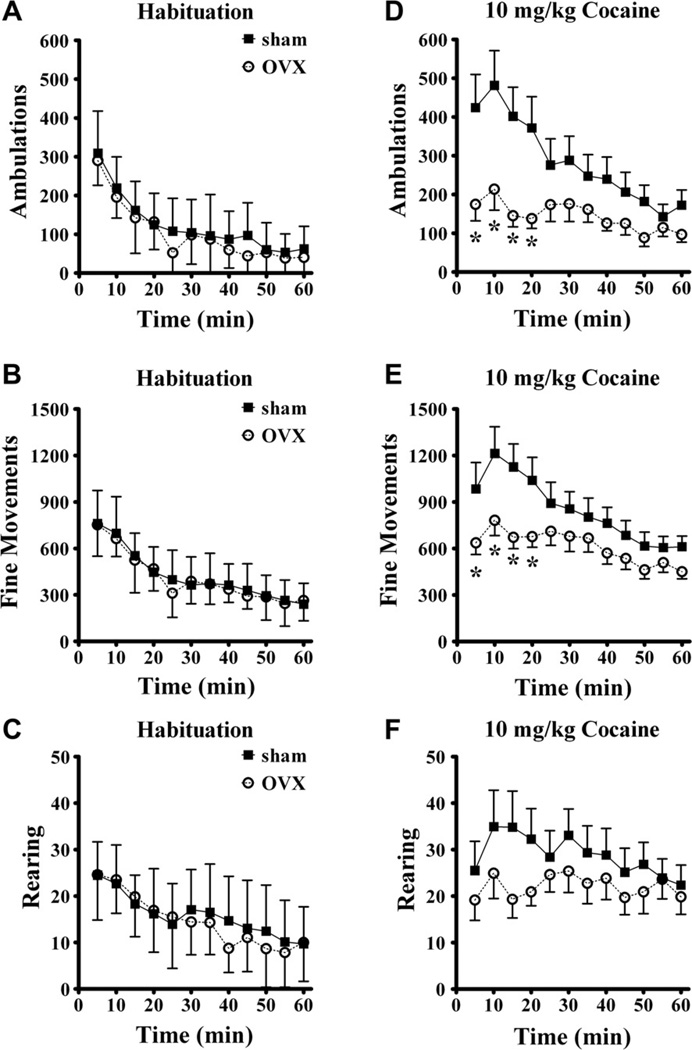
The effect of ovarian hormones on spontaneous motor behavior during exploration of a novel open-field environment and following 10 mg/kg cocaine injection i.p. was investigated. Fig. 1 shows ambulations, fine movements, and rearing during both conditions. Motor activity was highest when rats were introduced to the chamber and activity waned over the one hour session (Fig.1, left side panels). ANOVA confirmed that activity varied with time (p < 0.001 for each behavior). These three behavioral topographies did not reveal any effect of ovariectomy during habituation. ANOVA found no main effect of ovariectomy and no interaction with time.
Fig. 1.
Motor activity during habituation to a novel open-field device and following 10 mg/kg cocaine injection i.p. The habituation data from all sham (N = 16) and OVX (N = 13) rats for each of the three behavioral topographies horizontal ambulations (A), fine movements (B) and rearing (C) showed no main effect of ovariectomy or an interaction with time. Ambulations (D, significant main effects of surgery and time and interaction with time) and fine movements (E, significant effect of time and interaction of surgery and time) were lower in the OVX rats following cocaine administration, especially at the earliest time points. Ovariectomy did not significantly affect rearing behavior. *significantly different than sham group at same time point. Group means ± standard errors are shown in this and all figures.
After the 1 h habituation period 10 mg/kg cocaine was injected i.p. Fig. 1 (right side panels) shows that ovariectomy attenuated the motor stimulation induced by cocaine, especially at early time points following injection. Cocaine-induced less ambulation in OVX females, as shown by a main effect of surgery (F(1,25) = 4.9, p = 0.036). Ambulations varied over time (F(11,275) = 10.1, p < 0.001) and time and surgery interacted (F(11,275) = 3.7, p < 0.001). The ovariectomy-related decreases in cocaine-stimulated fine movements were time-specific as demonstrated by the time by surgery interaction (F(11,275) = 2.56, p = 0.004). The main effect of ovariectomy on fine movements was not significant (F(1,25) = 2.95, p = 0.098) and the main effect of time after injection was highly significant (p < 0.001). In contrast, ovariectomy did not cause a significant change in rearing behavior. There was no main effect of surgery for rearing (p = 0.32) and no interaction with time (p = 0.12), but time after injection did vary (p = 0.012). In summary, ovariectomy did not alter initial exploration of the novel environment but did attenuate the stimulant effects of cocaine, especially immediately following injection with ambulatory movements being most sensitive.
3.2. Neurochemistry
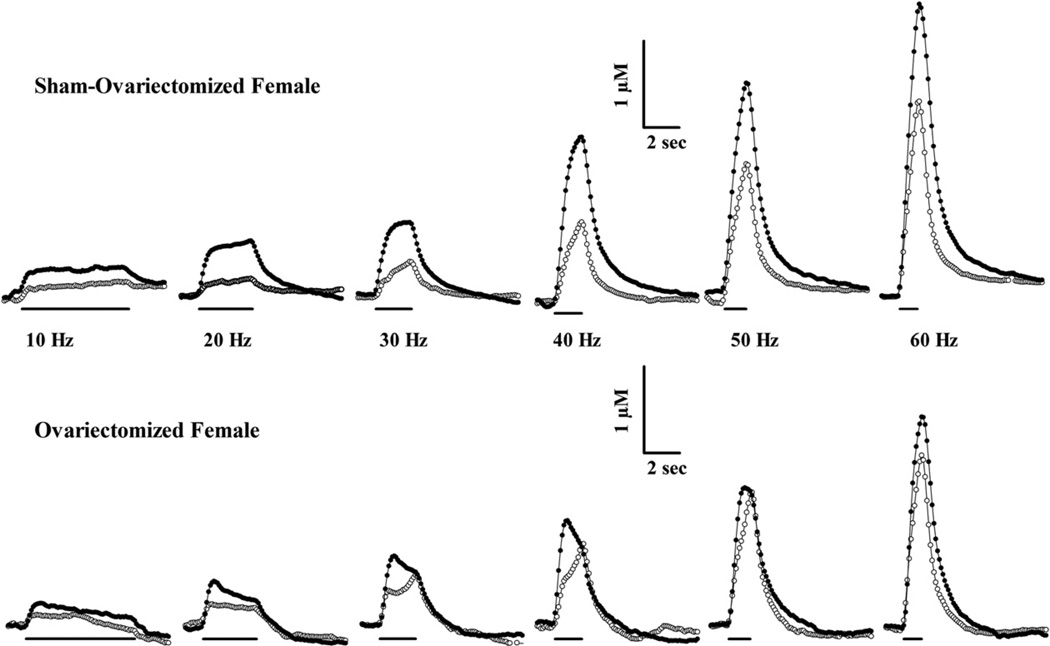
Dopamine efflux evoked by a range of stimulation frequencies was determined in dorsal striatum by fast-scan cyclic voltammetry. Example recordings of those overflow curves are shown in Fig. 2. The amplitudes of these curves were similar at baseline in these individual sham and OVX female rats. Injection of 10 mg/kg cocaine i.p. increased stimulated dopamine overflow at each frequency in the sham female but effects were more modest in the OVX rat. The maximal concentration of dopamine evoked at each frequency was determined and averaged across groups of rats.
Fig. 2.
Fast-scan cyclic voltammetry at carbon-fiber microelectrodes was used to assess electrically-stimulated dopamine efflux in dorsal striatum of anesthetized sham and OVX female rats, in vivo. Representative recordings at 100 ms intervals in sham and ovariectomized female rats are shown before (open circles) and after 10 mg/kg cocaine (filled circles). Sixty pulse electrical stimulations (300 µA) of the medial forebrain bundle evoked extracellular dopamine in each rat at the frequencies indicated. The identical scale bars for each rat apply to all frequencies. The lines under the recordings indicate the duration of the electrical stimulation injection.
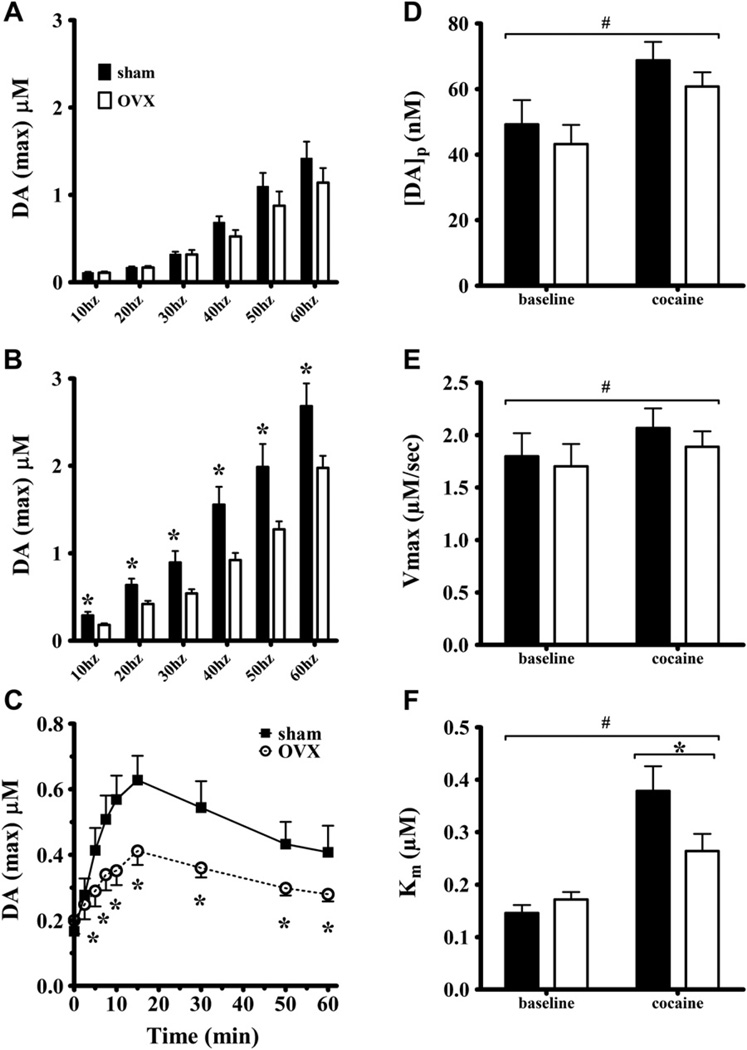
Cocaine effects on stimulated extracellular dopamine levels were determined as a time course at one frequency (20 Hz) and across a range of frequencies when cocaine effects were maximal. A global ANOVA showed that the effect of cocaine on log-transformed dopamine concentrations was greater in sham than OVX rats as shown by the main effect of surgery (F(1,25) = 4.64, p = 0.04). Frequency exerted a significant effect on extracellular dopamine levels (F(5,119) = 852, p < 0.001). The interaction of surgery and frequency was not significant (F(5,119) = 1.38, p = 0.24). The effect of cocaine was significant (F(1,25) = 191, p < 0.001) as well as its interactions with surgery (F(1,25) = 4.75, p = 0.039) and frequency (F(5,114) = 15.4, p < 0.001). The three-way interaction (surgery by frequency by dose) was not significant (F(5,114) = 1.96, p = 0.09).
Fig. 3A shows that ovariectomy did not affect stimulated efflux across the range of frequencies at baseline (F(1,25) = 1.08, p = 0.31). The effect of frequency was significant (F(5,114) = 428, p < 0.001) but its interaction with surgery was not (p = 0.14). The effects of ovariectomy on cocaine-stimulated dopamine efflux are shown in Fig. 3B. Ovariectomy attenuated the ability of cocaine to increase extracellular dopamine across the range of frequencies as ANOVA indicated a main effect of surgery (F(1,25) = 9.66, p = 0.005). The effect of frequency was significant (F(5,118) = 739, p < 0.001) but the interaction of surgery and frequency was not (p = 0.27). Post-hocs showed that shams had more dopamine release at every frequency tested.
Fig. 3.
The maximal concentration of stimulated dopamine efflux was determined from overflow curves like those shown in Fig. 2. Group averages of those maximal concentrations are shown across the range of stimulation frequencies at baseline (A) and following 10 mg/kg cocaine i.p. (B). The time course of cocaine-induced dopamine efflux following 20 Hz stimulations (C) showing peak or maximal extracellular dopamine concentrations (DAmax). In vivo dopamine release and uptake kinetics at baseline and following 10 mg/kg cocaine i.p. in dorsal striatum were determined from overflow curves following 10–60 Hz stimulations that were analyzed simultaneously to resolve release (D) and uptake parameters, Vmax (E) and Km (F) (see Methods). * indicates sham and ovex are significantly different, # indicates significantly different from baseline.
A further analysis examined the frequency dependence of the effects of ovarian hormones on uptake inhibition by cocaine by determining the percent increase (drug/baseline) in stimulated extracellular dopamine efflux at each frequency (transformed data not shown). ANOVA using repeated measures on frequency determined the effect of surgery on the percent increase of stimulated dopamine efflux. There was a significant main effect of surgery (F(1,25) = 4.71, p = 0.04) and frequency (F(5,114) = 14, p < 0.001) and their interaction (F(5,114) = 2.52, p = 0.033). Post-hoc analysis for the main effect of frequency, considering sham and OVX rats together, showed that the percent increase at 20 Hz was significantly greater than that of all other frequencies. For the surgery by frequency interaction, the significant effect of ovariectomy on cocaine-induced percent increase was enhanced in sham females only at 20 and 30 Hz. This frequency dependence matches that known to be most enhanced by cocaine’s competitive uptake inhibition (Wightman and Zimmerman, 1990) and supports the postulate that ovarian hormones enhance the uptake inhibition potency of cocaine.
The effects of ovariectomy were also simultaneously determined on the time course of cocaine-stimulated dopamine efflux at a single frequency (20 Hz). Fig. 3C shows that cocaine increased extracellular dopamine more in sham than OVX females at all but the earliest time points. The effect of surgery was not significant (F(1,25) = 3.28, p = 0.082). The effect of time after injection was significant (F(8,194) = 30.6, p < 0.001), as was the interaction of the surgery and time (F(8,194) = 4.93, p < 0.001). Post-hoc analysis indicated that dopamine concentrations were greater in sham than OVX females at every time point after 2.5 min (p < 0.05).
The baseline and cocaine dopamine efflux data were analyzed to resolve release and uptake kinetics. Fig. 3D displays [DA]p or the dopamine release capacity of sham and OVX rats. Cocaine increased [DA]p in both groups but ovariectomy did not alter either baseline or cocaine-stimulated release. ANOVA showed an effect of cocaine (F(1,25) = 35.5, p < 0.001) but no overall effect of surgery (p = 0.28) and no interaction (p = 0.60). Post-hoc analysis indicated that [DA]p was significantly greater after cocaine than baseline in both sham and OVX rats. ANOVA for Vmax (Fig. 3E) reported no effect of surgery (p = 0.46) and no interaction of surgery and dose (p = 0.80). Cocaine increased Vmax (F(1,25) = 7.97, p = 0.01) probably because the transporter was not saturated at baseline by the extracellular dopamine elicited by the somewhat brief stimulation trains. The greater enhancement of extracellular dopamine levels by cocaine in sham relative to OVX rats was due to a larger decrease in transporter affinity (Km) (Fig. 3F). The main effect of surgery on Km was not significant (F(1,25) = 1.71, p < 0.20). Dose exerted an effect on Km (F(1,25) = 43.8, p < 0.001) and there was an interaction (F(1,25) = 6.04, p = 0.02). Neuman–Keuls post-hoc analysis indicated that the Km for sham rats was greater than that for OVX after cocaine administration. Km values following cocaine administration were greater than baseline values for both groups.
3.3. Neuroanatomy
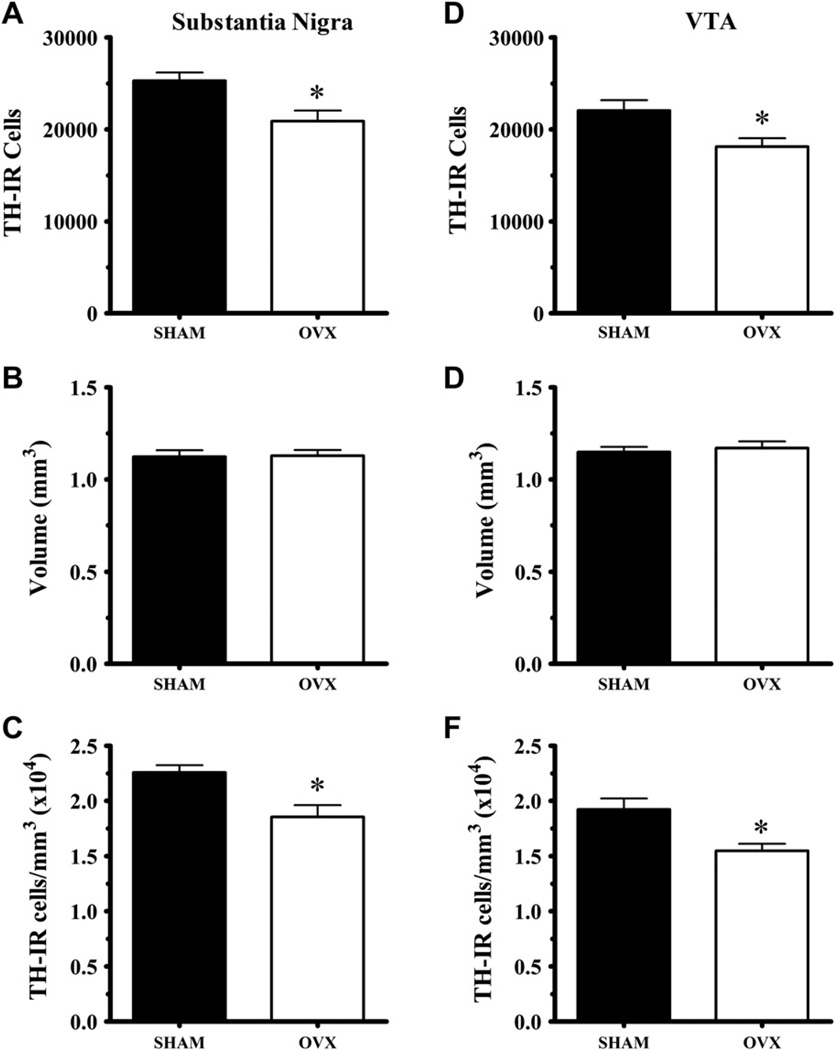
Fig. 4 shows the estimated numbers of TH-IR cells in SN and VTA, the volumes of both regions and the calculated densities. The effects of ovariectomy were consistent in each region. Ovariectomy decreased cell number and density of dopamine neurons. The number of TH-IR cells was significantly decreased in SN (t(27) = 3.07, p = 0.005) and VTA (t(25) = 2.64, p = 0.014). Ovariectomy did not alter the volume of either SN (p = 0.82) or VTA (p = 0.64). TH-IR cell densities were greater in sham than OVX rats in SN (t(25) = 3.94, p < 0.001) and VTA (t(25) = 3.59, p = 0.001).
Fig. 4.
Ovariectomy altered dopamine anatomy in the midbrain but not in the forebrain. OVX rats had fewer TH-IR neurons in both the SN and VTA (A, D) but maintained the volume of both midbrain regions (B, E). As a result the OVX rats had a lower density of DA neurons in the SN and VTA (C, F). The number of non-TH-IR, cresyl violet stained cells was not affected by ovariectomy (data not shown). Likewise, the number of TH-IR terminals and their density in dorsal striatum was not affected by ovariectomy (data not shown).
Cresyl violet stained cells were also counted in both regions. The numbers for the SN were 3809 ± 340 for sham (N = 11) and 2749 ± 392 for OVX (N = 11) rats. This difference was not significant (t(20) = 2.04, p = 0.055). Similarly for VTA, there were 2356 ± 303 for sham (N = 11) and 1834 ± 218 for OVX (N = 11) rats. The non-significant decline (rather than rise) indicates that ovariectomy did not cause a phenotypic change from TH-IR positive to negative.
Dopamine terminal number and density in dorsal striatum were determined in the sham (N = 12) and OVX (N = 11) female brains (data not shown). Ovariectomy did not alter the estimated number of terminals determined by stereological counting (t(21) = 0.90, p = 0.38), the volume of CP counted (t(21) = 0.12, p = 0.90), or density of terminals (t(21) = 0.94, p = 0.36). Thus, the effect of ovariectomy was limited to change at the cell body region.
3.4. Correlations
We conducted pair-wise comparisons to investigate associations between anatomic and functional variables. Table 1 shows correlations between the structural variables (uterine weight and dopamine neuron density) and the functional variables (behavior and dopamine efflux). These results vary markedly by endocrine status of the rats: interactions found in sham females are absent or even opposite in OVX rats. Strong correlations were found in sham rats between structural and cocaine-stimulated, but not baseline measurements. SN density was more often associated with behavioral and neurochemical values than was VTA density and caudate putamen (CP) terminal density.
Table 1.
Pair-wise correlations (r).
| Behavioral and neurochemical variables | Physical variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Uterus weight | SN TH-IR density | VTA TH-IR density | CP TH-IR density | ||||||
| Baseline | Cocaine | Baseline | Cocaine | Baseline | Cocaine | Baseline | Cocaine | ||
| SHAM | Fine movements | −0.03 | 0.76** | 0.03 | 0.83*** | −0.20 | 0.33 | 0.12 | 0.20 |
| Ambulations | −0.28 | 0.75** | −0.07 | 0.79*** | −0.25 | 0.32 | 0.03 | 0.18 | |
| Rearing | −0.71* | 0.67* | −0.60* | 0.83*** | −0.34 | 0.33 | 0.03 | 0.16 | |
| 10 Hz DA (µM) | 0.45 | 0.85*** | 0.26 | 0.60* | 0.18 | 0.11 | −0.40 | −0.36 | |
| 20 Hz | 0.33 | 0.48 | 0.25 | 0.38 | 0.19 | −0.01 | −0.03 | −0.53 | |
| 30 Hz | 0.15 | 0.45 | −0.01 | 0.41 | −0.23 | −0.09 | −0.21 | −0.47 | |
| 40 Hz | 0.07 | 0.64* | −0.32 | 0.56* | −0.48 | 0.03 | −0.50 | −0.43 | |
| 50 Hz | −0.07 | 0.62* | −0.23 | 0.58* | −0.55* | 0.04 | −0.48 | −0.35 | |
| 60 Hz | 0.06 | 0.63* | −0.16 | 0.49 | −0.36 | 0.17 | −0.44 | −0.38 | |
| DAp | −0.04 | 0.48 | −0.32 | −0.21 | −0.40 | 0.19 | −0.35 | −0.08 | |
| Vmax | −0.11 | 0.36 | −0.31 | 0.14 | −0.35 | 0.19 | −0.36 | 0.09 | |
| Km | −0.14 | −0.01 | −0.24 | −0.09 | −0.13 | −0.13 | 0.27 | −0.43 | |
| OVEX | Fine movements | −0.27 | 0.02 | 0.39 | −0.62* | 0.40 | −0.34 | −0.17 | −0.08 |
| Ambulations | −0.17 | 0.32 | 0.53 | −0.24 | 0.46 | −0.06 | −0.10 | −0.03 | |
| Rearing | 0.22 | 0.11 | −0.16 | −0.64* | 0.04 | −0.60* | −0.27 | 0.36 | |
| 10 Hz DA (µM) | −0.17 | −0.06 | 0.08 | 0.07 | 0.24 | 0.24 | 0.00 | −0.13 | |
| 20 Hz | −0.21 | 0.14 | −0.04 | −0.05 | 0.08 | −0.08 | 0.14 | −0.07 | |
| 30 Hz | 0.60 | 0.23 | −0.45 | −0.08 | −0.37 | −0.04 | 0.32 | 0.20 | |
| 40 Hz | 0.72* | 0.46 | −0.50 | −0.30 | −0.34 | −0.28 | 0.28 | −0.08 | |
| 50 Hz | 0.72* | 0.48 | −0.52 | −0.20 | −0.44 | 0.02 | 0.16 | 0.01 | |
| 60 Hz | 0.48 | 0.42 | −0.49 | −0.21 | −0.37 | −0.03 | 0.15 | 0.01 | |
| DAp | 0.44 | 0.24 | −0.55* | −0.38 | −0.49 | −0.32 | 0.07 | −0.07 | |
| Vmax | 0.29 | −0.01 | −0.56* | −0.33 | −0.48 | −0.28 | 0.10 | −0.13 | |
| Km | 0.05 | 0.00 | −0.33 | −0.52 | −0.33 | −0.32 | −0.13 | −0.02 | |
p < 0.05;
p < 0.01;
p < 0.005.
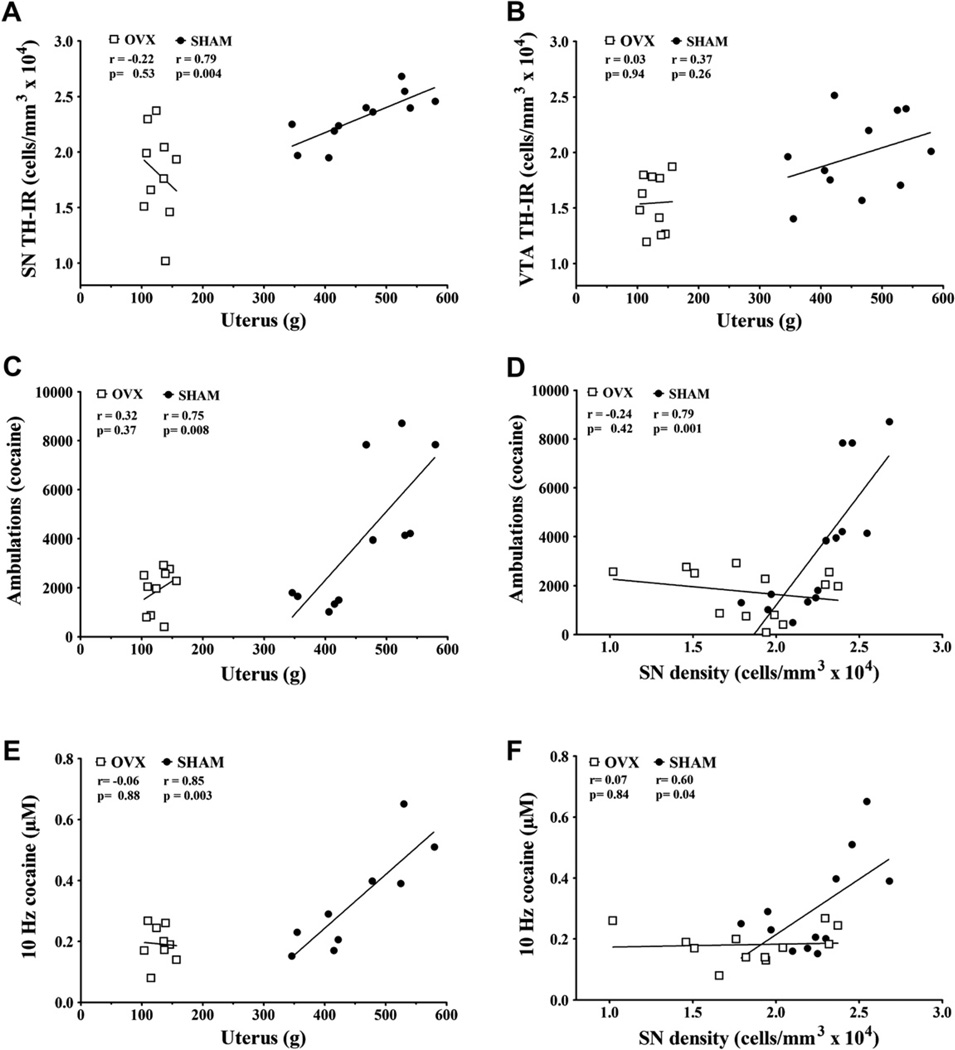
Fig. 5 illustrates correlations between structural and functional variables that were particularly striking. We have focused on the correlations for SN density because it was more often and better correlated than SN dopamine neuron number. Uterine weight is strongly correlated with SN density (Fig. 5A) but not VTA density (Fig. 5B) for sham rats only. Cocaine-stimulated ambulations correlated significantly with uterine weight (Fig. 5C) and SN density (Fig. 5D) in sham but not OVX rats. Similarly, cocaine-stimulated dopamine efflux at 10 Hz correlated strongly with uterine weight (Fig. 5E) and SN density (Fig. 5F) in sham but not OVX rats. Behavioral and neurochemical endpoints were related to each other. After cocaine administration, ambulations and fine movements were significantly associated with stimulated dopamine efflux at a single 10 Hz frequency (p = 0.02 for both, data not shown).
Fig. 5.
Correlations between anatomic and functional endpoints. Linear regression analysis reported R2 and p values for sham and OVX rats, which are presented on all panels.
Table 1 displays a number of other pair-wise correlations. In general, cocaine-stimulated behaviors and dopamine efflux correlated positively with uterine weight in sham rats, and these correlations were lost after ovariectomy. This contrasted with baseline behaviors and dopamine efflux that generally did not exhibit correlations with uterine weight. Rearing behavior exhibited interesting relationships. During habituation rearing was negatively correlated with both uterine weight and SN density. These became positive associations following cocaine in the sham rats but negative in the OVX rats (as was fine movements) for SN density. SN density was nearly related to VTA density in sham rats (p = 0.055) and VTA density exhibited correlation patterns somewhat similar to SN density. Differences between the two were that VTA density was not related to uterine weight (p = 0.26) and cocaine-stimulated behaviors were correlated with SN but not VTA density in sham rats. The calculated kinetic parameters showed few significant associations with perhaps a general trend between high dopamine cell density and low Vmax and [DA]p in dorsal striatum.
4. Discussion
The present findings show that uterine weight correlates with dopamine neuron density in the SN, cocaine-stimulated behavior and dopamine efflux in gonadally intact female rats. High-reacting females exhibited high uterine weight, high dopamine neuron density and high cocaine-stimulated behavior and dopamine efflux. Evidence for an endocrine basis for these correlative relationships is that they were lost in OVX rats. Correlations between SN cell density and cocaine-stimulated behavior and dopamine efflux were stronger than similar relationships observed for SN cell number, emphasizing the need to assess not only neuron number but also spatial relationships. This study is the first to demonstrate that natural anatomic variability in uterine weight and dopamine cell density in female rats is strongly associated with individual differences in drug responses.
Effects of ovarian hormones on cocaine pharmacokinetics probably do not contribute to the observed findings. There are no sex differences in brain or plasma levels of cocaine in rats, primates or humans (Bowman et al., 1999; Evans and Foltin, 2010; Mendelson et al., 1999) and we have shown that ovariectomy does not affect brain levels of cocaine in rats (Bowman et al., 1999). Estradiol replacement after ovariectomy might even lower brain cocaine levels (Niyomchai et al., 2006). The lack of evidence for different cocaine pharmacokinetics in our previous study suggests that the enhanced cocaine-stimulated behavior and dopamine efflux observed in intact females likely reflects physiologic effects of ovarian hormones. As the OVX group had experienced organizational effects of reproductive hormones through puberty until ovariectomy, these effects presumably reflect activational effects of ovarian hormones.
The critical role of ovarian hormones in maintaining relationships between dopamine neuron number, cocaine-stimulated dopamine release and behavior was demonstrated by comparing gonadally intact and OVX females. Further defining the roles of specific ovarian hormones could be useful but was too complex for this initial study as replacement experiments can be problematic (Strom et al., 2011; Theodorsson et al., 2008, 2010). Estradiol fluctuates over the 4–5 day rat estrous cycle and influences behavior over multiple time domains, ranging from trophic actions that maintain cell number over weeks to genomic actions mediated over days and rapid, nongenomic effects that occur within minutes (Becker and Hu, 2008; Cornil et al., 2006; Johnson et al., 2010b; Kuhn et al., 2010). Estradiol is uterotrophic as uterine wet weight is maximal on proestrus when estradiol is high and minimal on the first day of diestrus when it is low (Astwood, 1939; Katsuda et al., 1999; Mandl, 1951; Myatt et al., 1978; Nequin et al., 1979). The strong correlations with uterine weight suggest that activational effects of ovarian hormones occurring over days affect dopamine neuroanatomy, as is the case for hippocampal synapse density (Woolley et al., 1990; Woolley and Mcewen, 1992). This estradiol effect on synapse density in hippocampus was primarily due to enhanced numbers of spines per dendrite. Recent imaging studies in humans indicate increases in gray matter volume and decreases in CSF volume (Hagemann et al., 2011; Protopopescu et al., 2008): both would serve to increase neuron density. Fluctuating densities of dopamine uptake and release sites would significantly alter neurotransmitter concentration profiles (Venton et al., 2003) and could thereby be significant determinants of individual differences in psychostimulant responses in female rats. Dopamine cell bodies are thought to control motor activity primarily by releasing dopamine from their axon terminals in forebrain regions but psychostimulants also induce dopamine release to a lesser degree from dendritic projections in SN/VTA (White and Kalivas, 1998; Windels and Kiyatkin, 2006). Dendritically released dopamine autoregulates cell activity and is especially important for the initiation of behavioral sensitization to drugs (Kalivas and Weber, 1988; White and Kalivas, 1998). The present results suggest that SN density may contribute to a “pre-sensitized” behavioral setpoint of cocaine responsivity. It is a speculative hypothesis to propose that SN density mediates the observed effects on cocaine-stimulated dopamine efflux and behavior because both anatomy and dopamine efflux could be correlated with the actual causal substrate. Ovarian hormones are likely enhancing cocaine-stimulated dopamine efflux and behavior by more than one mechanism and locus.
Uterine weight correlated strongly with cocaine-stimulated dopamine efflux at almost every stimulation frequency although basal efflux was unassociated. OVX rats were not different from shams during habituation or for basal electrically-stimulated dopamine efflux and there were no correlations with baseline behavior or efflux. These results suggest that mechanisms unique to the action of cocaine are primarily influenced by ovarian hormones. Cocaine increased dopamine efflux in both groups of female rats by two kinetic mechanisms, increasing release and decreasing uptake. The present results show that cocaine increased [DA]p, the dopamine release rate, an effect reported previously (Addy et al., 2010; Jones et al., 1995; Lee et al., 1998) and mediated intracellularly by synapsin (Venton et al., 2006). Cocaine increased [DA]p equivalently in both sham and OVX rats and therefore was not ovarian hormone dependent and did not explain the greater increase in efflux in shams. The larger percent baseline increases in efflux in sham females were significantly greater than those of OVX rats at 20 and 30 Hz, low stimulation frequencies known to be affected by competitive uptake inhibitors (Wightman and Zimmerman, 1990). Kinetic analysis confirmed this specific effect as cocaine increased the Km more in sham than OVX females. Thus, ovarian hormones enhanced the uptake inhibition potency of cocaine but not its ability to increase release through synapsin-dependent mechanisms.
Other voltammetry studies have reported higher potency for uptake inhibition in cocaine-sensitized male rats without an apparent mechanism (Addy et al., 2010; Lee et al., 1998). Estradiol can induce rapid effects through direct mechanisms. Estradiol decreases the affinity of D2 receptors (Bazzett and Becker, 1994; Levesque and Di Paolo, 1988) and attenuated autoreceptor affinity should enhance stimulated dopamine efflux (Jones et al., 1996; Rubinstein et al., 2011). Alternatively, the affinity of the dopamine transporter for cocaine but not dopamine might be selectively enhanced by estradiol through phosphorylation or a similar mechanism (Blakely and Bauman, 2000; Ramamoorthy et al., 2011). Thus, the literature supports mechanisms through which ovarian hormones could be acting to increase the uptake inhibition potency of cocaine. The most rapid effects of estradiol or other ovarian hormones might not correlate with uterine weight because they occur on a different (briefer) time domain. Thus, uterine weight and SN density may not change rapidly enough to track the peak uptake inhibition occurring at 20 and 30 Hz that is enhanced by ovarian hormones. Furthermore, similar terminal density in sham and OVX rats could contribute to the lack of difference in behaviors during habituation and dopamine efflux at baseline. It is possible that plasticity in terminal density partially offsets the effects of OVX so that differences are observed only after the stronger challenge to the dopamine system caused cocaine.
The strong correlation of SN density with cocaine-stimulated behavior and DA efflux contrasted with the lack of correlation of these parameters with terminal number. One possible explanation is that the terminal tree is continually being sculpted in response to afferent input, especially negative feedback mediated by D2 receptors. Previous studies show that partial SN lesions and D2 receptor blockade cause adapative upregulation in the terminal tree, while chronic cocaine causes a downregulation (Finkelstein et al., 2000; Horne et al., 2011; Parish et al., 2001; Parish et al., 2002). Therefore, loss of dopamine neurons in OVX rats could have caused an adaptive upregulation in the terminal tree. This is suggested by the fact that OVX rats had fewer SN neurons but the same number of terminals. The significant negative correlations in OVX rats (opposite to those in shams) between SN density and cocaine-stimulated behaviors further suggest terminal compensation due to either loss of ovarian hormones, very low SN density or the combination of both. The strong correlation especially between DA cell density but not terminal numbers or density and cocaine-stimulated behavior suggests that factors directed by the cell body (cell firing, synthesis of critical terminal elements like storage vesicles) play a stronger role than has been appreciated in determining the behavioral response to psychomotor stimulants. The present results in female rats indicate that ovarian hormones influence SN density more than VTA density and that SN density is more strongly linked to cocaine-stimulated locomotor behaviors than VTA density at this low dose of cocaine. These findings suggest that the balance of the contribution of dorsal and ventral striatal dopamine mechanisms to cocaine-stimulated behavior in females may differ from the strong link between ventral striatal dopamine mechanisms and non-stereotyped locomotor behaviors that have been established in male rats (Kelly et al., 1977; Sharp et al., 1987).
Investigating individual differences in vulnerability to drug abuse has focused on finding associations between a behavioral phenotype, usually novelty-reactivity, and greater drug response (Cummings et al., 2011; Harro, 2010; Hooks et al., 1991). Recent studies of individual differences in female rats have shown a broad range of cocaine-stimulated activity (Mandt et al., 2009;Walker et al., 2001). Female rats selectively-bred for high locomotor response to novel environments (HR) acquired cocaine self-administration, took more cocaine infusions and showed higher breakpoints than HR males and both groups of LR rats (Cummings et al., 2011; Davis et al., 2008). The HR phenotype exerted a greater effect than estrous cycle stage on novelty-induced locomotion and cocaine self-administration. Female rats exhibiting the highest novelty-stimulated locomotion showed greater locomotor responses to cocaine at multiple points in the estrous cycle, representing an additional interaction of HR phenotype and ovarian hormones (Sell et al., 2005). We postulate that at least two mechanisms augment psychostimulant responses in female rats. A longer established, likely genetic, predisposition and a transient enhancement during the estrous cycle linked to surging ovarian hormone concentrations. We postulate that individual differences in cumulative and/or maximal levels of ovarian hormones across all cycle stages support the chronic mechanism. Understanding individual differences in female drug sensitivity will require accounting for effects of phenotype and the chronic and transient effects of ovarian hormones.
Electrically-stimulated dopamine release monitored with fast-scan cyclic voltammetry has been a useful tool to investigate neurochemical effects of drugs and regional differences in dopaminergic neurotransmission. Some studies have attempted to link electrically-stimulated dopamine efflux to behavioral effects of drugs. Multiple studies have shown that the time courses of drug effects on electrically-stimulated dopamine efflux were similar to their respective behavioral time courses (Budygin et al., 2000; Budygin et al., 2007; Garris et al., 2003; Oleson et al., 2009). In contrast, the present studies found correlations in individual rats among the magnitude of cocaine-stimulated dopamine efflux and multiple behavioral topographies, uterine weight and SN density. The highest correlation was for dopamine efflux elicited by 10 Hz stimulations. Dopamine concentrations elicited by this lowest frequency of stimulation may best reflect the dopamine efflux that mediates cocaine-stimulated locomotion, which was shown to be unaffected by deletion of NMDA receptors that mediate phasic dopamine behaviors (Palmiter et al., 2009; Zweifel et al., 2008). Efflux elicited at 10 Hz was also strongly affected by cocaine sensitization (Addy et al., 2010). These results demonstrate that the electrically-stimulated dopamine efflux paradigm can detect drug-induced changes relevant to behavioral changes and anatomic substrates.
In summary, the present study demonstrates that a strong relationship exists in intact female rats among dopamine neuron number, cocaine-stimulated dopamine efflux and cocaine-stimulated behavior. This finding suggests that individual differences in dopamine anatomy/function may represent a vulnerability factor for psychostimulant addiction. The lack of these relationships in OVX animals suggest that this vulnerability may be important in females and mediated in part by ovarian hormones. Given the important role of dopaminergic function during the initiation of addiction, this individual vulnerability may contribute to the “telescoped” development of addictions that has been observed in human females.
Acknowledgments
This work was generously supported by NIDA grant #DA019114 These experiments comply with all applicable United States laws and regulations.
References
- Addy NA, Daberkow DP, Ford JN, Garris PA, Wightman RM. Sensitization of rapid dopamine signaling in the nucleus accumbens core and shell after repeated cocaine in rats. J. Neurophysiol. 2010;104:922–931. doi: 10.1152/jn.00413.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astwood EB. Changes in the weight and water content of the uterus of the normal adult rat. Am. J. Physiol. 1939;126:162–170. [Google Scholar]
- Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol. Biochem. Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur. J. Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Bauman AL. Biogenic amine transporters: regulation in flux. Curr. Opin. Neurobiol. 2000;10:328–336. doi: 10.1016/s0959-4388(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM. Effects of sex and gonadectomy on cocaine metabolism in the rat. J. Pharmacol. Exp. Ther. 1999;290:1316–1323. [PubMed] [Google Scholar]
- Brady K, Randall R. Gender differences in substance abuse disorders. Psych. Clin. N. Am. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Kilpatrick MR, Gainetdinov RR, Wightman RM. Correlation between behavior and extracellular dopamine levels in rat striatum: comparison of microdialysis and fast-scan cyclic voltammetry. Neurosci. Lett. 2000;281:9–12. doi: 10.1016/s0304-3940(00)00813-2. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Mathews TA, Lack AK, Diaz MR, McCool BA, Jones SR. Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacology (Berl) 2007;193:495–501. doi: 10.1007/s00213-007-0812-1. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm. Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol. Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol. Sex Differ. 2011;2:3. doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred high-responder and low-responder rats. Pharmacol. Biochem. Behav. 2008;90:331–338. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm. Behav. 2010;58:13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm. Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK. Axonal sprouting following lesions of the rat substantia nigra. Neuroscience. 2000;97:99–112. doi: 10.1016/s0306-4522(00)00009-9. [DOI] [PubMed] [Google Scholar]
- Garris PA, Budygin EA, Phillips PEM, Venton BJ, Robinson DL, Bergstrom BP, Rebec GV, Wightman RM. A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience. 2003;118:819–829. doi: 10.1016/s0306-4522(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. Independent influences of sex steroids of systemic and central origin in a rat model of Parkinson’s disease: a contribution to sex-specific neuroprotection by estrogens. Horm. Behav. 2010;57:23–34. doi: 10.1016/j.yhbeh.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Hagemann G, Ugur T, Schleussner E, Mentzel HJ, Fitzek C, Witte OW, Gaser C. Changes in brain size during the menstrual cycle. PloS One. 2011;6 doi: 10.1371/journal.pone.0014655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harro J. Inter-individual differences in neurobiology as vulnerability factors for affective disorders: implications for psychopharmacology. Pharmacol. Ther. 2010;125:402–422. doi: 10.1016/j.pharmthera.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Booze RM, Welch M, Browning CE, Mactutus CF. Acute and repeated intravenous cocaine-induced locomotor activity is altered as a function of sex and gonadectomy. Pharmacol. Biochem. Behav. 2005;82:170–181. doi: 10.1016/j.pbb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharmacol. Biochem. Behav. 1991;38:467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Horne MK, Lee J, Parish CL, Tomas D. Chronic cocaine administration reduces striatal dopamine terminal density and striatal dopamine release which leads to drug-seeking behaviour. Neuroscience. 2011;174:143–150. doi: 10.1016/j.neuroscience.2010.11.055. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Day AE, Ho CC, Walker QD, Francis R, Kuhn CM. Androgen decreases dopamine neurone survival in rat midbrain. J. Neuroendocrinol. 2010a;22:238–247. doi: 10.1111/j.1365-2826.2010.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Ho CC, Day AE, Walker QD, Francis R, Kuhn CM. Oestrogen receptors enhance dopamine neurone survival in rat midbrain. J. Neuroendocrinol. 2010b;22:226–237. doi: 10.1111/j.1365-2826.2010.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Wightman RM. Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens. J. Pharmacol. Exp. Ther. 1995;274:396–403. [PubMed] [Google Scholar]
- Jones SR, Lee TH, Wightman RM, Ellinwood EH. Effects of intermittent and continuous cocaine administration on dopamine release and uptake regulation in the striatum: in vitro voltammetric assessment. Psychopharmacology. 1996;126:331–338. doi: 10.1007/BF02247384. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J. Pharmacol. Exp. Ther. 1988;245:1095–1102. [PubMed] [Google Scholar]
- Kantak K, Goodrich C, Uribe V. Influence of sex, estrous cycle and drug-onset age on cocaine self-administratio in rats (Rattus norvegicus) Experimental and Clinical Psychopharmacology. 2007;15:37–47. doi: 10.1037/1064-1297.15.1.37. [DOI] [PubMed] [Google Scholar]
- Katsuda SI, Yoshida M, Watanabe T, Kuroda H, Ando-Lu J, Takahashi M, Hayashi H, Maekawa A. Estrogen receptor mRNA in uteri of normal estrous cycling and ovariectomized rats by in situ hybridization. Proc. Soc. Exp. Biol. Med. 1999;221:207–214. doi: 10.1046/j.1525-1373.1999.d01-78.x. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Iverson LL, Iversen SD, Snyder SH. Handbook in Psychopharmacology. New York: Plenum Press; 1977. Drug-induced Motor Behaviour; pp. 295–332. [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, Walker QD. The emergence of gonadal hormone influences on dopaminergic function during puberty. Horm. Behav. 2010;58:122–137. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Gee KR, Ellinwood EH, Seidler FJ. Altered cocaine potency in the nucleus accumbens following 7-day withdrawal from intermittent but not continuous treatment: voltammetric assessment of dopamine uptake in the rat. Psychopharmacology (Berl) 1998;137:303–310. doi: 10.1007/s002130050623. [DOI] [PubMed] [Google Scholar]
- Levesque D, Di Paolo T. Rapid conversion of high into low striatal D2-dopamine receptor agonist binding states after an acute physiological dose of 17 beta-estradiol. Neurosci. Lett. 1988;88:113–118. doi: 10.1016/0304-3940(88)90324-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp. Clin. Psychopharmacol. 2006;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29:943–951. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- Mandl AM. The phases of the oestrous cycle in the adult white rat. J. Exp. Biol. 1951;28:576–584. [Google Scholar]
- Mandt BH, Allen RM, Zahniser NR. Individual differences in initial low-dose cocaine-induced locomotor activity and locomotor sensitization in adult outbred female Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2009;91:511–516. doi: 10.1016/j.pbb.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Miller DB, Ali SF, O’Callagham JP, Laws SC. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann. N.Y. Acad. Sci. 1998;844:153–165. [PubMed] [Google Scholar]
- Morissette M, Le Saux M, D’Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura LM, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J. Steroid. Biochem. Mol. Biol. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Myatt L, Chaudhuri G, Elder MG, Lim L. The oestrogen receptor in the rat uterus in relation to intra-uterine devices and the oestrous cycle. Biochem. J. 1978;176:523–529. doi: 10.1042/bj1760523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nequin LG, Alvarez J, Schwartz NB. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol. Reprod. 1979;20:659–670. doi: 10.1095/biolreprod20.3.659. [DOI] [PubMed] [Google Scholar]
- Niyomchai T, Akhavan A, Festa ED, Lin SN, Lamm L, Foltz R, Quinones-Jenab V. Estrogen and progesterone affect cocaine pharmacokinetics in female rats. Brain Res. Bull. 2006;68:310–314. doi: 10.1016/j.brainresbull.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Talluri S, Childers SR, Smith JE, Roberts DCS, Bonin KD, Budygin EA. Dopamine uptake changes associated with cocaine self-administration. Neuropsychopharmacology. 2009;34:1174–1184. doi: 10.1038/npp.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJY, Paladini CA, Phillips PEM. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc. Nat. Acad. Sci. U.S. A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CL, Finkelstein DI, Drago J, Borrelli E, Horne MK. The role of dopamine receptors in regulating the size of axonal arbors. J. Neurosci. 2001;21:5147–5157. doi: 10.1523/JNEUROSCI.21-14-05147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CL, Stanic D, Drago J, Borrelli E, Finkelstein DI, Horne MK. Effects of long-term treatment with dopamine receptor agonists and antagonists on terminal arbor size. Eur. J. Neurosci. 2002;16:787–794. doi: 10.1046/j.1460-9568.2002.02132.x. [DOI] [PubMed] [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol. Biochem. Behav. 2008;89:314–323. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, McEwen B, Silbersweig D, Stern E. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18:985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S. Progesterone attenuates cocaine-induced responses. Horm. Behav. 2010;58:22–32. doi: 10.1016/j.yhbeh.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: role of transporter phosphorylation. Pharmacol. Ther. 2011;129:220–238. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol. Biochem. Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, Alvarez VA, Lovinger DM. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D(2) autoreceptors. Nat. Neurosci. 2011;14 doi: 10.1038/nn.2862. 1033–U1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Sanchez MG, Bourque M, Morissette M, Di Paolo T. Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 2010;16:e43–e71. doi: 10.1111/j.1755-5949.2010.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savageau MM, Beatty WW. Gonadectomy and sex differences in the behavioral repsonses to amphetamine and apomorphine of rats. Pharmacol. Biochem. Behav. 1981;14:17–21. doi: 10.1016/0091-3057(81)90097-6. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell SL, Dillon AM, Cunningham KA, Thomas ML. Estrous cycle influence on individual differences in the response to novelty and cocaine in female rats. Behav. Brain Res. 2005;161:69–74. doi: 10.1016/j.bbr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Sharp T, Zetterstrom T, Ljungberg T, Ungerstedt U. A direct comparison of amphetamine-induced behaviours and regional brain dopamine release in the rat using intracerebral dialysis. Brain Res. 1987;401:322–330. doi: 10.1016/0006-8993(87)91416-8. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol. Biochem. Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Strom JO, Theodorsson A, Theodorsson E. Mechanisms of estrogens’ dose-dependent neuroprotective and neurodamaging effects in experimental models of cerebral ischemia. Int. J. Mol. Sci. 2011;12:1533–1562. doi: 10.3390/ijms12031533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorsson A, Strom JO, Theodorsson E. Order of magnitude differences between methods for maintaining physiological 17 beta-oestradiol concentrations in ovariectomized rats. Scand. J. Clin. Lab. Invest. 2008;68:814–822. doi: 10.1080/00365510802409703. [DOI] [PubMed] [Google Scholar]
- Theodorsson E, Strom JO, Holm L, Theodorsson A. Different methods for administering 17 beta-estradiol to ovariectomized rats result in opposite effects on ischemic brain damage. BMC Neurosci. 2010;11 doi: 10.1186/1471-2202-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PEM, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J. Neurosci. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Zhang H, Garris PA, Phillips PEM, Sulzer D, Wightman RM. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J. Neurochem. 2003;87:1284–1295. doi: 10.1046/j.1471-4159.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- Walker QD, Francis R, Cabassa J, Kuhn CM. Effect of ovarian hormones and estrous cycle on stimulation of the hypothalamo-pituitary-adrenal axis by cocaine. J. Pharmacol. Exp. Ther. 2001;297:291–298. [PubMed] [Google Scholar]
- Walker QD, Kuhn CM. Cocaine increases stimulated dopamine release more in periadolescent than adult rats. Neurotoxicol. Teratol. 2008;30:412–418. doi: 10.1016/j.ntt.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31:1193–1202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res. Brain Res. Rev. 1990;15:135–144. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Windels F, Kiyatkin EA. Dopamine action in the substantia nigra pars reticulata: iontophoretic studies in awake, unrestrained rats. Eur. J. Neurosci. 2006;24:1385–1394. doi: 10.1111/j.1460-9568.2006.05015.x. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, Mcewen BS. Naturally-occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Mcewen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous-cycle in the adult-rat. J. Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J. Neurosci. Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]