Abstract
AIM: To investigate human epidermal growth factor receptor 2 (HER2)-phosphatidylinositol 3-kinase (PI3K)-v-Akt murine thymoma viral oncogene homolog signaling pathway.
METHODS: We analyzed 231 formalin-fixed, paraffin-embedded gastric cancer tissue specimens from Japanese patients who had undergone surgical treatment. The patients’ age, sex, tumor location, depth of invasion, pathological type, lymph node metastasis, and pathological stage were determined by a review of the medical records. Expression of HER2 was analyzed by immunohistochemistry (IHC) using the HercepTestTM kit. Standard criteria for HER2 positivity (0, 1+, 2+, and 3+) were used. Tumors that scored 3+ were considered HER2-positive. Expression of phospho Akt (pAkt) was also analyzed by IHC. Tumors were considered pAkt-positive when the percentage of positive tumor cells was 10% or more. PI3K, catalytic, alpha polypeptide (PIK3CA) mutations in exons 1, 9 and 20 were analyzed by pyrosequencing. Epstein-Barr virus (EBV) infection was analyzed by in situ hybridization targeting EBV-encoded small RNA (EBER) with an EBER-RNA probe. Microsatellite instability (MSI) was analyzed by polymerase chain reaction using the mononucleotide markers BAT25 and BAT26.
RESULTS: HER2 expression levels of 0, 1+, 2+ and 3+ were found in 167 (72%), 32 (14%), 12 (5%) and 20 (8.7%) samples, respectively. HER2 overexpression (IHC 3+) significantly correlated with intestinal histological type (15/20 vs 98 /205, P = 0.05). PIK3CA mutations were present in 20 cases (8.7%) and significantly correlated with MSI (10/20 vs 9/211, P < 0.01). The mutation frequency was high (21%) in T4 cancers and very low (6%) in T2 cancers. Mutations in exons 1, 9 and 20 were detected in 5 (2%), 9 (4%) and 7 (3%) cases, respectively. Two new types of PIK3CA mutation, R88Q and R108H, were found in exon1. All PIK3CA mutations were heterozygous missense single-base substitutions, the most common being H1047R (6/20, 30%) in exon20. Eighteen cancers (8%) were EBV-positive and this positivity significantly correlated with a diffuse histological type (13/18 vs 93/198, P = 0.04). There were 7 cases of lymphoepithelioma-like carcinomas (LELC) and 6 of those cases were EBV-positive (percent/EBV: 6/18, 33%; percent/all LELC: 6/7, 86%). pAkt expression was positive in 119 (53%) cases but showed no correlation with clinicopathological characteristics. pAkt expression was significantly correlated with HER2 overexpression (16/20 vs 103/211, P < 0.01) but not with PIK3CA mutations (12/20 vs 107/211, P = 0.37) or EBV infection (8/18 vs 103/211, P = 0.69). The frequency of pAkt expression was higher in cancers with exon20 mutations (100%) than in those with exon1 (40%) or exon9 (56%) mutations. One case showed both HER2 overexpression and EBV infection and 3 cases showed both PIK3CA mutations and EBV infection. However, no cases showed both PIK3CA mutations and HER2 overexpression. One EBV-positive cancer with PIK3CA mutation (H1047R) was MSI-positive. Three of these 4 cases were positive for pAkt expression. In survival analysis, pAkt expression significantly correlated with a poor prognosis (hazard ratio 1.75; 95%CI: 1.12-2.80, P = 0.02).
CONCLUSION: HER2 expression, PIK3CA mutations and EBV infection in gastric cancer were characterized. pAkt expression significantly correlates with HER2 expression and with a poor prognosis.
Keywords: Human epidermal growth factor receptor 2, Phosphatidylinositol 3-kinase, Catalytic, Alpha polypeptide, Epstein-Barr virus, Akt, Gastric cancer
INTRODUCTION
Gastric cancer is one of the most common cancer types and the second leading cause of cancer-related deaths worldwide[1]. Genetic and epigenetic alterations play important roles in the development and progression of these tumors[1,2]. Considerable attention has been given to the potential role of the phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway in gastric cancer[3,4]. Various alterations, such as activation of growth factor receptors, PI3K, catalytic, alpha polypeptide (PIK3CA) mutations and inactivation of phosphatase and tensin homolog (PTEN) lead to activation of the PI3K-Akt signaling pathway. With regards to growth factor receptors, there is growing evidence that human epidermal growth factor receptor 2 (HER2) is a key driver of tumorigenesis and an important biomarker in gastric cancer. The amplification or overexpression of HER2 is observed in 7%-34% of these cases[5-9].
PIK3CA is mutated in a wide variety of human tumor types[10,11], including gastric cancers[12-15]. Activating mutations in this gene up-regulate the PI3K-Akt signaling pathway, making it a potentially useful therapeutic target. For example, oncogenic mutations of PIK3CA reportedly render breast cancers more resistant to treatment with the anti-HER2 receptor antibody trastuzumab[16]. Thus, this signaling pathway is thought to be one of the mechanisms underlying resistance to trastuzumab. Trastuzumab has recently been approved for treatment of advanced gastric cancers[5,6].
Pyrosequencing-based methods facilitate the identification of low-frequency tumor mutations and allow a more accurate assessment of tumor mutation burden[17]. PIK3CA mutations have been detected in 4%-25% of gastric cancers[12-15]. However, in most previous studies, only exons 9 and 20 hot spot mutations in PIK3CA were analyzed by DNA sequencing. Moreover, the association between HER2 expression and PIK3CA mutations in gastric cancer has not been reported.
A significant correlation has been found between Ep-stein-Barr virus (EBV) and the methylation of multiple genes in gastric cancers[18-20]. EBV infection reportedly induces PTEN expression loss through CpG island methylation of its promoter, leading to activation of the PI3K-Akt signaling pathway, in EBV-associated gastric cancer[21].
The aim of our present study was to systematically characterize HER2 expression, PIK3CA mutations, and EBV infection, all of which are involved in the PI3K-Akt signaling pathway, in a large cohort of gastric cancers (n = 231). We wished to determine the prevalence of each of these factors with a high precision and thereby correlate them with clinicopathological and molecular features of gastric lesions, including microsatellite instability (MSI) and phospho Akt (pAkt) expression.
MATERIALS AND METHODS
Tissue samples
A total of 231 formalin-fixed, paraffin-embedded (FFPE) gastric cancer tissue specimens from Japanese patients who had undergone surgical treatment was analyzed in this study. The patients’ age, sex, tumor location, depth of invasion, pathological type, lymph node metastasis, and pathological stage were determined by a review of their medical records. Clinicopathological findings were determined according to the criteria of the Japanese Research Society for Gastric Cancer (Table 1). Our institutional review committee approved the study.
Table 1.
Clinicopathological characteristics of patients with gastric cancer
| Variables (n = 231) | n (%) | |
| Sex | Male | 157 (68) |
| Female | 74 (32) | |
| Age (yr) | Median (range) | 71 (25-91) |
| Location | Cardias | 82 (35) |
| Body | 62 (27) | |
| Antrum | 83 (36) | |
| Unknown | 4 (2) | |
| Depth of invasion | T2 | 125 (54) |
| T3 | 92 (40) | |
| T4 | 14 (6) | |
| Lymph node metastasis | N0 | 65 (28) |
| N+ | 158 (68) | |
| N1 | 73 (32) | |
| N2 | 56 (24) | |
| N3 | 29 (13) | |
| Unknown | 8 (3) | |
| Stage | IB | 49 (21) |
| II | 45 (19) | |
| IIIA + IIIB | 82 (35) | |
| IV | 51 (22) | |
| Unknown | 4 (2) | |
| Lauren histotype | Intestinal | 113 (49) |
| Diffuse | 112 (48) | |
| Others | 6 (3) |
Immunohistochemistry
HER2 expression was analyzed using the HercepTestTM kit (DAKO, Carpinteria, CA) by manual sample processing in accordance with the manufacturer’s instructions. Standard criteria for HER2 positivity (0, 1+, 2+ and 3+) were used. Tumors that scored 3+ were considered HER2-positive. For the immunohistochemical analysis of pAkt, FFPE specimens were processed using SignalStain Boost Detection Reagent (Cell Signaling Technology, Beverly, MA). Briefly, 5-μm-thick sections were dewaxed in xylene, rehydrated in ethanol, and heated with target retrieval solution (DAKO) in an autoclave for antigen retrieval. Endogenous peroxidase was blocked by incubation with 0.3% hydrogen peroxide in methanol for 10 min. The tissue sections were then washed twice with tris-buffered saline (TBS) and preblocked with 10% goat serum in TBS for 60 min. After washing with TBS, the sections were incubated with an anti-phospho-Akt (Ser473) polyclonal antibody (D9E, Cell Signaling Technology) at a dilution of 1:100 for 30 h at 4 °C. The sections were washed three times in TBS and incubated with SignalStain Boost Detection Reagent for 45 min. After three further washes in TBS, a diamino-benzidine tetrahydrochloride working solution was applied. Finally, the sections were counterstained with hematoxylin. Tumors were considered pAkt-positive when the percentage of positive tumor cells was 10% or more[22]. Only clear staining of the tumor cell nucleus and/or cytoplasm was considered positive.
Mutation analysis of the PIK3CA gene by pyrosequencing
Genomic DNA was extracted from tumor specimens and mutations in exon9 and exon20 of the PIK3CA gene were analyzed by pyrosequencing as described previously[23,24]. We also developed a pyrosequencing assay to detect PIK3CA exon1 mutations using the primer sets exon1-RS1 (5’-GGGAAGAATTTTTTGATGAAACA-3’ for the biotinylated forward primer and 5’-GGTTGCCTACTGGTTCAATTACTT-3’ for the reverse primer) and exon1-RS2 (5’-CGGCTTTTTCAACCCTTTTT-3’ for the forward primer and 5’-ATTTCTCGATTGAGGATCTTTTCT-3’ for the biotinylated reverse primer). Each polymerase chain reaction (PCR) mix contained the forward and reverse primers (each 10 μmol/L), a 25 mmol/L dNTP mix with dUTP, 75 mmol/L MgCl2, 1 × PCR buffer, 1.0 U of exTaq, and 2 μL of template DNA in a total volume of 25 μL. PCR conditions were as follows: initial denaturing at 95 °C for 5 min; 50 cycles of 94 °C for 20 s, 50 °C for 20 s and 74 °C for 40 s; and a final extension at 72 °C for 1 min. The PCR products (each 25 μL) were sequenced using the PyroMark kit and Pyrosequencing PSQ96 HS System (Qiagen, Valencia, CA).
In situ hybridization for EBER1
The presence of EBV in the carcinoma tissues was evaluated by in situ hybridization (ISH) targeting of EBV-encoded small RNA (EBER-ISH) with an EBER-RNA probe (Dako Cytomation).
Microsatellite instability analysis
MSI was analyzed by PCR using the mononucleotide markers (BAT25 and BAT26). Based on the number of markers showing instability per tumor sample, cancers were divided into two groups; those with one or more of the two markers displaying MSI and those with no instability (microsatellite stable).
Statistical analysis
For all statistical analysis, the JMP program was used. All P values were two-sided and statistical significance was set at P ≤ 0.05. For categorical data, the χ2 test was used. For survival analysis, Kaplan-Meier method and log-rank test were used. For analysis of cancer-specific mortality, we excluded surgery-related deaths (deaths within one month of surgery).
RESULTS
HER2 expression in gastric cancer tissues
HER2 expression levels of 0, 1+, 2+ and 3+ were found in 167 (72%), 32 (14%), 12 (5%) and 20 (8.7%) samples, respectively (Figure 1). HER2 overexpression (IHC 3+) significantly correlated with intestinal histological type (15/20 vs 98/205, P = 0.05, Table 2). Three-year survival rates were 29% in patients with HER2 overexpression and 59% in cases without HER2 overexpression, respectively [hazard ratio (HR) 1.73; 95%CI: 0.87-3.14, P = 0.24].
Figure 1.
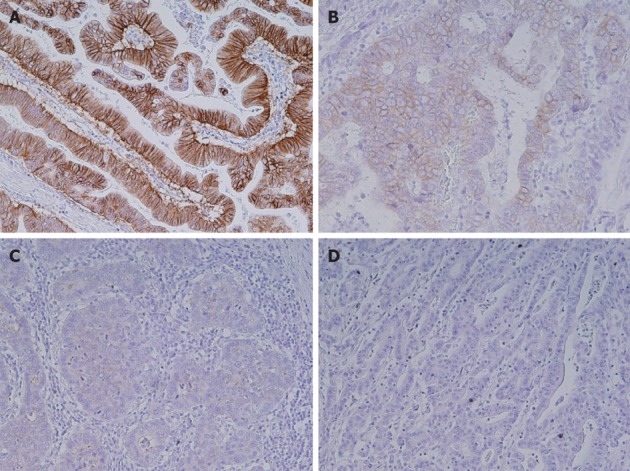
Immunohistochemical analysis of human epidermal growth factor receptor 2 in gastric cancer tissues. A: Human epidermal growth factor receptor 2 (HER2) 3+; B: HER2 2+; C: HER2 1+; D: HER2 0. Original magnification, ×200.
Table 2.
Clinicopathological characteristics of patients with gastric cancer based on human epidermal growth factor receptor 2 expression, phosphatidylinositol 3-kinase, catalytic, alpha polypeptide mutations and Epstein-Barr virus infection n (%)
| HER2 | PIK3CA | EBV | ||||||||
| Positive (n = 20) | Negative (n = 211) | P value | Mutation (n = 20) | Wild type (n = 211) | P value | Positive (n = 18) | Negative (n = 204) | P value | ||
| Sex | Male | 15 (75) | 142 (67) | 0.48 | 13 (65) | 144 (68) | 0.77 | 14 (78) | 138 (68) | 0.38 |
| Female | 5 (25) | 69 (33) | 7 (35) | 70 (32) | 4 (22) | 66 (32) | ||||
| Age | Median | 69 (50-84) | 71 (25-91) | 0.26 | 71 (25-85) | 70 (38-91) | 0.40 | 72 (48-90) | 70 (38-91) | 0.41 |
| Location | Cardias | 10 (50) | 72 (34) | 0.49 | 5 (25) | 77 (36) | 0.31 | 8 (44) | 73 (36) | 0.70 |
| Body | 5 (25) | 57 (27) | 4 (20) | 58 (27) | 5 (28) | 55 (27) | ||||
| Antrum | 5 (25) | 78 (37) | 10 (50) | 73 (35) | 5 (28) | 75 (37) | ||||
| Unknown | 0 | 4 (2) | 1 (5) | 2 (1) | 0 | 1 (0) | ||||
| Depth | T2 | 12 (60) | 113 (54) | 0.48 | 8 (40) | 117 (55) | 0.15 | 12 (67) | 106 (52) | 0.35 |
| T3 | 8 (40) | 84 (40) | 9 (45) | 83 (39) | 6 (33) | 85 (42) | ||||
| T4 | 0 | 14 (6) | 3 (15) | 11 (5) | 0 | 13 (6) | ||||
| L/N meta | N0 | 5 (25) | 60 (28) | 0.71 | 4 (20) | 61 (29) | 0.37 | 3 (17) | 57 (28) | 0.28 |
| N+ | 14 (70) | 144 (68) | 16 (80) | 142 (67) | 14 (77) | 140 (69) | ||||
| N1 | 5 (25) | 68 (32) | 8 (40) | 65 (31) | 8 (44) | 63 (31) | ||||
| N2 | 6 (30) | 50 (24) | 6 (30) | 50 (24) | 2 (11) | 53 (26) | ||||
| N3 | 3 (15) | 26 (12) | 2 (10) | 27 (13) | 4 (22) | 24 (12) | ||||
| Unknown | 1 (5) | 7 (3) | 0 | 8 (4) | 1 (6) | 7 (3) | ||||
| Stage | I | 5 (25) | 44 (21) | 0.89 | 1 (5) | 48 (23) | 0.14 | 3 (17) | 41 (20) | 0.98 |
| II | 3 (15) | 42 (20) | 7 (35) | 38 (18) | 4 (22) | 39 (19) | ||||
| III | 6 (30) | 76 (36) | 8 (40) | 74 (35) | 6 (33) | 75 (37) | ||||
| IV | 5 (25) | 46 (22) | 4 (20) | 47 (22) | 4 (22) | 46 (23) | ||||
| Unknown | 1 (5) | 3 (1) | 0 | 4 (2) | 1 (6) | 3 (1) | ||||
| Lauren | Intestinal | 15 (75) | 98 (46) | 0.05 | 14 (70) | 99 (47) | 0.13 | 5 (28) | 105 (51) | 0.04 |
| histotype | Diffuse | 5 (25) | 107 (51) | 6 (30) | 106 (50) | 13 (72) | 93 (46) | |||
| LELC | 0 | 6 (3) | 2 (10) | 4 (2) | 5 (28) | 0 | ||||
| Others | 0 | 6 (3) | 0 | 6 (3) | 0 | 6 (3) | ||||
| MSI | 2 (10) | 28 (13) | 0.72 | 10 (50) | 20 (9) | < 0.01 | 1 (6) | 26 (13) | 0.36 | |
| pAkt | 16 (84) | 103 (51) | < 0.01 | 12 (63) | 107 (53) | 0.37 | 8 (47) | 103 (52) | 0.69 | |
| 3 yr OS (%) | 29.4 | 59.2 | 0.24 | 57.3 | 56.8 | 0.59 | 57.4 | 57.3 | 0.98 | |
MSI: Microsatellite instability; LELC: Lymphoepithelioma-like carcinoma; HER2: Human epidermal growth factor receptor 2; PIK3CA: Phosphatidylinositol 3-kinase, catalytic, alpha polypeptide mutations; EBV: Epstein-Barr virus; pAkt: Phospho Akt; OS: Overall survival.
Mutations of the PIK3CA gene in gastric cancer tissues
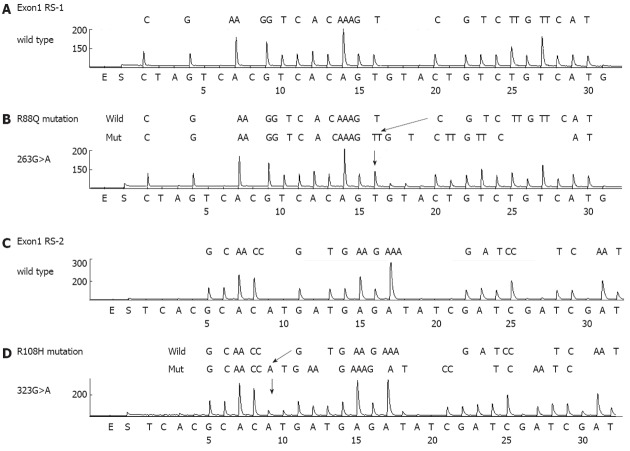
PIK3CA mutations were present in 20 cases (8.7%) (Table 2 and Figure 2). The mutation frequency was high (21%) in T4 cancers and low (6%) in T2 cancers. Mutations in exons 1, 9 and 20 of PIK3CA were detected in 5 (2%), 9 (4%) and 7 (3%) cases, respectively (Table 3). One case had multiple PIK3CA mutations (R108H and E542K). The exon20/exon9 prevalence ratio was 0.78 (7/9). Two new types of PIK3CA mutations, R88Q and R108H, were detected in exon1. All mutations were heterozygous missense single-base substitutions and the most common mutation was H1047R (6/20; 30%) in exon20. PIK3CA mutations were also found to significantly correlate with MSI (10/20 vs 9/211, P < 0.01) but not with other clinicopathological characteristics. The three-year survival rates were 57% in patients with PIK3CA mutations and 57% in cases without PIK3CA mutations, respectively (HR 1.37; 95%CI: 0.68-3.26, P = 0.59).
Figure 2.
Phosphatidylinositol 3-kinase, catalytic, alpha polypeptide mutations detected by pyrosequencing in gastric cancer tissues. A: Exon1 RS1 wild type; B: 263G>A (R88Q) mutation; C: Exon1 RS2 wild type; D: 323G>A (R108H) mutation.
Table 3.
Frequencies of phosphatidylinositol 3-kinase, catalytic, alpha polypeptide mutations detected in gastric cancer tissues
| Mutation | Overall frequency | Percent/total cases | Percent/mutated cases | Microsatellite instability | |
| Exon1 | R88Q | 1 | 0.4 | 5 | 1 |
| R108H | 4 | 1.7 | 20 | 1 | |
| Total | 5 | 2.2 | 2 | ||
| Exon9 | E542K | 5 | 2.2 | 25 | 1 |
| E545K | 2 | 0.9 | 10 | 1 | |
| E545G | 2 | 0.9 | 10 | 1 | |
| Total | 9 | 4.0 | 3 | ||
| Exon20 | H1047Y | 1 | 0.4 | 5 | 1 |
| H1047R | 6 | 2.6 | 30 | 4 | |
| Total | 7 | 3.0 | 5 |
EBV infection
Eighteen samples in our cohort (8%) were EBV-positive and this positivity significantly correlated with diffuse histological type (13/18 vs 93/198, P = 0.04) (Table 2 and Figure 3). There were 7 cases of LELC and 6 of those cases were EBV-positive (percent/EBV: 6/18, 33%; percent/all LELC: 6/7, 86%). The three-year survival rates were 57% in patients with EBV infection and 57% in those without EBV infection (HR 0.81; 95%CI: 0.36-2.31, P = 0.98).
Figure 3.
In situ hybridization analysis of Epstein-Barr virus-encoded small RNA-1 and human epidermal growth factor receptor 2 immunohistochemical expression in gastric cancer tissues. A: Gastric adenocarcinoma positive for Epstein-Barr virus-encoded small RNA-1 (EBER-1); B: Gastric adenocarcinoma negative for EBER-1; C: Immunohistochemical analysis of human epidermal growth factor receptor 2 (HER2) in an Epstein-Barr virus-positive and HER2-positive case. Original magnification, ×200.
Association of HER2 overexpression, PIK3CA mutations and EBV infection
One of our cases showed both HER2 overexpression and EBV infection and 3 cases showed both PIK3CA mutations and EBV infection. However there were no cases showing both PIK3CA mutations and HER2 overexpression. Three of the 4 cases were positive also for pAkt expression. PIK3CA mutations were present in 3 EBV-positive cancers, including 2 cases of LELC (2/5, 40%). One EBV-positive cancer with a PIK3CA mutation (H1047R) was MSI-positive.
pAkt expression
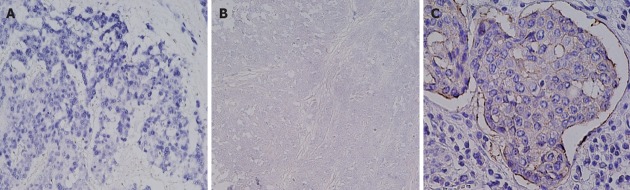
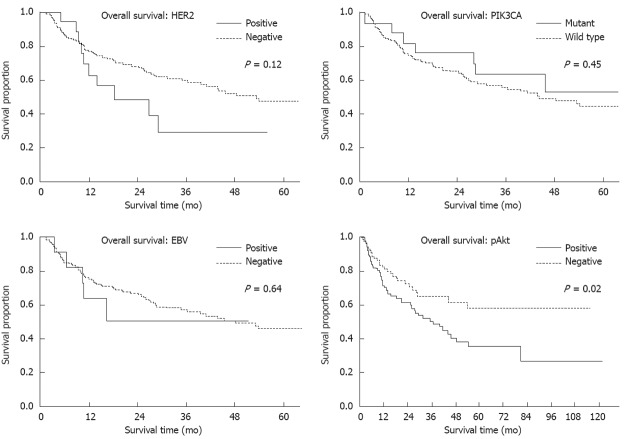
pAkt expression was positive in 119 (53%) of our cases but this showed no correlation with clinicopathological characteristics (Figure 4A). On the other hand, pAkt expression was found to be significantly correlated with HER2 overexpression (16/19 vs 103/204, P < 0.01) but not with PIK3CA mutations (12/19 vs 107/204, P = 0.37) or EBV infection (8/17 vs 103/198, P = 0.69) (Table 2). The frequency of pAkt expression was higher in cancers with exon20 mutations (100%) than in those with exon1 (40%) or exon9 (56%) mutations of PIK3CA, although this difference did not reach statistical significance (Figure 4B). The five-year survival rates were 37% in patients with pAkt expression and 59% in those without pAkt expression (HR 1.75; 95%CI: 1.12-2.80, P = 0.02) (Figure 5). Hence, pAkt expression significantly correlates with a poor prognosis in gastric cancer.
Figure 4.
Immunohistochemical analysis and assessment of phospho Akt positivity based on molecular alterations in gastric cancer tissues. A: Gastric adenocarcinoma showing phospho Akt (pAkt) positivity. Original magnification, ×200; B: pAkt expression significantly correlates with human epidermal growth factor receptor 2 (HER2) overexpression (P < 0.01) but not with phosphatidylinositol 3-kinase, catalytic, alpha polypeptide (PIK3CA) mutations (P = 0.37) or Epstein-Barr virus (EBV) infection (P = 0.69).
Figure 5.
Survival analysis of gastric cancer patients. Three year survival of human epidermal growth factor receptor 2 (HER2)-positive vs HER2-negative, 29.1 mo vs 59.4 mo; Phosphatidylinositol 3-kinase, catalytic, alpha polypeptide (PIK3CA) mutation vs wild type, 63.7 mo vs 56.3 mo; Epstein-Barr virus (EBV)-positive vs EBV-negative, 51.3 mo vs 57.6 mo; And phospho Akt (pAkt)-positive vs pAkt-negative, 50.7 mo vs 64.8 mo. Five year survival of pAkt-positive vs pAkt-negative cases, 35.5 mo vs 58.1 mo.
DISCUSSION
In our present study, we systematically characterized HER2 expression, PIK3CA mutations and EBV infection, all of which are involved in the PI3K-Akt signaling pathway, in a large cohort of patients with gastric cancer (n = 231). We aimed to determine the prevalence of these characteristics with a high level of precision and to correlate them with clinicopathological and molecular features, such as MSI and pAkt expression.
HER2 overexpression (IHC 3+) was present in 20 samples (8.4%), a value that is within the range (7%-34%) reported in the current literature[5-9]. HER2 overexpression was found to significantly correlate with the intestinal histological type. Hence, the frequency of HER2 expression may depend on, at least in part, the distribution of histology in a cohort of gastric cancer samples. Some studies have suggested that HER2 positivity in gastric cancer is associated with poor outcomes and aggressive disease, but the results are conflicting. We found for the first time in our present analyses that HER2 overexpression significantly correlates with pAkt expression in gastric cancer tissues. Moreover, pAkt expression correlated with a poor prognosis in these patients. Thus, the HER2-Akt axis may play an important role in gastric cancer.
Pyrosequencing-based methods facilitate the identification of low-frequency tumor mutations and allow a more accurate assessment of tumor mutation burden[17,23,24]. We characterized PIK3CA mutations in gastric cancer tissues using pyrosequencing for the first time. The overall prevalence of PIK3CA mutations was found in our analysis to be 8.7%, a value that is within the previously reported range (4% to 25%)[10,12-15]. The mutation frequency was found to be high (21.4%) in T4 cancers and low (6.4%) in T2 cancers in our sample cohort. Thus, PIK3CA mutations appear to be late events in gastric carcinogenesis, leading to tumor progression. These patients might therefore be appropriate for targeted therapies directed against the PI3K pathway.
The most common PIK3CA mutation found in our analysis was H1047R, which was also found previously[15]. Importantly, two new types of mutations were found in exon1. To our knowledge, PIK3CA mutations involving residues 88 and 108 (R88Q and R108H) have been never reported previously in gastric cancer, nor described in the COSMIC database, despite the large number of previous studies in which this region was investigated. These mutations have been detected in several other types of cancer tissues[25]. Importantly also, these mutations have been reported to be gain-of-function[26-28]. Our present results thus have potential clinical implications since the mutational status of PIK3CA could stratify patients for genotype-based molecular therapies targeting the PI3K pathway. Hence, exon1 of PIK3CA should be analyzed in gastric cancer patients in these clinical settings.
PIK3CA mutations were found to be significantly associated with the MSI phenotype in our experiments. An association between PIK3CA mutations and MSI has been reported, or at least suggested, for both gastric and colon cancers[12,13,29]. We found in our present study that PIK3CA mutations in cancers with MSI are distributed in exon1, exon9 and exon20. These results further support the notion that PIK3CA is one of the most important oncogenes activated by missense mutations in MSI-positive gastric cancers.
The frequency of pAkt expression was found to be higher in cancers with exon20 mutations (100%) than in those with exon1 (40%) or exon9 (56%) mutations in PIK3CA. These results further support the notion that the functional significance of PIK3CA mutations depends on the mutation type and that the H1047R hotspot mutation has high oncogenic activity.
The previous ToGA study has shown that the addition of trastuzumab to the chemotherapeutic regimen improves survival in patients with advanced gastric or gastro-esophageal junction cancer[5,6]. PIK3CA mutation is one of the mechanisms underlying the resistance to trastuzumab in breast cancer[30]. Trastuzumab is likely to be effective for HER2-overexpressing breast cancers with no PIK3CA mutations, with possible rescue using HER2-TKIs in cases of relapse[31]. For HER2-overexpressing breast cancer with PIK3CA mutations, inhibitors against molecules of the PI3K pathway are possibly more effective than anti-HER2 agents, which are unlikely to be beneficial[32]. In our present study, PIK3CA mutations were not found in gastric cancers with HER2 overexpression. Thus, it is unlikely that PIK3CA mutation is a major mechanism underlying the resistance to trastuzumab in gastric cancer.
HER2 overexpression was found in only one of the 18 EBV-positive gastric cancers in our sample cohort. This result can be explained, at least in part, by the fact that HER2 overexpression and EBV infection significantly correlate with intestinal and diffuse histological types, respectively. On the other hand, PIK3CA mutations were identified in 3 EBV-positive cancers, including 2 cases of LELC (2/5, 40%). Although not analyzed in our current study, EBV infection reportedly inactivates PTEN through the CpG island methylation of its promoter in EBV-associated gastric cancer[21]. Thus, alterations in the PI3K-Akt signaling pathway in EBV-positive gastric cancers may differ from those in EBV-negative cancers.
Finally, pAkt expression was found to correlate with a poor prognosis in gastric cancer. A significant association between increased pAkt expression and poor prognosis has been reported previously in patients with T3/T4 gastric cancer but not in those with T1/T2 cancer[33]. It has been reported also that pAkt expression is associated with increased resistance to multiple chemotherapeutic agents in gastric cancer patients, when chemotherapeutic sensitivities were tested using MTT assays[34]. Thus, Akt activation appears to lead to a poor prognosis and resistance to chemotherapeutic agents in gastric cancer. A positive correlation between a decrease in the pAkt levels after gefitinib administration and tumor apoptotic index in gastric cancer has also been reported[35]. Further analyses regarding the pAkt status in cancer tissues before and after chemotherapy and molecular targeted therapy will be necessary. Not all Akt activation events can be explained by HER2 expression, PIK3CA mutations, and EBV infection in gastric cancer. We have reported previously that a dominant negative insulin-like growth factor (IGF)-1 receptor blocks the Akt-1 activation induced by IGF-1 and IGF-2 in gastric cancer cell lines[36]. Thus, molecular alterations, such as the overexpression of IGF-1 receptor, might be involved in the activation of Akt in gastric cancer and this issue needs to be clarified in the near future.
COMMENTS
Background
Personalized therapy has begun also in advanced gastric cancer through the use of trastuzumab, an anti-human epidermal growth factor receptor 2 (HER2) antibody. Many drugs targeting the phosphatidylinositol 3-kinase (PI3K)-Akt pathway have now been developed and clinical trials are ongoing. An appropriate biomarker is necessary for successful molecular targeted therapy. The alterations of molecules in the PI3K-Akt pathway could be a good biomarker for such drugs.
Research frontiers
Various alterations, such as activation of growth factor receptors, PI3K, catalytic, alpha polypeptide (PIK3CA) mutations and Epstein-Barr virus (EBV) infection lead to activation of the PI3K-Akt signaling pathway. However, clinicopathological and molecular correlates among such alterations have not been clearly addressed. In the present study, the authors identify new clinicopathological and molecular correlations between HER2 expression, PIK3CA mutations, EBV infection and phospho Akt (pAkt) expression in gastric cancer.
Innovations and breakthroughs
This is the first study to systematically characterize HER2 expression, PIK3CA mutations and EBV infection, all of which are involved in the PI3K-Akt signaling pathway, in a large cohort of patients with gastric cancer. The prevalence of these characteristics was thereby determined with a high level of precision and correlations with the clinicopathological and molecular features of gastric cancers, such as microsatellite instability and pAkt expression, could be assessed accurately for the first time.
Applications
The results have potentially important clinical implications since the mutational status of PIK3CA can be used to stratify cancer patients for genotype-based molecular therapies that target the HERs-PI3K pathway.
Terminology
PI3K-Akt pathway: Akt is believed to transduce the major downstream PI3K signals in cancer. Akt regulates cell growth and survival pathways by phosphorylating substrates such as GSK3, forkhead transcription factors, and the TSC2 tumor suppressor protein; PIK3CA: PIK3CA encodes a key enzymatic subunit of PI3K. Gain of function mutations in PIK3CA occur frequently in several cancer types. Hotspots of PIK3CA mutations are located in exons 9 and 20.
Peer review
The authors investigated HER2 expression, PIK3CA mutations and EBV infection in patients with gastric cancer. The results demonstrated that pAkt expression significantly correlates with the prognosis and the HER2 expression status in gastric cancer. This article is important for the further development of molecular targeted therapy in patients with advanced gastric cancer.
Footnotes
Supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, to Yamamoto H and Shinomura Y
Peer reviewer: Takaaki Arigami, MD, PhD, Department of Surgical Oncology and Digestive Surgery, Field of Oncology, Kagoshima University Graduate School of Medical and Dental Sciences, 8-35-1 Sakuragaoka, Kagoshima 891-0175, Japan
S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Hamilton JP, Sato F, Greenwald BD, Suntharalingam M, Krasna MJ, Edelman MJ, Doyle A, Berki AT, Abraham JM, Mori Y, et al. Promoter methylation and response to chemotherapy and radiation in esophageal cancer. Clin Gastroenterol Hepatol. 2006;4:701–708. doi: 10.1016/j.cgh.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Tamura G. Alterations of tumor suppressor and tumor-related genes in the development and progression of gastric cancer. World J Gastroenterol. 2006;12:192–198. doi: 10.3748/wjg.v12.i2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 4.Mueller A, Bachmann E, Linnig M, Khillimberger K, Schimanski CC, Galle PR, Moehler M. Selective PI3K inhibition by BKM120 and BEZ235 alone or in combination with chemotherapy in wild-type and mutated human gastrointestinal cancer cell lines. Cancer Chemother Pharmacol. 2012;69:1601–1615. doi: 10.1007/s00280-012-1869-z. [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 6.Sawaki A, Ohashi Y, Omuro Y, Satoh T, Hamamoto Y, Boku N, Miyata Y, Takiuchi H, Yamaguchi K, Sasaki Y, et al. Efficacy of trastuzumab in Japanese patients with HER2-positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the Trastuzumab for Gastric Cancer (ToGA) study. Gastric Cancer. 2012;15:313–322. doi: 10.1007/s10120-011-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 9.Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 10.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 11.Lee JW, Soung YH, Kim SY, Lee HW, Park WS, Nam SW, Kim SH, Lee JY, Yoo NJ, Lee SH. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene. 2005;24:1477–1480. doi: 10.1038/sj.onc.1208304. [DOI] [PubMed] [Google Scholar]
- 12.Li VS, Wong CW, Chan TL, Chan AS, Zhao W, Chu KM, So S, Chen X, Yuen ST, Leung SY. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer. 2005;5:29. doi: 10.1186/1471-2407-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velho S, Oliveira C, Ferreira A, Ferreira AC, Suriano G, Schwartz S, Duval A, Carneiro F, Machado JC, Hamelin R, et al. The prevalence of PIK3CA mutations in gastric and colon cancer. Eur J Cancer. 2005;41:1649–1654. doi: 10.1016/j.ejca.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Ligresti G, Militello L, Steelman LS, Cavallaro A, Basile F, Nicoletti F, Stivala F, McCubrey JA, Libra M. PIK3CA mutations in human solid tumors: role in sensitivity to various therapeutic approaches. Cell Cycle. 2009;8:1352–1358. doi: 10.4161/cc.8.9.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbi S, Cataldo I, De Manzoni G, Bersani S, Lamba S, Mattuzzi S, Bardelli A, Scarpa A. The analysis of PIK3CA mutations in gastric carcinoma and metanalysis of literature suggest that exon-selectivity is a signature of cancer type. J Exp Clin Cancer Res. 2010;29:32. doi: 10.1186/1756-9966-29-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kataoka Y, Mukohara T, Shimada H, Saijo N, Hirai M, Minami H. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol. 2010;21:255–262. doi: 10.1093/annonc/mdp304. [DOI] [PubMed] [Google Scholar]
- 17.Weidlich S, Walsh K, Crowther D, Burczynski ME, Feuerstein G, Carey FA, Steele RJ, Wolf CR, Miele G, Smith G. Pyrosequencing-based methods reveal marked inter-individual differences in oncogene mutation burden in human colorectal tumours. Br J Cancer. 2011;105:246–254. doi: 10.1038/bjc.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang GH, Lee S, Kim WH, Lee HW, Kim JC, Rhyu MG, Ro JY. Epstein-barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol. 2002;160:787–794. doi: 10.1016/S0002-9440(10)64901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusano M, Toyota M, Suzuki H, Akino K, Aoki F, Fujita M, Hosokawa M, Shinomura Y, Imai K, Tokino T. Genetic, epigenetic, and clinicopathologic features of gastric carcinomas with the CpG island methylator phenotype and an association with Epstein-Barr virus. Cancer. 2006;106:1467–1479. doi: 10.1002/cncr.21789. [DOI] [PubMed] [Google Scholar]
- 20.Chang MS, Uozaki H, Chong JM, Ushiku T, Sakuma K, Ishikawa S, Hino R, Barua RR, Iwasaki Y, Arai K, et al. CpG island methylation status in gastric carcinoma with and without infection of Epstein-Barr virus. Clin Cancer Res. 2006;12:2995–3002. doi: 10.1158/1078-0432.CCR-05-1601. [DOI] [PubMed] [Google Scholar]
- 21.Hino R, Uozaki H, Murakami N, Ushiku T, Shinozaki A, Ishikawa S, Morikawa T, Nakaya T, Sakatani T, Takada K, et al. Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res. 2009;69:2766–2774. doi: 10.1158/0008-5472.CAN-08-3070. [DOI] [PubMed] [Google Scholar]
- 22.Gori S, Sidoni A, Colozza M, Ferri I, Mameli MG, Fenocchio D, Stocchi L, Foglietta J, Ludovini V, Minenza E, et al. EGFR, pMAPK, pAkt and PTEN status by immunohistochemistry: correlation with clinical outcome in HER2-positive metastatic breast cancer patients treated with trastuzumab. Ann Oncol. 2009;20:648–654. doi: 10.1093/annonc/mdn681. [DOI] [PubMed] [Google Scholar]
- 23.Nosho K, Kawasaki T, Ohnishi M, Suemoto Y, Kirkner GJ, Zepf D, Yan L, Longtine JA, Fuchs CS, Ogino S. PIK3CA mutation in colorectal cancer: relationship with genetic and epigenetic alterations. Neoplasia. 2008;10:534–541. doi: 10.1593/neo.08336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baba Y, Nosho K, Shima K, Hayashi M, Meyerhardt JA, Chan AT, Giovannucci E, Fuchs CS, Ogino S. Phosphorylated AKT expression is associated with PIK3CA mutation, low stage, and favorable outcome in 717 colorectal cancers. Cancer. 2011;117:1399–1408. doi: 10.1002/cncr.25630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudd ML, Price JC, Fogoros S, Godwin AK, Sgroi DC, Merino MJ, Bell DW. A unique spectrum of somatic PIK3CA (p110alpha) mutations within primary endometrial carcinomas. Clin Cancer Res. 2011;17:1331–1340. doi: 10.1158/1078-0432.CCR-10-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyake T, Yoshino K, Enomoto T, Takata T, Ugaki H, Kim A, Fujiwara K, Miyatake T, Fujita M, Kimura T. PIK3CA gene mutations and amplifications in uterine cancers, identified by methods that avoid confounding by PIK3CA pseudogene sequences. Cancer Lett. 2008;261:120–126. doi: 10.1016/j.canlet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Oda K, Okada J, Timmerman L, Rodriguez-Viciana P, Stokoe D, Shoji K, Taketani Y, Kuramoto H, Knight ZA, Shokat KM, et al. PIK3CA cooperates with other phosphatidylinositol 3’-kinase pathway mutations to effect oncogenic transformation. Cancer Res. 2008;68:8127–8136. doi: 10.1158/0008-5472.CAN-08-0755. [DOI] [PubMed] [Google Scholar]
- 28.Gymnopoulos M, Elsliger MA, Vogt PK. Rare cancer-specific mutations in PIK3CA show gain of function. Proc Natl Acad Sci USA. 2007;104:5569–5574. doi: 10.1073/pnas.0701005104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corso G, Velho S, Paredes J, Pedrazzani C, Martins D, Milanezi F, Pascale V, Vindigni C, Pinheiro H, Leite M, et al. Oncogenic mutations in gastric cancer with microsatellite instability. Eur J Cancer. 2011;47:443–451. doi: 10.1016/j.ejca.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Zhang Q, Zhang J, Sun S, Guo H, Jia Z, Wang B, Shao Z, Wang Z, Hu X. PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer. 2011;11:248. doi: 10.1186/1471-2407-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crawford A, Nahta R. Targeting Bcl-2 in Herceptin-Resistant Breast Cancer Cell Lines. Curr Pharmacogenomics Person Med. 2011;9:184–190. doi: 10.2174/187569211796957584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakami D, Tsujitani S, Osaki T, Saito H, Katano K, Tatebe S, Ikeguchi M. Expression of phosphorylated Akt (pAkt) in gastric carcinoma predicts prognosis and efficacy of chemotherapy. Gastric Cancer. 2007;10:45–51. doi: 10.1007/s10120-006-0410-7. [DOI] [PubMed] [Google Scholar]
- 34.Oki E, Baba H, Tokunaga E, Nakamura T, Ueda N, Futatsugi M, Mashino K, Yamamoto M, Ikebe M, Kakeji Y, et al. Akt phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int J Cancer. 2005;117:376–380. doi: 10.1002/ijc.21170. [DOI] [PubMed] [Google Scholar]
- 35.Rojo F, Tabernero J, Albanell J, Van Cutsem E, Ohtsu A, Doi T, Koizumi W, Shirao K, Takiuchi H, Ramon y Cajal S, et al. Pharmacodynamic studies of gefitinib in tumor biopsy specimens from patients with advanced gastric carcinoma. J Clin Oncol. 2006;24:4309–4316. doi: 10.1200/JCO.2005.04.2424. [DOI] [PubMed] [Google Scholar]
- 36.Min Y, Adachi Y, Yamamoto H, Imsumran A, Arimura Y, Endo T, Hinoda Y, Lee CT, Nadaf S, Carbone DP, et al. Insulin-like growth factor I receptor blockade enhances chemotherapy and radiation responses and inhibits tumour growth in human gastric cancer xenografts. Gut. 2005;54:591–600. doi: 10.1136/gut.2004.048926. [DOI] [PMC free article] [PubMed] [Google Scholar]