Abstract
AIM: To investigate whether the expression of kallikrein 12 (KLK12) is related to the development of gastric cancer (GC) and to determine the role of KLK12 in gastric cancer cells growth, invasion and migration.
METHODS: Between September 2007 and March 2008, 133 patients with histologically confirmed GC were recruited for the study. Expression of KLK12 was detected in samples from GC patients by quantitative real-time reverse transcription polymerase chain reaction and immunohistochemistry. The relationship between KLK12 protein expression and clinicopathological features of GC was analyzed. The difference in 5-year survival rates between the high KLK12 protein expression group and the low KLK12 expression group was compared. Additionally, the expression of KLK12 was examined in various human GC cell lines, including MKN-28, SGC-7901 and MKN-45. Small interfering RNA (siRNA) was used to inhibit KLK12 expression in MKN-45 cells. Cell clones stably transfected with KLK12 siRNA were tested for KLK12 expression by quantitative real-time reverse transcription-polymerase chain reaction and Western blotting. Furthermore, a series of functional assays were performed in this study to assess the biological features of transfected cells. Cell proliferation was assessed using the methylthiazolyltetrazoliumassay. Finally, cell migration and invasion were assessed using transwell chamber assays.
RESULTS: Of the 133 GC patients included in the study, 126 (94.7%) showed a higher expression level of KLK12 mRNA when compared to noncancerous tissue specimens. Expression of KLK12 mRNA was significantly higher in GC tissues than in normal tissue (P < 0.001). KLK12 protein expression was detected in 96 of 133 (72.2%) GC samples with moderate or strong staining primarily in the cytoplasm. In contrast, negative immunostaining for KLK12 protein was observed in the corresponding normal gastric mucosal tissue. Overexpression of KLK12 protein was significantly associated with lymph node metastasis (P = 0.001), histological type (P < 0.001) and tumor-node-metastasis stage (P = 0.005), while no significant correlation was observed between expression of KLK12 protein and sex, age, depth of invasion, tumor size or lymphatic invasion. Furthermore, patients with high KLK12 expression had a significantly poorer 5-year survival rate than those with low KLK12 expression (P = 0.002). Expression of KLK12 mRNA was significantly higher in MKN-45 GC cells compared to normal mucosal cells or two other GC cell lines (P < 0.01). Expression of KLK12 in MKN-45 cells was downregulated after transfection with siRNA. Knockdown of KLK12 markedly decreased the proliferation of MKN-45 cells when compared with parent or mock-transfected cells (P = 0.001), especially from the 3rd to the 5th day of the assay. In migration assays, fewer KLK12 siRNA cells migrated through the chambers (22.00 ± 1.81) when compared to the parent (46.47 ± 2.42) or mock-transfected cells (45.40 ± 1.99); these differences were statistically significant (P < 0.001). However, in the invasion assay, the number of KLK12 siRNA cells that invaded the chambers was 18.40 ± 1.12, closely similar to both the parent (18.67 ± 0.98) and mock-transfected cells (18.53 ± 0.92). There was no significantly difference between the three groups in the invasion assay (P = 0.054).
CONCLUSION: The KLK12 gene is markedly overexpressed in GC tissue, and its expression status may be a powerful prognostic indicator for patients with GC. KLK12 might serve as a novel diagnosis and prognosis biomarker in GC.
Keywords: Gastric cancer, Human kallikrein 12, Immunohistochemistry, Prognosis, Small interfering RNA, Migration, Invasion
INTRODUCTION
Gastric cancer (GC) is the fourth most common malignancy, and the second most common cause of cancer mortality worldwide[1]. Although morbidity and mortality rates for GC are steadily decreasing steadily in many countries, the overall outcomes for patients with GC have not changed significantly in recent decades[2]. Additionally, none of the potential biomarkers proposed throughout the years for GC have presented the desired properties to be incorporated into routine clinical practice. The human tissue kallikrein (KLK) genes are a newly identified subgroup of putative serine proteases, consisting of 15 genes located within approximately 256 kb on chromosome 19q13.3-4[3-5]. Due to their protease activity and expression in many tissues and cell types, KLKs have been implicated in a wide range of physiological processes and the pathogenesis of human diseases[6,7]. Over the last several decades, a steadily increasing number of studies have suggested that human KLKs are involved in human carcinogenesis and that several KLKs may be promising biomarkers of prostate, ovarian, testicular, and breast cancers[8-10]. The most notable kallikrein protein biomarker is KLK3, also known as prostate specific antigen (PSA)[11]. PSA is currently the only biochemical test for prostate cancer, although the specificity of the test is not optimal[12]. Recently, a considerable amount of work has focused on identifying novel KLK-derived molecular markers of GC[13-16]. The human KLK12 gene is a member of the KLK family, encoding human kallikrein 12 protein (hK12). Similar to other kallikreins, KLK12 is an enzyme with serine protease activity that participates in several biological processes[17]. Moreover, some researchers have shown that KLK12 might also play a role in human carcinogenesis[17-19]. However, no information is available regarding KLK12 expression in human GC.
To explore the vital role of KLK12 in the tumorigenesis and progression of GC, we examined expression patterns of KLK12 in GC tissues, analyzed the relationship between hK12 expression and clinicopathological factors of GC. Furthermore, a series of function assays utilizing small interfering RNA (siRNA)-mediated downregulation of KLK12 expression were performed.
MATERIALS AND METHODS
Patients and samples
Prior to operation, no patient had received any type of treatment. All research examinations were approved by the Ethics Committee Board of Renji Hospital. Moreover, participants in this study signed an informed consent form so that their samples could be used for research purposes from September 2007 to March 2008. A computerized database with the medical history of each patient was created for an extensive statistical analysis. Selection criteria included confirmation of GC diagnosis by histopathology and the availability of sufficient tumor tissue for RNA extraction. Tumor stage was defined according to the 7th edition of International Union Against Cancer tumor-node-metastasis classification. All specimens were snap-frozen in liquid nitrogen immediately after surgery, and then stored at -80 °C until analysis.
Cell lines and cell culture
The human GC cell lines MKN-28, SGC-7901 and MKN-45 and the normal gastric mucosal cell line GES-1 were obtained from Shanghai Institute of Digestive Disease (Shanghai, China). Cell lines were cultured in Dulbecco’s modified Eagle’s medium (Gibco, United States) with 10% fetal bovine serum (FBS, Gibco, United States).
Total RNA extraction and cDNA synthesis
Total RNA was extracted from the human tissues and GC cells using TRIzol Reagent (Invitrogen, United States) according to the manufacturer’s instructions. The RNA concentration and purity were determined using the absorbance ratio at 260/280 acquired by a spectrophotometer. cDNA synthesis from 4 μg of total RNA was performed with a reverse transcription system kit (Promega, United States) according to the manufacturer’s protocol. Briefly, samples were preincubated at 70 °C for 10 min, cooled on ice, then added to a reaction mixture consisting of 3 μL dNTP mixture, 2 μL M-MLV reverse transcriptase, 10 μL reverse transcription 5 × buffer, 1 μL Rnasin and 2 μL oligo-(dT)15 primer in a final volume of 20 μL. The reaction mixture was sequentially incubated at 95 °C for 15 min, 99 °C for 15 s and 62 °C for 40 s. The cDNA was stored at -20 °C before use.
Quantitative real-time reverse transcription polymerase chain reaction assay
Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) was carried out in 96-well polypropylene microplates on an ABI Prism 7500 sequence detection PCR system (Applied Biosystems, United States) with the following primers: KLK12: 5’-GCCTCAACCTCTCCATCGTC-3’ (forward) and 5’-CTTGAAGGACTCCCCCACAC-3’ (reverse); β-actin: 5’-CATGTACGTTGCTATCCAGGC-3’(forward) and 5’-CTCCTTAATGTCACGCACGAT-3’ (reverse). The 20 μL PCR reaction consisted of (2×) SYBR® Premix Ex TagTM (Takara, Japan), 2 μL cDNA and 0.4 μL of each gene-specific primer. The thermal cycling protocol included an initial denaturation step at 95 °C for 30 s followed by 45 cycles at 95 °C for 5 s for denaturation and 62 °C for 40 s for primer annealing and extension. After the last cycle, a melting curve analysis was performed. All PCR reactions were run in triplicate. KLK12 mRNA expression was calculated from the standard curve, and quantitative normalization in each sample was performed using β-actin gene expression as an internal control. Relative quantification was performed using the 2-∆∆Ct method.
Immunohistochemistry analysis
Immunohistochemistry (IHC) studies of hK12 were performed on surgical specimens from patients with GC using the avidin-biotin-peroxidase method (KIT-9702, Maixin Bio, China) on formalin-fixed, paraffin-embedded tissue specimens. All sections were incubated with polyclonal sheep anti-human KLK12 antibody (AF3095, R and D Systems, United States) at a dilution of 1:50. Slides were counterstained with hematoxylin. All sections were evaluated independently and blinded to outcome data by a pathologist three times. A cutoff of more than 30% of the tumor cells with moderate or strong KLK12 cytoplasmic staining in a gastric tumor section was considered to be positive.
siRNA transfection
The expression vector pBSKH1 (SIBS, China) was used for expression of siRNA. The siRNA designed to target the KLK12 gene (sense strand: 5’-AAACAGUGACAGCCACGUATT-3’, anti-sense strand: 5’-UACGUGGCUG UCACUGUUUGG-3’) was inserted into the pBSKH1 vector according to the manufacturer’s instructions, and then, the vector was transfected into the GC cells by the LipofectamineTM 2000 (Invitrogen, United States). A mock vector-transfected clone of the cell line was used as a control. Stably transfected cell clones were tested for KLK12 expression by quantitative real-time RT-PCR and Western blotting.
Western blotting analysis
Cells were harvested 72 h after transfection, and whole-cell lysates were prepared for protein extraction. The protein concentrations of the samples were determined by the bicinchoninic acid protein assay. Proteins were separated in 10% sodium dodecyl sulphate polyacrylamide gels, electrophoretically transferred to polyvinylidenedifluoride membranes and incubated in a blocking solution of 5% skim milk powder for 1 h at room temperature. Membranes were then incubated with polyclonal sheep anti-human KLK12 antibody (1:500, AF3095, R and D Systems, United States) and anti-β-actin antibody (1:1000, sc-47778, Santa Cruz, United States) overnight at 4 °C. The membranes were washed 3 times in tris-buffered saline tween-20 (TBST) and incubated for 1 h at room temperature with a 1:1000 dilution of secondary antibody conjugated to horseradish peroxidase (Invitrogen, United States). After incubation with a secondary antibody, the membranes were washed in TBST and developed using electrochemiluminescence according to the manufacturer’s instructions.
In vitro cell proliferation assay
Cell proliferation was evaluated using the methylthiazolyltetrazolium (MTT) assay. Cells were grown in a monolayer culture and plated at a density of 4 × 103cells/well into separate wells of a 96-well culture plate. The cells were incubated with MTT after 1, 2, 3, 4 or 5 d. Absorbance was measured at 570 nm using a microplate reader (SpectraMax 250, Molecular devices, United States). The experiments were performed in triplicate.
In vitro migration and invasion assays
The motility and invasiveness of plasmid-transfected cells were evaluated in 24-well transwell chambers with upper and lower culture compartments separated by polycarbonate membranes with 8-μm pores (CytoSelectTM 24 Well Cell Migration and Invasion Assay Combo Kit, 8-μm, CBA100-C, Cell Biolab, United States). Prior to plating cells into the transwells, the 24-well migration plate was allowed to warm up to room temperature for 10 min. A cell suspension containing 1.0 × 106 cells/mL in serum-free media was plated into the top chamber. Five hundred microliters of media containing 10% FBS was placed into the bottom chamber to act as a chemoattractant. Then, 300 μL of the cell suspension solution was added to the inside of each insert and incubated 24 h in a cell culture incubator. The cells that migrated through the 8-μm pores and adhered to the lower surface of the membrane were transferred to a clean well containing 400 μL of 0.09% crystal violet as a cell staining solution and washed several times in a beaker with water. The migratory cells were counted using light microscopy under high magnification, with 5 individual fields per insert. In a similar fashion, the invasiveness of plasmid-transfected cells was also evaluated using this kit (Invasion Chamber Plate, Cell Biolab, United States). Cells, media, experimental conditions, and the analysis performed were similar to those for the migration assays. Triplicate assays were performed for each group of cells in both the migration and invasion assays.
Statistical analysis
Statistical analysis were performed using SPSS 11.0 software (Shanghai Jiaotong University, China). The data are expressed as the mean ± SD. The Student’s t test and one-way analysis of variance test were used to compare data between the different groups. The χ2 test was used to assess the relationship between hK12 expression levels and clinicopathological characteristics of GC. Survival curves were drawn according to the Kaplan-Meier method, and the log-rank test was applied to compare the survival curves. Differences were considered significant at the P < 0.05 level.
RESULTS
KLK12 mRNA level is upregulated in GC patients
There were 133 GC patients included in the study. The age of the patients ranged from 18 to 87 years (median 61 years). Overall, 126 of the 133 patients (94.7%) showed a higher expression level of KLK12 mRNA in GC tissue specimens compared to noncancerous tissue specimens. The mean expression value of KLK12 mRNA in cancer tissues was significantly higher than the value in relevant normal tissues (2.75 ± 0.78 vs 1.10 ± 0.56, P < 0.001, Figure 1).
Figure 1.
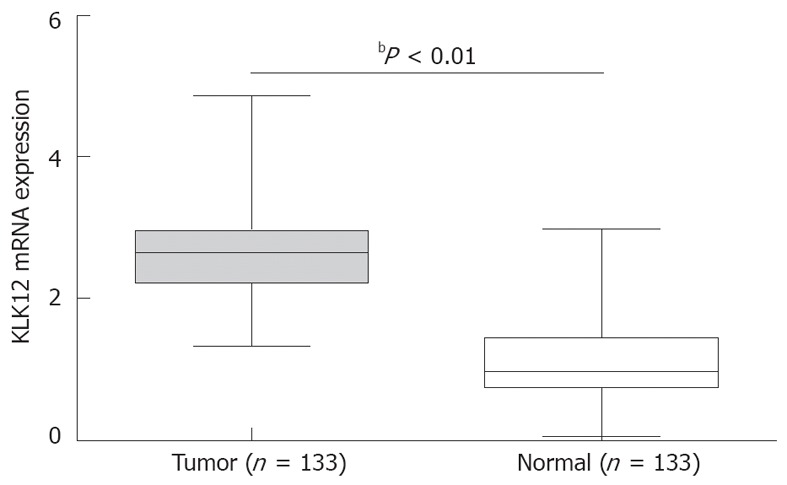
Upregulation of kallikrein 12 mRNA expression in gastric cancer. Quantitative real-time reverse transcription polymerase chain reaction showed that the mean expression value of kallikrein 12 (KLK12) mRNA in cancer tissues was significantly higher than the value in relevant normal tissues. Data are shown as mean ± SD, using the Student’s t test (bP < 0.01 between tumor and normal group, horizontal bars represent medians).
Immunostaining demonstrates hK12 overexpression in GC tissues
To further validate the expression and localization of hK12 in surgical specimens, IHC was performed on paraffin-fixed GC tissues and matched noncancerous mucosa of 133 patients. Dark-brown immunostaining was most prevalent in cancer cells, whereas the staining levels were lower in stromal cells or fibroblasts of GC tissues. hK12 expression was detected in 72.2% (96/133) GC samples with moderate or strong staining (Figure 2A), primarily located in the cytoplasm (Figure 2A and B). In contrast, negative immunostaining for hK12 was observed in normal gastric mucosal sections (Figure 2C and D).
Figure 2.
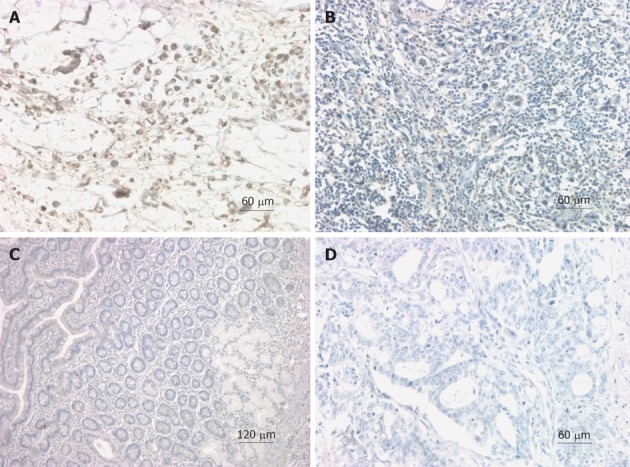
Expression of human kallikrein 12 protein in gastric cancer and noncancerous mucosal tissues detected by immunohistochemistry. A: Strong positive human kallikrein 12 protein (hK12) immunostaining in gastric cancer (GC) tissues, hK12 staining was observed in the cytoplasm of cancer cells; B: Weak positive hK12 immunostaining in GC tissues; C: Negative hK12 immunostaining in relevant normal tissues; D: Negative hK12 immunostaining in GC tissues (original magnification A, B, D ×200, C ×100).
Clinicopathological significance of hK12 expression in GC
The clinicopathological factors analyzed in relation to hK12 expression in tumor tissues are shown in Table 1. The incidence of lymph node metastasis was significantly higher (P = 0.001) in the high-expression group (76 of 95, 80.0%) than in the low-expression group (20 of 38, 52.6%). The histological type and pathological stage also correlated with the groups. However, no significant difference was observed with regard to sex, age, depth of invasion, tumor size or lymphatic invasion. The 5-year actuarial overall survival rates in patients with high hK12 levels and patients with low hK12 levels were 62.2% and 31.3%, respectively (Figure 3). The difference in survival rates between these two groups was statistically significant (P = 0.002).
Table 1.
Relationship between human kallikrein 12 protein expression and clinicopathological features in 133 patients with gastric cancer
| Feature | n | hK12 expression | P value1 | |
| High (n = 96) | Low (n = 37) | |||
| Sex | ||||
| Male | 83 | 59 | 24 | 0.716 |
| Female | 50 | 37 | 13 | |
| Age (yr) | ||||
| ≥ 61 | 69 | 47 | 22 | 0.277 |
| < 61 | 64 | 49 | 15 | |
| Depth of invasion (T) | ||||
| T1 + T2 | 21 | 14 | 7 | 0.539 |
| T3 + T4 | 112 | 82 | 30 | |
| Tumor size (cm) | ||||
| ≥ 5 | 77 | 59 | 18 | 0.180 |
| < 5 | 56 | 37 | 19 | |
| Lymph node metastasis | ||||
| Positive | 95 | 76 | 19 | 0.001 |
| Negative | 38 | 20 | 18 | |
| Lymphatic invasion | ||||
| Absent | 47 | 33 | 14 | 0.708 |
| Present | 86 | 63 | 23 | |
| Histological type | ||||
| Undifferentiated | 94 | 77 | 17 | < 0.001 |
| Differentiated | 39 | 19 | 20 | |
| Stage | ||||
| I + II | 44 | 25 | 19 | 0.005 |
| III + IV | 89 | 71 | 18 | |
P value was determined by the χ2 test. hK12: Human kallikrein 12 protein.
Figure 3.
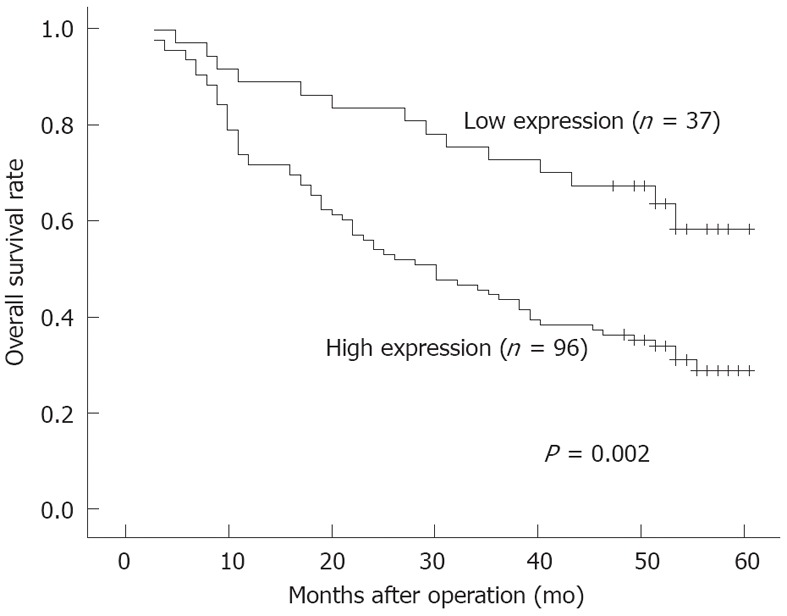
Overall survival of patients with gastric cancer according to human kallikrein 12 protein expression in the cancer tissues. Patients in the high human kallikrein 12 protein (hK12) expression group had a significantly poorer prognosis than those in the low hK12 expression group. Survival curves are drawn according to the Kaplan-Meier method, using the log-rank test to compare the survival rates (P = 0.002).
Expression of KLK12 mRNA in GC cell lines
Furthermore, three well-characterized GC cell lines, MKN-28, SGC-7901 and MKN-45, and the normal gastric mucosal cell line GES-1 were chosen to examine KLK12 mRNA expression due to their diverse differentiation features. MKN-45 cells expressed the highest level of KLK12 across the four cell lines (Figure 4). Therefore, MKN-45 cells were chosen to do a series of functional experiments involving knockdown of KLK12 expression.
Figure 4.
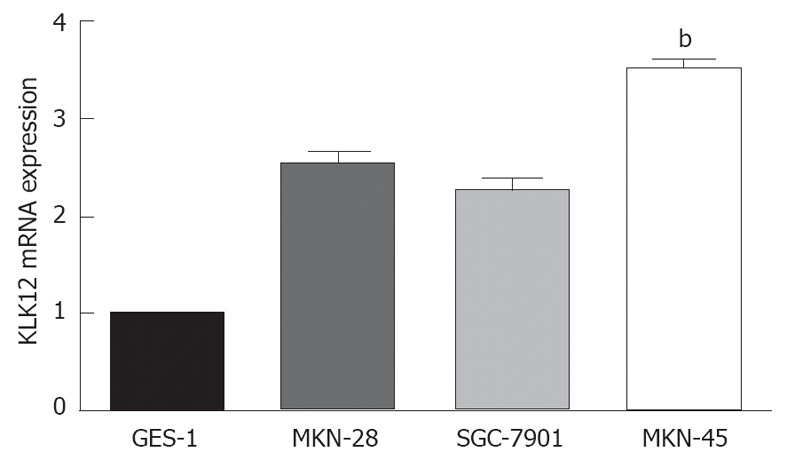
Expression of kallikrein 12 mRNA in gastric cancer cell lines and normal gastric mucosal cell line. Gastric cancer cell lines show higher levels of kallikrein 12 (KLK12) mRNA expression than normal gastric mucosal cell line, while MKN-45 cells expressed the highest level of KLK12 across the four cell lines. Data are shown as mean ± SD, using the one-way analysis of variance test (bP < 0.01 vs other cell lines).
KLK12 siRNA-transfected GC cell line stably suppresses both KLK12 mRNA and hK12
As shown in Figure 5A, MKN-45 cells transfected with siRNA targeting KLK12 showed a remarkable decrease in the level of KLK12 mRNA compared to mock-transfected or parent MKN-45 cells, as determined by quantitative real-time RT-PCR. Furthermore, we performed Western blotting analysis to verify the efficiency of the KLK12 siRNA. The stable KLK12-suppressed clone was confirmed to express markedly lower levels (about one fourth) of hK12 than the MKN-45 parental cells (Figure 5B), confirming that KLK12 siRNA decreased KLK12 expression, consistent with the quantitative real-time RT-PCR results.
Figure 5.
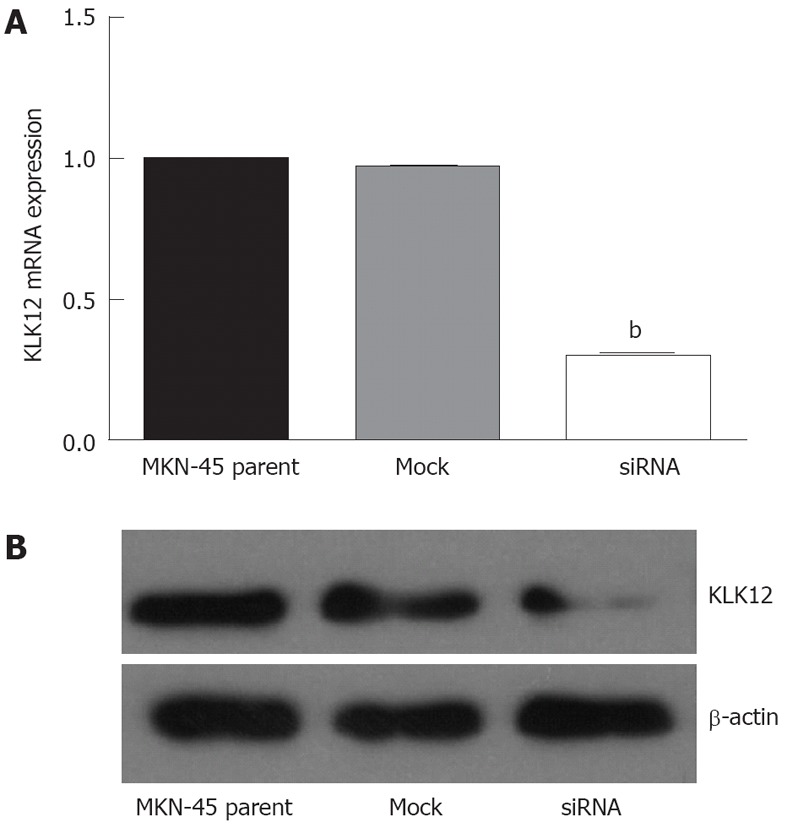
Efficiency of small interfering RNA in silencing the kallikrein 12 mRNA, and protein expression in MKN-45 cells. A: MKN-45 cells transfected with small interfering RNA targeting human kallikrein 12 (KLK12) gene showed a remarkable decrease in the level of KLK12 mRNA compared to mock-transfected or parent MKN-45 cells. Data are shown as mean ± SD, using the one-way analysis of variance test (bP < 0.01 vs other cell lines); B: Western blotting analysis showed a reduced protein expression in KLK12 small interfering RNA (siRNA) transfected cells. The protein levels are measured by Image J software (National Institutes of Health, United States) with β-actin protein normalization.
Knockdown of KLK12 affects the proliferative ability of MKN-45 GC cells
We analyzed whether suppressing the KLK12 expression would alter the growth rate of MKN-45 GC cells. As shown in Figure 6, transfection with KLK12 siRNA remarkably decreased the proliferative ability of MKN-45 cells when compared with the parent and mock-transfected cells (P = 0.001), especially from the 3rd to 5th days of the assay.
Figure 6.
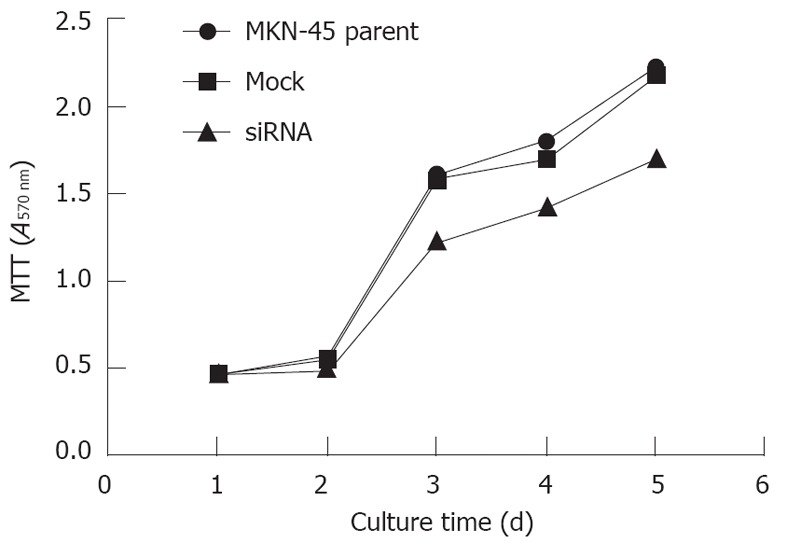
Reduction of cell proliferation by methylthiazolyltetrazolium assay after silencing the kallikrein 12. The proliferative ability significantly decreased in MKN-45 cells after transfection with kallikrein 12 small interfering RNA (siRNA), especially from the 3rd to 5th days of the assay. Data are shown as mean ± SD, using the one-way analysis of variance test (P = 0.001). MTT: Methylthiazolyltetrazolium.
Knockdown of KLK12 affects the migratory ability of MKN-45 GC cells
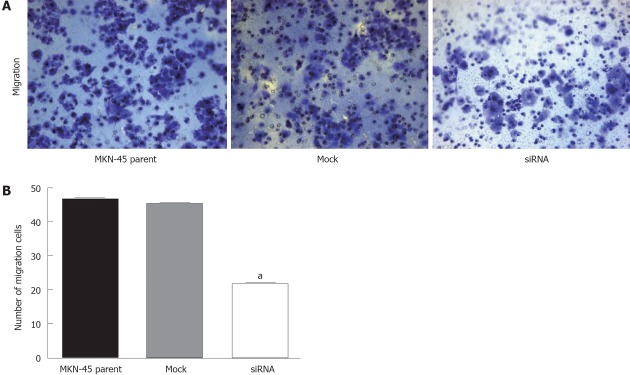
We evaluated whether suppression of KLK12 expression could alter the ability of in vitro migration and invasion of MKN-45 cells. As shown in Figure 7, fewer KLK12 siRNA-transfected cells migrated through the chambers (22.00 ± 1.81) when compared to the parent (46.47 ± 2.42) or mock-transfected (45.40 ± 1.99); these differences were statistically significant (P < 0.001, Figure 7A and B). However, in the invasion test, the number of KLK12 siRNA-transfected cells that invaded the chambers in 18.40 ± 1.12, closely similar to both the parent (18.67 ± 0.98) and mock-transfected cells (18.53 ± 0.92). There was no significant difference between the three groups in the invasion assay (P = 0.054).
Figure 7.
Effect of cell migration by kallikrein 12 knockdown. A: 24-well transwell chambers with upper and lower culture compartments separated by polycarbonate membranes with 8-μm pores were used for migration or invasion assay. The chambers were stained by 0.09% crystal violet and cells were counted using light microscopy under high magnification (magnification ×10); B: The migration or invasion cells were counted in 5 individual fields per insert. Values were the number of cells. Data are shown as mean ± SD, using the one-way analysis of variance test (aP < 0.05 vs other cell lines).
DISCUSSION
The KLK12 gene is a new member of the KLK gene family, some members of which are implicated in the initiation and progression of cancer[20-22]. KLK12 is encoded by 5 coding exons, and is structurally similar to serine proteases and other known KLKs. KLK12 is expressed in a variety of tissues including the salivary gland, stomach, uterus, lung, thymus, prostate, colon, brain, breast, thyroid, and trachea. Initially, it was reported that expression of KLK12 is downregulated at the mRNA level in breast cancer tissues and is upregulated by steroid hormones in breast and prostate cancer cell lines[17]. Memari et al[19] demonstrated that more than 95% of prostate cancers were KLK12 positive in a tissue microarray study. To the best of our knowledge, this study is the first investigation to analyze KLK12 expression in GC. We performed quantitative real time RT-PCR, IHC and a series of functional assays utilizing siRNA-mediated downregulation of KLK12 expression to determine the role and explore the mechanism of KLK12 in GC. Our results showed a drastic difference in KLK12 expression between GC and normal mucosal tissues. Substantially elevated expression of the KLK6 gene has been observed in clinical tissue samples and several GC cell lines[13], while other authors have reported that cancerous stomach tissues were found to present significantly decreased levels of KLK11 and KLK13 mRNA transcripts in comparison with their normal counterparts[15,16]. Moreover, our study showed that high hK12 expression was significantly correlated with the lymph node metastasis (P = 0.001), histological type (P < 0.001) and pathological stage (P = 0.005) of GC. High expression of hK12 was also associated with a poor prognosis for patients with GC. These findings suggest that enhanced expression of KLK12 might play an important role in various pathological processes of GC. MKN-45, a poorly differentiated GC cell line, was chosen for functional experiments because KLK12 mRNA expression was higher in MKN-45 cells compared to other cell lines. Additionally, hK12 expression was significantly higher in undifferentiated GC. In our experiments, we established clones with suppressed KLK12 expression by gene silencing using RNA interference (RNAi) techniques. RNAi is mediated by siRNAs that are produced from long double stranded RNAs of exogenous or endogenous origin by an endonuclease of the RNase-III type called Dicer[23]; this technique has emerged as a powerful tool for understanding gene functioning. As quantitative real-time RT-PCR and Western blotting revealed, KLK12 siRNA remarkably reduced KLK12 expression of MKN-45 cells. MTT proliferation assays showed with KLK12 siRNA dramatically decreased the proliferation of MKN-45 cells when compared with MKN-45 parent and mock-transfected cells. The differences were significant, especially from the 3rd day to the 5th day after transfection, which is in accordance with the Western blotting data showing knockdown of KLK12 expression was greatest 72 h after transfection. Metastasis and local recurrence are known to be the primary causes of death after radical surgery of GC patients. Tumor metastasis is a complicated process and involves many steps including migration of cancer cells to, and invasion through, the basement membrane[24]. In our experiments, significantly fewer KLK12-suppressed cells migrated across polycarbonate membranes when compared to MKN-45 parent and mock-transfected cells (P < 0.001). The invasiveness of all three cell lines was comparable, and the KLK12-suppressed cells displayed no difference in the invasion assay compared to parent or mock-transfected cells. Other KLKs have been shown to degrade components of the extracellular matrix. Magklara et al[25] reported that hK6 can degrade fibrinogen and collagen type I, basic constituents of the extracellular matrix, as well as collagen type IV, a major component of the basement membrane in vitro. The lysis of certain components of the extracellular matrix is linked with an altered regulation of tumor metastasis. However, Memari et al[26] reported that KLK12 is secreted as an inactive pro-enzyme, which is able to autoactivate to gain enzymatic activity. However, active KLK12 quickly loses its activity due to autodegradation, and its activity can also be rapidly inhibited by zinc ions and by alpha2-antiplasmin through covalent complex formation. According to these results, it is reasonable to conclude that the increased KLK12 expression may not play an important role in metastasis. In conclusion, we present an early report that KLK12 was remarkably overexpressed in GC tissues and that high KLK12 expression levels were associated with the lymph node metastasis, histological type, pathological stage and poor patient prognosis. Our findings also demonstrate that knockdown of KLK12 expression leads to reduced proliferation and migratory ability with little effect on invasiveness in MKN-45 GC cells. Consequently, KLK12 might serve as a novel diagnostic and prognostic biomarker, as well as a potential therapeutic target, in GC.
COMMENTS
Background
Gastric cancer (GC) is the fourth most common malignancy and the second most common cause of cancer mortality worldwide. Although morbidity and mortality rates for GC are deceasing steadily in many countries, the overall outcome for patients with GC has not changed significantly in recent decades. It is necessary to find a reliable biomarker for GC to be incorporated into routine clinical practice.
Research frontiers
The human kallikrein 12 (KLK12) gene is a new member of the KLK gene family. Similar to other kallikreins, KLK12 is a proteolytic enzyme with serine protease activity, and participates in several biological processes. Moreover, KLK12 may also play a role in human carcinogenesis of cancers such as breast and prostate cancer. However, little is known about KLK12 in human GC.
Innovations and breakthroughs
The results of this study provide strong evidence suggesting that KLK12 expression is upregulated in GC tissue and correlated with lymph node metastasis, poor histological type, advanced clinical stage and decreased overall survival rate. Furthermore, the authors found that knockdown of KLK12 markedly decreased the proliferative and migratory abilities of MKN-45 GC cells.
Applications
These results demonstrate that KLK12 might serve as a novel diagnostic and prognostic biomarker in GC. This study may represent a future strategy for therapeutic intervention in the treatment of patients with GC.
Terminology
The KLK genes are a newly identified subgroup of putative serine proteases, consisting of 15 genes located within approximately 256 kb on chromosome 19q13.3-4.
Peer review
This is a good descriptive study in which the authors investigate expression of KLK12 in GC tissues. The biological effects of KLK12 knockdown in the GC cell line MKN-45 were also studied. The results are interesting and suggest knockdown of KLK12 overexpression in GC may be a potential therapeutic target. The study was well designed and the data are convincing.
Footnotes
Supported by Scientific Research Fund from Shanghai Science and Technology Committee, No. 08411964200
Peer reviewer: Takashi Yao, Professor, Department of Human Pathology, Juntendo University School of Medicine, 2-1-1, Hongo, Bunkyo-ku, Tokyo 113-8421, Japan
S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jackson C, Cunningham D, Oliveira J. Gastric cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:34–36. doi: 10.1093/annonc/mdp122. [DOI] [PubMed] [Google Scholar]
- 3.Qin H, Kemp J, Yip MY, Lam-Po-Tang PR, Morris BJ. Localization of human glandular kallikrein-1 gene to chromosome 19q13.3-13.4 by in situ hybridization. Hum Hered. 1991;41:222–226. doi: 10.1159/000154005. [DOI] [PubMed] [Google Scholar]
- 4.Harvey TJ, Hooper JD, Myers SA, Stephenson SA, Ashworth LK, Clements JA. Tissue-specific expression patterns and fine mapping of the human kallikrein (KLK) locus on proximal 19q13.4. J Biol Chem. 2000;275:37397–37406. doi: 10.1074/jbc.M004525200. [DOI] [PubMed] [Google Scholar]
- 5.Yousef GM, Diamandis EP. Tissue kallikreins: new players in normal and abnormal cell growth? Thromb Haemost. 2003;90:7–16. [PubMed] [Google Scholar]
- 6.Chao J, Shen B, Gao L, Xia CF, Bledsoe G, Chao L. Tissue kallikrein in cardiovascular, cerebrovascular and renal diseases and skin wound healing. Biol Chem. 2010;391:345–355. doi: 10.1515/BC.2010.042. [DOI] [PubMed] [Google Scholar]
- 7.Simmer JP, Richardson AS, Smith CE, Hu Y, Hu JC. Expression of kallikrein-related peptidase 4 in dental and non-dental tissues. Eur J Oral Sci. 2011;119 Suppl 1:226–233. doi: 10.1111/j.1600-0722.2011.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H, Scorilas A, Katsaros D, Yousef GM, Massobrio M, Fracchioli S, Piccinno R, Gordini G, Diamandis EP. Human kallikrein gene 5 (KLK5) expression is an indicator of poor prognosis in ovarian cancer. Br J Cancer. 2001;84:643–650. doi: 10.1054/bjoc.2000.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamandis EP, Yousef GM. Human tissue kallikreins: a family of new cancer biomarkers. Clin Chem. 2002;48:1198–1205. [PubMed] [Google Scholar]
- 10.Borgoño CA, Diamandis EP. The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer. 2004;4:876–890. doi: 10.1038/nrc1474. [DOI] [PubMed] [Google Scholar]
- 11.Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, Petros JA, Andriole GL. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 12.Lin DW. Beyond PSA: utility of novel tumor markers in the setting of elevated PSA. Urol Oncol. 2009;27:315–321. doi: 10.1016/j.urolonc.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Nagahara H, Mimori K, Utsunomiya T, Barnard GF, Ohira M, Hirakawa K, Mori M. Clinicopathologic and biological significance of kallikrein 6 overexpression in human gastric cancer. Clin Cancer Res. 2005;11:6800–6806. doi: 10.1158/1078-0432.CCR-05-0943. [DOI] [PubMed] [Google Scholar]
- 14.Feng B, Xu WB, Zheng MH, Ma JJ, Cai Q, Zhang Y, Ji J, Lu AG, Qu Y, Li JW, et al. Clinical significance of human kallikrein 10 gene expression in colorectal cancer and gastric cancer. J Gastroenterol Hepatol. 2006;21:1596–1603. doi: 10.1111/j.1440-1746.2006.04228.x. [DOI] [PubMed] [Google Scholar]
- 15.Konstantoudakis G, Florou D, Mavridis K, Papadopoulos IN, Scorilas A. Kallikrein-related peptidase 13 (KLK13) gene expressional status contributes significantly in the prognosis of primary gastric carcinomas. Clin Biochem. 2010;43:1205–1211. doi: 10.1016/j.clinbiochem.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Wen YG, Wang Q, Zhou CZ, Yan DW, Qiu GQ, Yang C, Tang HM, Peng ZH. Identification and validation of Kallikrein-ralated peptidase 11 as a novel prognostic marker of gastric cancer based on immunohistochemistry. J Surg Oncol. 2011;104:516–524. doi: 10.1002/jso.21981. [DOI] [PubMed] [Google Scholar]
- 17.Yousef GM, Magklara A, Diamandis EP. KLK12 is a novel serine protease and a new member of the human kallikrein gene family-differential expression in breast cancer. Genomics. 2000;69:331–341. doi: 10.1006/geno.2000.6346. [DOI] [PubMed] [Google Scholar]
- 18.Planque C, Li L, Zheng Y, Soosaipillai A, Reckamp K, Chia D, Diamandis EP, Goodglick L. A multiparametric serum kallikrein panel for diagnosis of non-small cell lung carcinoma. Clin Cancer Res. 2008;14:1355–1362. doi: 10.1158/1078-0432.CCR-07-4117. [DOI] [PubMed] [Google Scholar]
- 19.Memari N, Diamandis EP, Earle T, Campbell A, Van Dekken H, Van der Kwast TH. Human kallikrein-related peptidase 12: antibody generation and immunohistochemical localization in prostatic tissues. Prostate. 2007;67:1465–1474. doi: 10.1002/pros.20596. [DOI] [PubMed] [Google Scholar]
- 20.Avgeris M, Mavridis K, Scorilas A. Kallikrein-related peptidases in prostate, breast, and ovarian cancers: from pathobiology to clinical relevance. Biol Chem. 2012;393:301–317. doi: 10.1515/hsz-2011-0260. [DOI] [PubMed] [Google Scholar]
- 21.Avgeris M, Mavridis K, Scorilas A. Kallikrein-related peptidase genes as promising biomarkers for prognosis and monitoring of human malignancies. Biol Chem. 2010;391:505–511. doi: 10.1515/BC.2010.056. [DOI] [PubMed] [Google Scholar]
- 22.Mavridis K, Scorilas A. Prognostic value and biological role of the kallikrein-related peptidases in human malignancies. Future Oncol. 2010;6:269–285. doi: 10.2217/fon.09.149. [DOI] [PubMed] [Google Scholar]
- 23.Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 24.Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272:177–185. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Magklara A, Mellati AA, Wasney GA, Little SP, Sotiropoulou G, Becker GW, Diamandis EP. Characterization of the enzymatic activity of human kallikrein 6: Autoactivation, substrate specificity, and regulation by inhibitors. Biochem Biophys Res Commun. 2003;307:948–955. doi: 10.1016/s0006-291x(03)01271-3. [DOI] [PubMed] [Google Scholar]
- 26.Memari N, Jiang W, Diamandis EP, Luo LY. Enzymatic properties of human kallikrein-related peptidase 12 (KLK12) Biol Chem. 2007;388:427–435. doi: 10.1515/BC.2007.049. [DOI] [PubMed] [Google Scholar]