Abstract
Esophageal cancer is mainly found in Asia and east Africa and is one of the deadliest cancers in the world. However, it has not garnered much attention in the Western world due to its low incidence rate. An increasing amount of data indicate that esophageal cancer, particularly esophageal adenocarcinoma, has been rising by 6-fold annually and is now becoming the fastest growing cancer in the United States. This rise has been associated with the increase of the obese population, as abdominal fat puts extra pressure on the stomach and causes gastroesophageal reflux disease (GERD). Long standing GERD can induce esophagitis and metaplasia and, ultimately, leads to adenocarcinoma. Acid suppression has been the main strategy to treat GERD; however, it has not been proven to control esophageal malignancy effectively. In fact, its side effects have triggered multiple warnings from regulatory agencies. The high mortality and fast growth of esophageal cancer demand more vigorous efforts to look into its deeper mechanisms and come up with better therapeutic options.
Keywords: Esophageal cancer, Gastroesophageal reflux disease, Obesity
INTRODUCTION
While the incidence of most cancers is declining, esophageal cancer has been continuing its march as the fastest growing malignancy in the Western world[1,2]. This rise is largely derived from gastroesophageal reflux disease (GERD), which is associated with the proliferation of obesity. The continuous growth of the obese population foreshadows a future increase of GERD and its associated esophageal cancer. This demands immediate and more rigorous research on the molecular mechanisms of this common disease and its pathways which lead to esophageal malignancy. Current GERD treatment mainly relies on acid suppression drugs which have not been proved to change the risk of cancer development. Although the debate is still going on whether gastric acid or bile acid is ultimately responsible for GERD malignancy, based on the data from human studies, animal modeling, and in vitro simulation, perhaps it is time to explore other options.
STATISTICS OF ESOPHAGEAL CANCER: RISING NUMBERS
Cancer is the second leading cause of death in the world[3] after heart disease (21.09%), contributing 18.04% to the total number of deaths worldwide (Figure 1). Among all types of cancers, skin cancer is the most common one, which includes 2-3 million non-melanoma and 132 000 melanoma cases diagnosed each year, making up one third of the total cancer cases. According to Skin Cancer Foundation Statistics, one in every five Americans will develop skin cancer in their lifetime. This prevalence is largely due to depletion of ozone in the atmosphere, which weakens our planet’s protective shield from the brunt of the sun’s harmful rays. It is estimated that every 10% decrease in ozone levels will generate an additional 300 000 non-melanoma and 4500 melanoma cancer cases. However, the majority of skin cancer can be easily treated, while digestive cancers, such as esophageal cancer, are highly life-threatening.
Figure 1.
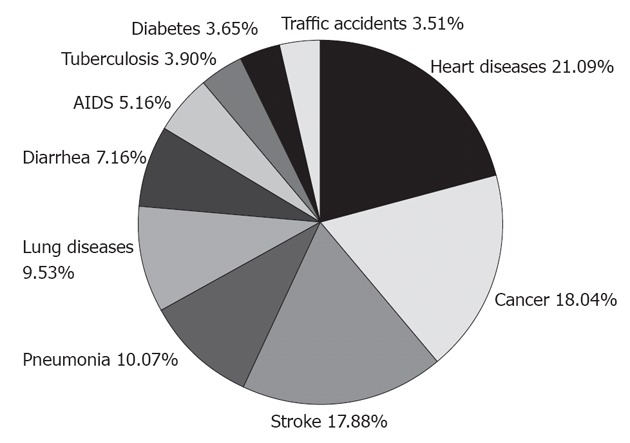
Top 10 leading causes of death worldwide. Cancer is the second highest one. Data extracted from World Health Organization documents. AIDS: Acquired immune deficiency syndrome.
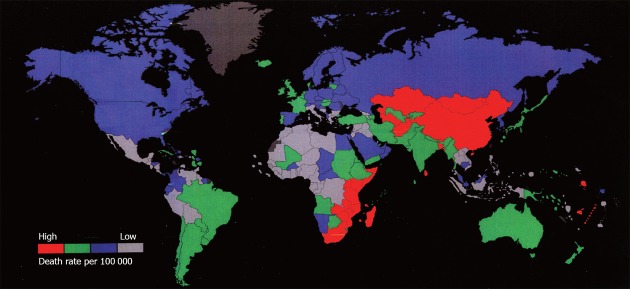
Esophageal cancer is found more commonly in males than in females, with a ratio of approximately 7:1. Currently, it is the 7th leading cancer in men globally, contributing 6.51% to the total number of male cancer cases (Figure 2). There are two main subtypes of esophageal cancer: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). ESCC occurs most often in the middle portion of the esophagus and accounts for 90%-95% of all cases of esophageal cancer worldwide, while EAC is primarily found in the lower esophagus. The type and incidence of esophageal cancer varies dramatically depending on the geographical location (Figure 3). The top 10 countries with the highest age-standardized death rate due to esophageal cancer are Nauru (30.3), Sao Tome (26.4), Mongolia (18.6), South Africa (18.2), Malawi (18.2), China (15.5), Lesotho (15.5), Kenya (13.9), Mozambique (13.5) and Uganda (13.4) (the death rate being deaths per 100 000 people). The highest rates are found in Asia, stretching from northern Iran through the central Asian republics to north-central China, often referred to as the “esophageal cancer belt”. For instance, in China, the majority of esophageal cancer diagnoses are ESCC and it is ranked as the 8th leading cause of death nationwide (Table 1), mostly in northern China where the incidence rate can be as high as 800 cases per 100 000 people. On the other hand, in the United States, more than 50% of esophageal cancer cases are EAC, and the rate is less than 5 in 100 000, making it the 29th leading cause of death. For this reason, esophageal cancer is not even on the current list of common cancers in the United States, according to the National Cancer Institute. In order to be on the list, esophageal cancer has to have at least 40 000 cases a year, while the current estimate for 2012 is only 17 460. Although the reason for this geographic variation still needs investigation, several factors have been suggested that might all contribute to the issue to a certain degree, such as Helicobacter infection, dietary pattern, and life habits[4]. As Eastern and Western countries become increasingly open to each other and people adapt more to each other, we expect this discrepancy will become less and less. As evident in China, EAC incidence was doubled from the 1970s to the 1980s, according to an examination of the medical records of esophageal cancer patients diagnosed from 1970 to 2001 in a local hospital[5].
Figure 2.
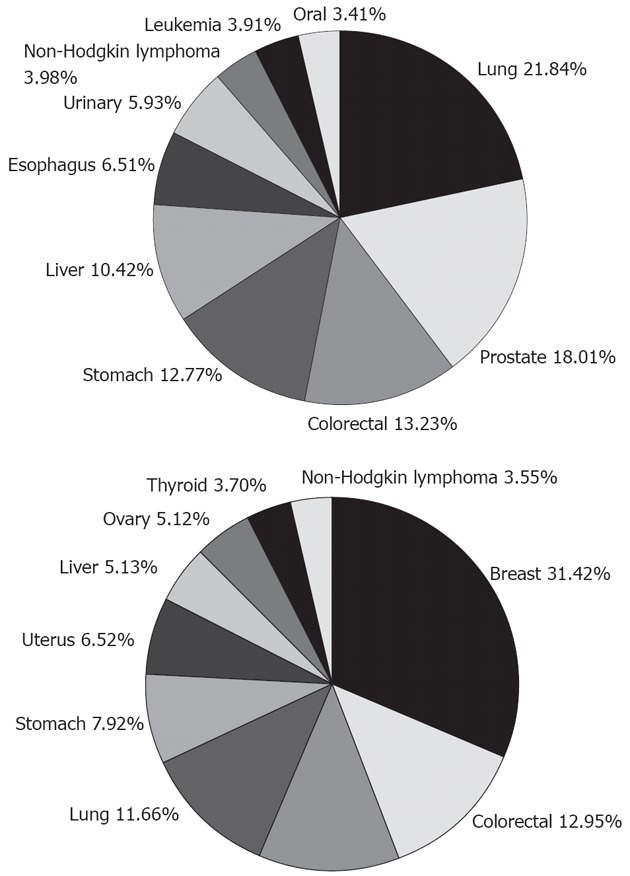
Top 10 of the most common cancers in men (up) and women (below) worldwide. Esophageal cancer is No. 7 in men. Data extracted from World Health Organization documents.
Figure 3.
Geographic distribution of esophageal cancer. China is a hot spot. Data from World Health Organization documents.
Table 1.
Top 10 leading causes of death in China vs the United States
| Rank | China | United States | ||||
| Diseases | Deaths | Death rate (%) | Diseases | Deaths | Death rate (%) | |
| 1 | Stroke | 2 125 802 | 23.92 | Coronary heart disease | 445 864 | 21.42 |
| 2 | Lung disease | 1 287 089 | 14.48 | Alzheimer/dementia | 172 765 | 8.30 |
| 3 | Coronary heart disease | 1 040 692 | 11.71 | Lung cancers | 165 402 | 7.95 |
| 4 | Lung cancers | 460 856 | 5.19 | Stroke | 146 664 | 7.05 |
| 5 | Liver cancer | 380 491 | 4.28 | Lung disease | 130 808 | 6.29 |
| 6 | Stomach cancer | 354 829 | 3.99 | Diabetes mellitus | 75 280 | 3.62 |
| 7 | Road traffic accidents | 292 481 | 3.29 | Colorectal cancers | 62 592 | 3.01 |
| 8 | Esophageal cancer | 212 537 | 2.39 | Hypertension | 62 156 | 2.99 |
| 9 | Other injuries | 209 836 | 2.36 | Pneumonia | 57 722 | 2.77 |
| 10 | Hypertension | 205 689 | 2.31 | Kidney disease | 50 889 | 2.45 |
Esophageal cancer is the No. 8 killer in China, while in the United States, it is No. 29. Here the death rate is the percentage of the total deaths nationwide. Data from World Health Organization, World Bank and National Institute of Health.
These numbers only tell one side of the story. Based on the annual reports on the status of cancer[1,2], although esophageal cancer is low in Americans, it has been rising by 6-fold annually and its increase rate now exceeds that for any other type of cancer. There are several possible reasons for this rise, such as excessive alcohol consumption, smoking, stress, and a diet low in vegetables, but the leading factor is GERD, a term that frequently appears in the media as well as in general conversations. A recent study showed that GERD increases the risk of esophageal cancer by 8.6 fold[6].
GERD: NOT A SMALL PROBLEM
GERD is the most common gastrointestinal diagnosis given during office visits and its direct medical costs, which primarily include drug costs, exceed $10 billion a year in the United States[7], whereas indirect costs resulting from reduced work productivity are estimated to be as much as $75 billion a year[8]. GERD occurs when the esophageal sphincter at the bottom of the esophagus weakens and allows stomach acid (often mixed with duodenal contents) to back up into the esophagus. The refluxate erodes the epithelial lining of the lower esophagus and gives a burning sensation in the middle of the chest, which is commonly described as “heartburn”. Patients with long standing GERD can develop esophagitis, an inflammation characterized histologically by a markedly thickened epithelium, elongation of the lamina propria papillae into the epithelium, and basal cell hyperplasia (Figure 4). Over time, this inflammation/injury cycle can induce esophageal mucosa transformation from squamous to a more protective intestinal columnar phenotype, known as Barrett’s esophagus (BE). From a physiological point of view, the secretory columnar epithelium is better prepared to withstand the erosive action of the acidic refluxate than squamous epithelium; however, this metaplastic change confers an increased risk of transformation to EAC. Studies have shown that people with BE can have as high as a 400-fold increased risk of EAC[9,10]. Today, over 60% of Americans experience occasional episodes of acid reflux, and about 25% deal with the problem on a weekly basis. The prevalence of the condition in Americans is increasing by approximately 5% annually[11]. Hospitalizations for all GERD-caused esophageal disorders doubled from 1998 to 2005, according to the United States Agency for Healthcare Research and Quality.
Figure 4.
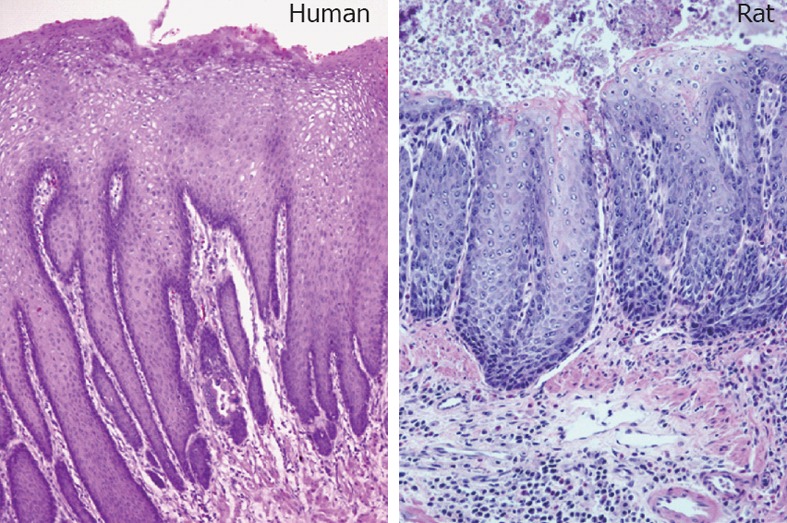
Gastroesophageal reflux disease-induced esophagitis in human and rat (hematoxylin and eosin staining). Gastroesophageal reflux disease rats were created by surgically anastomosing the duodenum to the gastroesophageal junction. These rats can develop esophageal adenocarcinoma within a year, in a pathological sequence similar to human esophageal malignancy.
OBESITY: THE DEVIL
The rise in GERD is associated with the rapidly growing obese population[12], which is usually measured by body mass index (BMI). BMI is calculated based on the weight and height of a person [BMI = weight/(height)2, kg/m2]. The World Health Organization regards a BMI of less than 18.5 as underweight and may indicate malnutrition, an eating disorder, or other health problems, while a BMI greater than 25 is considered overweight and above 30 is considered obese. A recent study showed that global obesity rates have doubled since 1980[13]. The health care costs resulting from excess weight are estimated at greater than $100 billion annually in the United States. According to the report released last year from the Centers for Disease Control and Prevention, more than 34% of adult Americans are obese, which is higher than Canadians (24%). It is predicted that by the year 2020, 77.6% of men will be overweight and 40.2% obese; the corresponding figures for women will be 71.1% and 43.3%, respectively[14]. This problem has also affected children, whose obesity rate has tripled in the last 30 years[15]. At the time of writing, 15.5% of children in the United States are obese. A recent study with a study population of 690 321 patients (age: 2-19 years) revealed that obese children have a 30%-40% higher risk of GERD, compared with children with a normal weight according to their BMI (BMI = 20 ± 3.8 kg/m2)[16]. In adults, the situation is worse. In 2007, a study showed that the total number of GERD episodes was 48% higher in obese patients than those with a normal BMI[17]. The link between increasing BMI and the presence of GERD was further strengthened by a meta-analysis of 20 independent studies, which established a dose-dependent association between these two conditions[18]. A similar connection has also been drawn between increasing BMI and esophageal cancer[19-23].
The precise pathophysiological pathway from obesity to GERD has not been fully elucidated. It has been shown that excess fat in the abdominal area can push on the stomach’s contents to back up, relax the lower esophagus muscle[24,25], disable the esophageal motor[26], impair stomach accommodations[27], and ultimately result in a higher frequency of esophageal acid exposure[12,28,29]. Therefore, a potential causal pathway from body size to esophageal cancer may be from normal to GERD to esophagitis to BE, and ultimately to EAC. In such a direct pathway, obesity could act by increasing the prevalence of GERD, by increasing the prevalence of BE among the GERD population, or by enhancing the risk of malignant transformation from BE to EAC. Although obesity is a major contributor, other factors (e.g., smoking, drinking, diet, or genetics) may also influence the steps on this pathway. For example, GERD smokers were found to have 12.3-fold higher risk of developing EAC than GERD non-smokers[6]. While the issue is quite complex, since the main pathway starts with GERD, interventions aimed at GERD should be expected to proportionally lower the risk of the subsequent steps in the pathway: BE and EAC.
TREATMENT: NO WINNERS
Current treatment for GERD patients includes acid suppressive medications and lower esophageal repair surgery. Aside from traditional antacids (Alka-Seltzer and Tums) which have side effects such as diarrhea and constipation, there are now two categories of medications to treat GERD. H-2 blockers (e.g., Zantac 75, Pepcid AC, Tagamet HB and Axid AR) reduce the amount of histamine-2, which produces acid in the stomach, and are recommended for people with less frequent/severe heartburn. A second medication is the proton pump inhibitors or proton pump inhibitors (PPIs) (e.g., Prilosec, Prevacid, Protonix and Nexium), which directly shuts down the H+/K+ ATPase pump of the parietal cells in the stomach that produce acid. These drugs are stronger than H2 blockers and are recommended for people with more persistent/acute symptoms. Control of acid reflux with PPIs has been found extremely effective for healing reflux esophagitis, but not for prevention of BE development or its progression to EAC. As matter of fact, more and more evidence is emerging about the long-term side-effects associated with these drugs, such as decreased absorption of vitamins/minerals[30], susceptibility to bacterial infections[31], bone fracture[32], and even elevated risk of developing EAC[6,33]. The Food and Drug Administration of the United States has issued warnings repeatedly over the years on high-dose or long-term use of PPIs.
For people who have responded to medication but continue to experience GERD symptoms, surgery to reconstruct the lower esophageal sphincter is usually an option. However, only about 5% of GERD patients undergo surgery and a follow-up study showed that almost two-thirds of the surgical patients were back on medication[34]. A more recent study reported that although surgical therapy achieved better remission of GERD symptoms than Prilosec, 36% of surgical-treated GERD patients ultimately received PPI medication, while 14% of PPI-treated GERD patients underwent subsequent surgery[35]. One final way to treat GERD is through endoscopic procedures, including stitching or using radio-frequency waves to reconstruct the lower esophageal sphincter, but in 2002 this was not recommended by the American Gastroenterological Association for GERD treatment.
CAUSE OF EAC: ACID OR BILE? WHAT TO BLAME?
The inadequacy of treatment options raises the question on the real scientific basis of acid suppression in GERD treatment. A direct chemical analysis of esophageal fluid showed that GERD patients contain about 10 times more bile acid in their lower esophagus than normal people[36]. This might give us a clue as to why regular use of acid suppressants has not lowered the risk of GERD malignancy. In support of this notion, animal studies showed that gastric reflux alone does not cause EAC at all; it is the duodenal contents per se that ultimately lead to esophageal malignancy[37-39]. Furthermore, some animal studies even suggested that gastric acid may play a protective role in GERD against malignancy. For example, one study showed that 87% of rats with surgically-created duodenal reflux alone developed EAC, while the percentage of rats with gastric-duodenal reflux was only 30%[40]. In agreement with the animal studies, a recent systematic review[41,42] examined publications indexed in MEDLINE from 1950 to 2010, and found 82 original human studies on the association of bile acids with GERD, among which, all in vivo studies detected bile acids in the esophageal aspirates of GERD patients, and what’s more, their concentrations were significantly higher than in normal people. It is clear that the refluxate of GERD patients frequently contains bile acids, sometimes even in millimolar concentrations. In addition to human studies and animal modeling, in vitro experiments have shown that bile acids, at equivalent concentrations to the ones found in the esophagus of GERD patients, can stimulate esophageal epithelial cells to produce inflammatory cytokines and chemokines, to generate reactive oxygen species, and to express intestinal genes. All these factors have the ability to facilitate esophageal epithelial metaplasia and even malignancy.
CONCLUSION
Although skin cancer is the most common cancer in the world, 95% of cases are either basal cell carcinoma or squamous cell carcinoma, which have less than 0.5% mortality. Even for the most deadly type of skin cancer - melanoma, which is very rare - the death rate is only about 15%. So is prostate cancer, the second most common cancer in the United States. On the other hand, although esophageal cancer patients have a mortality rate of approximately 85%, since it is less common than either skin cancer or prostate cancer, it receives little attention from government agencies or the research community. This can be seen by looking at the annual budget of the National Institute of Health (NIH) of the United States. In 2011, the NIH spent $284 million on 772 prostate cancer research projects, while only funding 30 esophageal cancer studies with $13 million (Figure 5). Through this article, we hope to attract attention to this disease since, due to its high mortality and fast growth, esophageal cancer could be catastrophic in the near future if we do not prepare ourselves with the proper knowledge.
Figure 5.
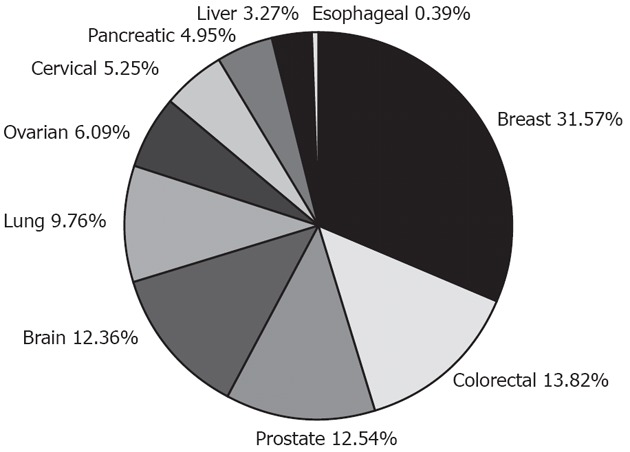
National Institute of Health expenditure on cancer-related studies in 2011. Esophageal cancer barely shows on the pie chart. Data from National Institute of Health documents.
Footnotes
Supported by The Department of Veterans Affairs of the United States
Peer reviewers: Hiroshi Nakagawa, Assistant Professor, Gastroenterology Division, University of Pennsylvania, 415 Curie Blvd., 638B CRB, Philadelphia, PA 19104, United States; Piero Marco Fisichella, MD, Department of Surgery, Loyola University Medical Center, 2160 S. 1st Ave, Maywood, IL 60153, United States; Rene Lambert, Professor, International Agency for Research on Cancer, 150 Cours Albert Thomas, 69372 Cedex 8 Lyon, France
S- Editor Wu X L- Editor Rutherford A E- Editor Li JY
References
- 1.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA, Edwards BK. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Cancer Facts and Figures. 2nd ed. American Cancer Society. 2008. [Google Scholar]
- 4.Chen MJ, Lee YC, Chiu HM, Wu MS, Wang HP, Lin JT. Time trends of endoscopic and pathological diagnoses related to gastroesophageal reflux disease in a Chinese population: eight years single institution experience. Dis Esophagus. 2010;23:201–207. doi: 10.1111/j.1442-2050.2009.01012.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Chen SH, Li YM. Epidemiological investigation of esophageal carcinoma. World J Gastroenterol. 2004;10:1834–1835. doi: 10.3748/wjg.v10.i12.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandeya N, Webb PM, Sadeghi S, Green AC, Whiteman DC. Gastro-oesophageal reflux symptoms and the risks of oesophageal cancer: are the effects modified by smoking, NSAIDs or acid suppressants? Gut. 2010;59:31–38. doi: 10.1136/gut.2009.190827. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen NJ, Hansen RA, Morgan DR, Gangarosa LM, Ringel Y, Thiny MT, Russo MW, Sandler RS. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128–2138. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 8.Wahlqvist P, Reilly MC, Barkun A. Systematic review: the impact of gastro-oesophageal reflux disease on work productivity. Aliment Pharmacol Ther. 2006;24:259–272. doi: 10.1111/j.1365-2036.2006.02996.x. [DOI] [PubMed] [Google Scholar]
- 9.Lagergren J. Adenocarcinoma of oesophagus: what exactly is the size of the problem and who is at risk? Gut. 2005;54 Suppl 1:i1–i5. doi: 10.1136/gut.2004.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corley DA. Obesity and the rising incidence of oesophageal and gastric adenocarcinoma: what is the link? Gut. 2007;56:1493–1494. doi: 10.1136/gut.2007.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2007;5:17–26. doi: 10.1016/j.cgh.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Pandolfino JE, El-Serag HB, Zhang Q, Shah N, Ghosh SK, Kahrilas PJ. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639–649. doi: 10.1053/j.gastro.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruhm CJ. Current and Future Prevalence of Obesity and Severe Obesity in the United States. Forum for Health Economics and Policy. 2007;10:1–26. [Google Scholar]
- 15.Pashankar DS, Corbin Z, Shah SK, Caprio S. Increased prevalence of gastroesophageal reflux symptoms in obese children evaluated in an academic medical center. J Clin Gastroenterol. 2009;43:410–413. doi: 10.1097/MCG.0b013e3181705ce9. [DOI] [PubMed] [Google Scholar]
- 16.Koebnick C, Getahun D, Smith N, Porter AH, Der-Sarkissian JK, Jacobsen SJ. Extreme childhood obesity is associated with increased risk for gastroesophageal reflux disease in a large population-based study. Int J Pediatr Obes. 2011;6:e257–e263. doi: 10.3109/17477166.2010.491118. [DOI] [PubMed] [Google Scholar]
- 17.El-Serag HB, Ergun GA, Pandolfino J, Fitzgerald S, Tran T, Kramer JR. Obesity increases oesophageal acid exposure. Gut. 2007;56:749–755. doi: 10.1136/gut.2006.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corley DA, Kubo A. Body mass index and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:2619–2628. doi: 10.1111/j.1572-0241.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- 19.Lagergren J, Bergström R, Nyrén O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130:883–890. doi: 10.7326/0003-4819-130-11-199906010-00003. [DOI] [PubMed] [Google Scholar]
- 20.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States) Cancer Causes Control. 2001;12:721–732. doi: 10.1023/a:1011290704728. [DOI] [PubMed] [Google Scholar]
- 21.Engeland A, Tretli S, Bjørge T. Height and body mass index in relation to esophageal cancer; 23-year follow-up of two million Norwegian men and women. Cancer Causes Control. 2004;15:837–843. doi: 10.1023/B:CACO.0000043434.21558.ea. [DOI] [PubMed] [Google Scholar]
- 22.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:872–878. doi: 10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 23.Merry AH, Schouten LJ, Goldbohm RA, van den Brandt PA. Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study. Gut. 2007;56:1503–1511. doi: 10.1136/gut.2006.116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crookes PF. Physiology of reflux disease: role of the lower esophageal sphincter. Surg Endosc. 2006;20 Suppl 2:S462–S466. doi: 10.1007/s00464-006-0039-y. [DOI] [PubMed] [Google Scholar]
- 25.Wu JC, Mui LM, Cheung CM, Chan Y, Sung JJ. Obesity is associated with increased transient lower esophageal sphincter relaxation. Gastroenterology. 2007;132:883–889. doi: 10.1053/j.gastro.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 26.Koppman JS, Poggi L, Szomstein S, Ukleja A, Botoman A, Rosenthal R. Esophageal motility disorders in the morbidly obese population. Surg Endosc. 2007;21:761–764. doi: 10.1007/s00464-006-9102-y. [DOI] [PubMed] [Google Scholar]
- 27.Iovino P, Angrisani L, Galloro G, Consalvo D, Tremolaterra F, Pascariello A, Ciacci C. Proximal stomach function in obesity with normal or abnormal oesophageal acid exposure. Neurogastroenterol Motil. 2006;18:425–432. doi: 10.1111/j.1365-2982.2006.00768.x. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA. 2003;290:66–72. doi: 10.1001/jama.290.1.66. [DOI] [PubMed] [Google Scholar]
- 29.El-Serag HB, Kvapil P, Hacken-Bitar J, Kramer JR. Abdominal obesity and the risk of Barrett’s esophagus. Am J Gastroenterol. 2005;100:2151–2156. doi: 10.1111/j.1572-0241.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 30.Evenepoel P. Alteration in digestion and absorption of nutrients during profound acid suppression. Best Pract Res Clin Gastroenterol. 2001;15:539–551. doi: 10.1053/bega.2000.0197. [DOI] [PubMed] [Google Scholar]
- 31.Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, Talmor D. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784–790. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 32.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 33.Leedham S, Jankowski J. The evidence base of proton pump inhibitor chemopreventative agents in Barrett’s esophagus--the good, the bad, and the flawed! Am J Gastroenterol. 2007;102:21–23. doi: 10.1111/j.1572-0241.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 34.Spechler SJ, Lee E, Ahnen D, Goyal RK, Hirano I, Ramirez F, Raufman JP, Sampliner R, Schnell T, Sontag S, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA. 2001;285:2331–2338. doi: 10.1001/jama.285.18.2331. [DOI] [PubMed] [Google Scholar]
- 35.Lundell L, Miettinen P, Myrvold HE, Hatlebakk JG, Wallin L, Engström C, Julkunen R, Montgomery M, Malm A, Lind T, et al. Comparison of outcomes twelve years after antireflux surgery or omeprazole maintenance therapy for reflux esophagitis. Clin Gastroenterol Hepatol. 2009;7:1292–1298; quiz 1260. doi: 10.1016/j.cgh.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 36.Kauer WK, Peters JH, DeMeester TR, Feussner H, Ireland AP, Stein HJ, Siewert RJ. Composition and concentration of bile acid reflux into the esophagus of patients with gastroesophageal reflux disease. Surgery. 1997;122:874–881. doi: 10.1016/s0039-6060(97)90327-5. [DOI] [PubMed] [Google Scholar]
- 37.Miwa K, Sahara H, Segawa M, Kinami S, Sato T, Miyazaki I, Hattori T. Reflux of duodenal or gastro-duodenal contents induces esophageal carcinoma in rats. Int J Cancer. 1996;67:269–274. doi: 10.1002/(SICI)1097-0215(19960717)67:2<269::AID-IJC19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Omura N, Kashiwagi H, Chen G, Suzuki Y, Yano F, Aoki T. Establishment of surgically induced chronic acid reflux esophagitis in rats. Scand J Gastroenterol. 1999;34:948–953. doi: 10.1080/003655299750025020. [DOI] [PubMed] [Google Scholar]
- 39.Miyashita T, Ohta T, Fujimura T, Ninomiya I, Fushida S, Hattori T, Miwa K. Duodenal juice stimulates oesophageal stem cells to induce Barrett’s oesophagus and oesophageal adenocarcinoma in rats. Oncol Rep. 2006;15:1469–1475. [PubMed] [Google Scholar]
- 40.Ireland AP, Peters JH, Smyrk TC, DeMeester TR, Clark GW, Mirvish SS, Adrian TE. Gastric juice protects against the development of esophageal adenocarcinoma in the rat. Ann Surg. 1996;224:358–370; discussion 370-371. doi: 10.1097/00000658-199609000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McQuaid KR, Laine L, Fennerty MB, Souza R, Spechler SJ. Systematic review: the role of bile acids in the pathogenesis of gastro-oesophageal reflux disease and related neoplasia. Aliment Pharmacol Ther. 2011;34:146–165. doi: 10.1111/j.1365-2036.2011.04709.x. [DOI] [PubMed] [Google Scholar]
- 42.Thompson R. Upper gastrointestinal tract: Bile acids may have a role in GERD and related neoplasia. Nat Rev Gastroenterol Hepatol. 2011;8:419. doi: 10.1038/nrgastro.2011.110. [DOI] [PubMed] [Google Scholar]