Abstract
In highland areas of unstable, low malaria transmission, the extent to which immunity to uncomplicated malaria develops with age and intermittent parasite exposure has not been well characterized. We conducted active surveillance for clinical malaria during April 2003–March 2005 in two highland areas of western Kenya (Kapsisiywa and Kipsamoite). In both sites, annual malaria incidence was significantly lower in persons ≥ 15 years of age than in persons < 5 years of age (Kapsisiywa: incidence = 382.9 cases/1,000 persons among persons < 1–4 years of age versus 135.1 cases/1,000 persons among persons ≥ 15 years of age; Kipsamoite: incidence = 233.0 cases/1,000 persons in persons < 1–4 years of age versus 43.3 cases/1,000 persons in persons ≥ 15 years of age). In Kapsisiywa, among persons with malaria, parasite density and axillary body temperature were also significantly lower in persons ≥ 15 years of age than in persons < 5 years of age. Even in highland areas of unstable and low malaria transmission, age is associated with development of clinical immunity to malaria.
Introduction
Malaria caused by Plasmodium falciparum is a leading cause of death among children less than five years of age in sub-Saharan Africa.1 Children living where malaria transmission is holo-endemic or hyper-endemic develop immunity and begin to control the level of parasite burden such that, as adults, they are at reduced risk of uncomplicated and severe disease, including death. Parameters of malaria transmission, such as the force of infection, and disease incidence have been shown to correlate with this clinical immunity and the development of overall immune responses to P. falciparum.2–4
Some studies of malaria in areas of low transmission support the premise that the risk of uncomplicated malaria is similar in adults and children, although severe disease and death from malaria in these areas still occurs most frequently in young children.5–7 Studies on age-related risk of malaria in highland areas, which have unstable, seasonal, and low levels of malaria transmission, have produced conflicting results.8–11 Some studies suggest that persons of all ages experience equal risk of malaria, but others, including several historical studies, suggest that adults may develop some degree of immunity and are at a reduced risk for clinical malaria compared with children.6,12–14
Most studies of highland malaria and many studies of malaria in low transmission areas have used retrospective chart reviews of hospital data or have studied a limited age range that may not be able to assess nuanced differences in malaria incidence with age. There is also a paucity of recent studies of age-related risk of malaria in low transmission settings. To address this gap in knowledge, we analyzed data from a prospective cohort study of uncomplicated malaria incidence in two highland areas of Kenya that experience very low, seasonal transmission. In this study, we also sought to understand how clinical parameters of immunity, including parasite density and measured temperature during an acute malaria episode, differ with age.
Materials And Methods
Study sites.
Two highland sites, comprising multiple villages, were the setting for this analysis. Both sites have low and unstable malaria transmission, as indicated by seasonal peaks in incidence and periodic epidemics, and are located in the Nandi North District of western Kenya: Kapsisiywa (elevation = 1,887–1,982 meters, population in 2003 = 3,412 persons) and Kipsamoite (elevation = 1,950–2,100 meters, population in 2003 = 3,250 persons).15 Most persons in both sites are of the Kalenjin Nandi tribe. A Kenyan Ministry of Health dispensary, capable of microscopy and malaria treatment, is located in each site.
Active surveillance for malaria.
Persons from the two cohorts were observed, the first cohort was studied during April–December 2003 and the second cohort was studied during January 2004–March 2005. Written informed consent was obtained from all participants before enrollment in either cohort; parents or guardians of children less than 15 years of age provided consent for children. A literate family member, or other witness, was present during the consent process of illiterate participants to ensure that the details of the study were conveyed effectively.
Enrollment in the first cohort in Kipsamoite was a continuation of a previous study in which persons had been randomly selected from the population to participate in a study of immune response to malaria disease.16 For enrollment into each cohort in Kapsisiywa and the second cohort in Kipsamoite, each site was divided into four geographic sub-regions of approximately equal population size. Within each sub-region, households were randomly selected for recruitment until approximately 15% of the underlying population had been enrolled. Refusal rates were < 1% in both communities.
Active surveillance for malaria disease was conducted by trained, local field assistants who made weekly visits to each enrolled household, including the head of the household and all present and enrolled residents. Clinical malaria was detected among study participants in three ways: 1) study participants reported a current illness or illness in the past week during a weekly household visit; 2) study participants contacted a field assistant when ill; or 3) study participants reported directly to the dispensary when ill. The case definition for suspected clinical malaria was history of fever, chills, headache, or severe malaise in a study participant in the past week. Parents or guardians were asked about illness in infants and young children consistent with the above case definition. The case definition did not differ by age. Field assistants at the participant's house, or clinical officers at the study dispensaries, took finger prick blood samples and measured axillary body temperature when malaria was suspected. All participants meeting the case definition were referred to the dispensary for blood smear results and treatment. For study participants who attended health facilities outside the study area, axillary temperature and parasite density were not available but data on the results of blood smears were recorded per self-report.
Microscopy testing and treatment of patients with clinical malaria.
Clinical malaria was confirmed by microscopy for persons with suspected malaria. Trained microscopists from the Division of Vector-Borne Diseases in the Kenyan Ministry of Health at each of the health dispensaries viewed Giemsa-stained thick and thin blood smears for the presence of Plasmodium species parasites, as described.11 In brief, slides were considered negative if no asexual parasites were observed after counting microscopic fields containing at least 200 leukocytes. The presence of any asexual Plasmodium parasite indicated a positive blood smear. Parasite densities were estimated per microliter of blood, assuming 8,000 leukocytes/μL of blood. Additionally, the presence and density of gametocytes were assessed in all positive blood smears. Both sites followed the same microscopy protocol for each cohort.
Participants with microscopy-confirmed malaria were treated with sulfadoxine/pyrimethamine according to the Kenyan Ministry of Health treatment guidelines until May 2004; at which time the national treatment guidelines for the first-line antimalarial switched to amodiaquine.
Entomologic surveillance.
To estimate the entomologic inoculation rate (EIR) at each site, pyrethrum spray catches (PSC) were performed every two weeks during April 2004–March 2005 in 120 randomly selected households by using standard methods.17 Collected mosquitoes were identified by species, and the blood-engorged abdomens of anopheline mosquitoes were tested by polymerase chain reaction (PCR) for human blood, and the head and thorax were tested by enzyme-linked immunosorbent assay for P. falciparum circumsporozoite protein (CSP). The EIR per year was calculated as 1.605 × (the human biting rate) × (sporozoite rate) × 365, where the constant 1.605 was used to account for the use of PSC rather than human landing catches.18–20 The human biting rate was defined as the number of blood-fed mosquitoes divided by the number of persons who slept in the sampled houses during the night of sampling, and the sporozoite rate was defined as the number of CSP enzyme-linked immunosorbent assay–positive vectors divided by the number of vectors collected.
Statistical analysis.
To assess the differences in malaria incidence by age, data were divided into three age groups: 0–4 years old, 5–14 years old, and ≥ 15 years old. Only microscopy-confirmed cases were considered. All data were summarized by month to ease adjustments for seasonality. Participants who were present for at least one week of the month were counted in the denominator for the respective month. Participants with a malaria diagnosis within 30 days of a prior positive blood smear were considered to have recrudescent disease.
Initial assessment of the age-specific frequency of malaria cases was conducted using the chi-square test. Negative binomial regression was used to model monthly malaria incidence as a function of age group, month, and year of observation. Variability in the seasonal pattern of malaria in both sites indicated that a negative binomial regression was more appropriate for modeling incidence than a typical Poisson model. Indicators for the month and year of observation were included to adjust for seasonal transmission patterns. Clinical characteristics, including parasite density and axillary body temperature (°C), were compared across age groups by using linear regression, adjusting for the study period of observation. Parasite density was log-transformed to preserve normality.
The malaria attributable fraction (MAF), or the proportion of fevers (axillary temperature ≥ 37.5°C) attributable to malaria, was assessed for each age group in each site. The MAF was estimated by the classic equation for the population attributable risk: MAF = p(RR – 1)/RR, where p is the prevalence of microscopy parasitemia among those with a fever and RR is the relative risk of having a fever in parasitemic versus non-parasitemic persons.21 Estimates of asymptomatic parasitemia came from cross-sectional studies conducted in 2002 in Kapsisiywa and Kipsamoite; 7.9% of participants were found to be asymptomatically parasitemic during the survey.11
Calculation of sensitivity and specificity at specific parasite densities was performed using methods developed by Smith and others.21 Sensitivity for a specific parasite density was defined as the number of symptomatic persons with parasitemia at that density divided by the total number of persons with parasitemia and measured fever. Specificity for a parasite density was defined as the number of asymptomatic persons without parasitemia at that density divided by the number of asymptomatic persons. Calculations for these results were also made with the inclusion of data on asymptomatic parasitemia from the 2002 parasite prevalence survey.11 Optimal parasite density was chosen as the density in which the graph of sensitivity and specificity crossed. Stata version 10 (StataCorp LP, College Station, TX) was used for all statistical analyses.
The Institutional Review Boards at Case Western Reserve University (Cleveland, OH), the Centers for Disease Control and Prevention (Atlanta, GA), the Kenya Medical Research Institute (Nairobi, Kenya), and the University of Minnesota (Minneapolis, MN) granted ethical approval for this study.
Results
Study population.
In Kapsisiywa, 631 participants (18.5% of the population) were enrolled in the active surveillance cohort in April 2003. In Kipsamoite, a previously established active surveillance cohort of 237 persons (7.3% of the population) was re-enrolled in the present study in April 2003. In January 2004, new cohorts of 549 participants in Kapsisiywa (16.1% of the 2003 population) and 575 participants in Kipsamoite (17.7% of the 2003 population) were enrolled for active malaria surveillance. Active surveillance follow-up rates were high and similar between age groups in Kapsisiywa (average percentage of cohort observed per month, 2003, 0–4 years, 94.7%, 5–14 years, 95.2%, ≥ 15 years, 91.4%; 2004–2005, 0–4 years, 87.4%, 5–14 years, 82.2%, ≥ 15 years, 87.1%) and in Kipsamoite (2003, 0–4 years, 96.3%, 5–14 years, 91.2%, ≥ 15 years, 94.4%; 2004–2005, 0–4 years, 94.7%, 5–14 years, 96.5%, ≥ 15 years, 92.0%).
In Kapsisiywa, 1,046 blood smears were obtained during the two study periods, of which 214 (20.5%) were positive for P. falciparum. In Kipsamoite, 401 blood smears were obtained and 50 (12.5%) were positive for P. falciparum. No smears were positive for P. malariae, P. vivax, or P. ovale in either site. Visits to outside health facilities comprised 31.1% of all clinic visits by study participants. However, visiting an outside clinic was not associated with age or positivity of a blood smear for P. falciparum.
Entomologic inoculation rate.
Malaria transmission parameters in these two sites were estimated from PSC for 2,651 houses with 9,060 residents during April 2004–March 2005. Eighty-five Anopheles mosquitoes were collected (84 Anopheles gambiae s.l. and 1 An. funestus). Thirty-seven of the Anopheles mosquitoes had fed on human blood, and one was positive for P. falciparum CSP. Therefore, the estimated EIR for the two areas combined was 0.028 infectious bites per person per year.
Malaria incidence and age distribution.
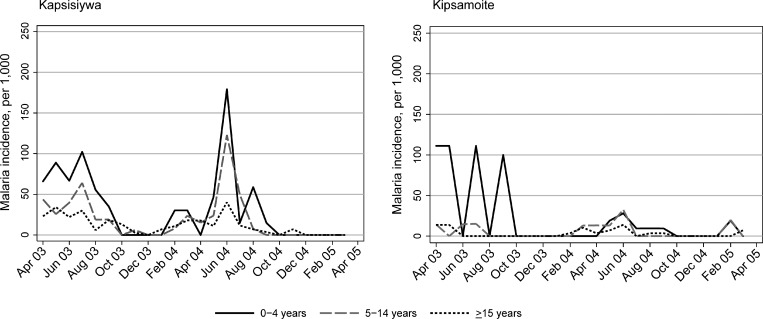
Annual incidence of malaria was considerably higher in Kapsisiywa than in Kipsamoite (Figure 1). Incidence of malaria over time was highly seasonal in both sites with peaks during March–August of each year.
Figure 1.
Monthly malaria incidence per 1,000 persons in Kapsisiywa and Kipsamoite, Kenya, April 2003–March 2005, according to age group (0–4 years, 5–14 years, and ≥ 15 years).
The distribution of malaria cases differed according to age in both study periods and sites (P < 0.05 for all comparisons). In both sites, malaria incidence was highest in children less than five years of age, and more than 50% of malaria incidence in both sites occurred in children less than five years of age, although children in this age group constituted only 15.1% and 12.8% of the study population in Kapsisiywa and 7.6% and 18.8% of the study population in Kipsamoite, in 2003 and 2004–2005, respectively (Table 1). In Kapsisiywa, incidence of malaria was 42% lower (95% confidence interval [CI] = 10–63%) (Table 1) in persons 5–14 years of age compared with those less than five years of age, and 61% lower (95% CI = 40 –75%) in persons ≥ 15 years of age after adjusting for seasonality. In Kipsamoite, malaria incidence was not significantly different for persons less than five years of age and those 5–14 years of age, but was significantly decreased in persons ≥ 15 years of age compared with those less than five years of age (61% decrease; 95% CI = 21–81%).
Table 1.
Cumulative malaria incidence in Kapsisiywa and Kipsamoite in Nandi North District, Kenya*
| Location, patient age, years | Average annual malaria incidence/1,000 persons (95% CI)† | % Of average malaria incidence† | % Of study population† | RR for malaria (95% CI) |
|---|---|---|---|---|
| Kapsisiywa | ||||
| 0–4 | 382.9 (130.0–635.8) | 51.7 | 14.5 | Reference |
| 5–14 | 222.9 (30.0–415.9) | 30.1 | 27.3 | 0.58 (0.37–0.90) |
| ≥ 15 | 135.0 (0–285.4) | 18.2 | 58.2 | 0.39 (0.25–0.60)‡ |
| Kipsamoite | ||||
| 0–4 | 233.0 (35.7–430.3) | 66.7 | 14.9 | Reference |
| 5–14 | 73.2 (0–183.8) | 20.9 | 28.5 | 0.68 (0.33–1.38) |
| ≥ 15 | 43.3 (0–128.1) | 12.4 | 56.6 | 0.39 (0.19–0.79)§ |
RR = relative risk; CI = confidence interval.
Weighted for the two active surveillance periods according to the total months of observation (9 months from April through December 2003 and 14 months from January 2004 through March 2005).
P value for trend with increasing age < 0.001, by chi-square test for trend.
P value for trend with increasing age = 0.035, by chi-square test for trend.
In a previous study, PCR testing for P. falciparum DNA was performed on blood samples obtained from symptomatic persons from this cohort during 11 months of 2004.22 The percentage of malaria incidence in children less than five years of age during these 11 months did not differ significantly between malaria as detected by PCR versus microscopy (Kapsisiywa = 58.8% by PCR and 61.4% by microscopy; P = 0.842; Kipsamoite = 42.3% by PCR and 53.1% by microscopy; P = 0.647).
Parasite density and measured temperature in persons with clinical malaria.
In Kapsisiywa, the parasite density in persons ≥ 15 years of age was 62.8% lower than in persons less than five years of age after adjusting for study period (95% CI = 13.9–83.9%, P = 0.021) (Table 2). Decreased parasite density was also seen in persons ≥ 15 years of age in Kipsamoite, but the number of persons with disease was small, and the differences were not statistically significant. Median parasite density was higher in Kipsamoite than Kapsisiywa, although this difference was statistically significant only in persons ≥ 15 years of age (P = 0.002).
Table 2.
Clinical characteristics of incident malaria episodes during April 2003–March 2005 in Kapsisiywa and Kipsamoite in Nandi North District, Kenya
| Location, patient age, years | Parasite density (per μL) | Axillary body temperature (°C) | |||||
|---|---|---|---|---|---|---|---|
| No. | Median | Range | P* | Mean | SD | P* | |
| Kapsisiywa | |||||||
| 0–4 | 50 | 1,920 | (80–168,000) | Reference | 38.4 | 1.21 | Reference |
| 5–14 | 53 | 6,440 | (40–179,200) | 0.039 | 38.0 | 1.21 | 0.086 |
| ≥ 15 | 65 | 560 | (80–228,000) | 0.021 | 37.5 | 1.18 | < 0.001 |
| Kipsamoite | |||||||
| 0–4 | 10 | 20,660 | (80–168,520) | Reference | 38.3 | 1.50 | Reference |
| 5–14 | 14 | 11,920 | (480–199,680) | 0.714 | 38.8 | 1.34 | 0.544 |
| ≥ 15 | 9 | 8,400 | (400–197,000) | 0.783 | 37.4 | 1.43 | 0.193 |
P values calculated from linear regression with log-transformed parasite density or axillary body temperature against age group, adjusted for study period.
Thirteen participants in Kapsisiywa and one participant in Kipsamoite had detectable gametocytes. All participants with gametocytes were less than 15 years of age (mean age = 6.5 years, range = 1.1–13.2 years), except for one participant in Kapsisiywa who was 74 years old.
In Kapsisiywa, measured axillary body temperature also decreased with increasing age (Table 2) (P for trend < 0.001), and significantly reduced temperatures were seen in the 5–14 and ≥ 15-year age groups. In Kipsamoite, a non-significant trend toward a decrease in temperature was seen in persons ≥ 15 years of age (P for trend = 0.132).
Parasite density cutoff value for detection of fever.
Because the parasite density of symptomatic clinical malaria varied by age, it may be beneficial to augment the clinical definition of malaria with a parasite density cutoff value for each age group. For the entire population of Kapsisiywa, the cutoff value that provided the maximum sensitivity (100%) with 93.6% specificity was 40 parasites/μL, which was the lowest density detectable by microscopy. For the entire population in Kipsamoite, sensitivity was maximized at a similarly low cutoff value (80 parasites/μL, 100% sensitivity, 93.1% specificity).
In Kapsisiywa, the optimal parasite density cutoff value did not differ by age. In Kipsamoite, the optimal parasite density cutoff value was higher among children 5–14 years of age (480 parasites/μL) and among those ≥ 15 years of age (400 parasites/μL). However, the number of smears obtained for these age groups was low, and no densities lower than the specified cutoff values were recorded. Therefore, a lower cutoff value might have been seen with a larger sample size in Kipsamoite.
Malaria attributable fraction.
In Kapsisiywa, the MAF was 23.2% (95% CI = 19.6–26.3%), which suggested that 23% of observed fevers in the area were attributable to malaria. In Kipsamoite, the MAF was 18.8% (95% CI = 13.7–22.3%). By age group, the MAF in Kapsisiywa was 18.1% in those less than five years of age, 29.5% in those 5–14 years of age, and 19.8% in those ≥ 15 years of age. In Kipsamoite, the MAF was 12.8%, 24.9%, and 13.7% in persons less than five years of age, 5–14 years of age, and ≥ 15 years of age, respectively.
Discussion
Our study findings add to the contemporary literature documenting incidence of uncomplicated malaria and parasite density in persons of all ages in highland areas of low and seasonal transmission. These analyses demonstrate a significant reduction in the incidence of uncomplicated malaria with increasing age in two highland areas of Kenya with seasonal, unstable and low, malaria transmission. In addition, a lower parasite density at the time of malaria episode was seen in adults ≥ 15 years of age compared with children < 5 years of age in Kapsisiywa, the area of higher transmission intensity. The study findings provide evidence that even in areas of low malaria transmission, where persons have less than one infectious bite per year, increased age is associated with development of significant clinical immunity to uncomplicated malaria. These findings are important for consideration of interventions and risk prediction during epidemics in highland areas because they suggest that older children and adults are at lower risk than children less than five years of age not only for severe malaria, as shown in earlier studies, but also for mild, uncomplicated disease.7,12 The study findings also have implications for what may be expected in areas where malaria transmission is reduced but not eliminated. Adults in our study appear to be at a lower risk for clinical malaria than children even in the presence of low transmission intensity, although, importantly, adults were not found to be free from risk and may still be at a higher risk of malaria than adults from areas with moderate and high transmission intensity.
Our study findings are consistent with studies conducted in areas of low, seasonal malaria transmission in Kenya, South Africa, eastern Sudan, and the highlands of Uganda.12,14,23–26 Early studies in western Uganda suggested that at high altitudes, the parasite rate in persons less than 20 years of age old was nearly twice as high as in those persons ≥ 20 years of age.14 Although not in a high altitude region, similar results were found in eastern Sudan where malaria transmission is seasonal but sporadic.24 In Kenya, a study of highland sites nearby Kapsisiywa and Kipsamoite found that the ratio of the number of hospital admissions for malaria in adults compared with children (the adult-to-child ratio) indicated much greater disease in children.12 Finally, a range of transmission settings studied in South Africa found that a shift in the pattern of disease towards higher incidence in children was only seen once the annual incidence exceeded 3–14 cases/1,000 persons, thresholds that are surpassed in Kapsisiywa and Kipsamoite during typical seasonal transmission.23,26
In contrast, results of other studies in the highlands of Uganda, Senegal, Mali, and from passive surveillance in Kipsamoite are not consistent with our findings and have suggested that the risk for uncomplicated malaria may remain constant with age.25,27,28 Because the study in Senegal28 and a recent systematic review5 surveyed a much younger age range, only up to 11 years of age, those results are not comparable with the primary findings of our study. Although the study in Mali included adults (through an age of 20 years), the reduced risk of uncomplicated malaria might not be fully realized until 20 or 30 years of age. Thus, studies that exclude middle-age community members may still not be able to detect differences in disease incidence as seen in this study.8,23,25 When the entire population was observed in Kabale District, Uganda, a site at a similar altitude as Kapsisiywa and Kipsamoite, an adult-to-child ratio of 1.5 was calculated and most adult cases (42% of cases in persons ≥ 15 years of age) were among persons 15–29 years of age.27
Reduced parasite exposure in older children and adults could explain the lower incidence of clinical malaria seen compared with persons less than five years of age. However, in this study population, we believe that adults and adolescents were likely to have greater, not lower, parasite exposure than children less than five years of age. The two greatest risk factors for exposure, living near or traveling to areas of higher vector density within the sites (e.g., areas near a swamp) and travel outside the sites to areas of higher malaria endemicity are likely to be much more frequent in adults and older children than in children less than five years of age. Use of insecticide-treated bed nets (ITNs) may have also modified parasite exposure. Free ITNs were provided by the Ministry of Health for children less than five years of age and pregnant women. Therefore, despite low reported rates of use at these two sites, children less than five years of age were more likely to sleep under an ITN than older persons.29 In a concurrent case–control study, ITN use was low, and there was no association between ITN use and malaria.30 Therefore, it is unlikely that ITN use contributed to protection in older persons, and our estimates of the increased risk seen in children less than five years of age may be conservative.
Furthermore, in these areas, we have found that frequencies and levels of IgG against pre-erythrocytic and blood-stage P. falciparum antigens and interferon-γ responses to pre-erythrocytic antigens are higher in adults than in children.16,31 These responses would be seen only in persons exposed to P. falciparum, and support the contention that adults in these highland areas have greater exposure to P. falciparum than children. Finally, exposure to malaria might have been reduced over the course of the study periods because of enhanced detection and prompt treatment of malaria cases. Although this suggestion might account for decreasing incidence trends over the course of the study, it is unlikely that this reduced exposure would act differentially by age to influence the associations seen in this study. Assessing changes in asymptomatic parasitemia over the course of the observation periods would have aided our estimate of the degree of clinical immunity that exists in this community and how that may change with age. Unfortunately, cross-sectional samples of the cohorts were not conducted during the period of observation.
Earlier studies indicate that immunity to malaria increases with the intensity of transmission and can be related in terms of the number of exposures in a lifetime and the interval of time between exposures.32,33 The number of episodes of malaria in an area with unstable, low transmission may be minor during any given year, but the recurrent annual exposure may provide sufficient immune boosting for residents to mount an effective immune response over time. It has been suggested that populations living in low but seasonal transmission zones may be able to mount immunity to severe malaria (e.g., severe malarial anemia) after three malaria episodes, but more data is needed to understand the number and frequency of clinical malaria episodes or malaria exposure required to develop immunity to uncomplicated malaria in areas of unstable transmission.33 Data from an area of low transmission in Mali (EIR < 1 infectious bite/person/year) demonstrated re-infection rates of 80% in all age groups over a 12-week period during peak seasonal transmission, suggesting that exposure to malaria is nearly universal during a season and may be enough to induce clinical immunity.34
When we looked at clinical markers of malaria immunity in Kapsisiywa, we found that clinical episodes of malaria and body temperature during a malaria episode decreased when persons reached 5–14 years of age, but parasite density decreased only when persons were ≥ 15 years of age. The findings suggest that in this area, development of clinical immunity may precede development of parasitemia control, a finding consistent with prior research in settings of low transmission.3 However, this pattern was not seen in Kipsamoite, where relatively small numbers of persons showed development of clinical malaria, which might have hindered our ability to detect meaningful changes in clinical markers of disease with age. Parasite density was significantly higher in the area of higher transmission only in persons ≥ 15 years of age, supporting the idea that control of parasitemia may require immunity that comes with increased age and increased exposure.
In addition, there appears to be an inverse association between age and gametocyte prevalence in the oldest age group. Further studies with larger numbers of study participants and with a P. falciparum zygote/ookinete surface protein (PfS25) reverse transcription-PCR, which is more sensitive than microscopy for detection of gametocytemia, are required to establish if parasite density and gametocyte prevalence are lower in persons ≥ 15 years of age in all highland areas.35 Such studies will be important for furthering our understanding of the different mechanisms by which immune control of clinical symptoms and parasite density may occur. The ability of a person to control parasitemia and gametocytemia will be critical to the success of malaria elimination and eradication efforts. Studies of parasite population would also help to define whether parasite diversity is a determinant of immunity. We are currently conducting such studies in this study population.
Using a case definition of malaria that included any parasitemia, we found that the proportion of fevers caused by malaria, or the MAF, was similar to those found in highland Uganda.27 In the study reported by Lindblade and others27 and the current study, the MAF was highest among persons 5–14 years of age, further demonstrating that the burden of malaria seems to change with age even in areas with low but seasonal transmission. In areas with higher, more stable transmission (EIR = 10 infectious bites/person/year), the MAF was much higher (50% overall), and was also found to decrease with age, peaking in persons 5–10 years of age.36
The optimal parasite density for a maximally sensitive and specific case definition was fairly low among persons with clinical malaria in this study. Several other studies have investigated parasite density thresholds in varying malaria transmission settings and, in general, higher altitudes seem to have a lower parasite density threshold and the threshold also tends to decrease with age, as was seen in this analysis.37,38
Of note from this study, 20% of persons with clinical malaria had a parasitemia less than 100 parasites/μL, the density at which sensitivity for detection of infection is decreased in many rapid diagnostic tests.39–41 Furthermore, in a subset of persons in this cohort who had symptoms of malaria, we found that PCR detected many infections not detected by microscopy, and that the MAF increased when assessed by PCR than when assessed by microscopy.22 Careful study will be required to ensure that field diagnostic systems used in highland areas of low transmission do not miss a small, but significant, proportion of persons with clinical malaria and low-density, or subpatent, parasitemia.
In summary, the present study suggests that in highland areas of unstable and low transmission, significant clinical immunity to uncomplicated malaria seems to develop with age and reduce the burden of disease in adults. This immunity may result from repeated intermittent exposure, changes in the nature of the immune response with age, or, most likely, a combination of these factors.7,33,42 Future studies will continue to investigate immune correlates of protection in these populations. The study findings suggest that interventions that decrease but do not completely eliminate seasonal malaria transmission may still enable development of some immune protection among adults. In addition, the study results support the importance of interventions targeted at young children even in low transmission settings. However, in both Kapsisiywa and Kipsamoite, there remained a substantial proportion of uncomplicated malaria in adults. Thus, although there is clearly a degree of age-related immunity in these populations, it is incomplete and area-wide interventions that include populations of all ages will remain critical to campaigns for malaria elimination and eradication.
ACKNOWLEDGMENTS
We thank the study participants in Kipsamoite and Kapsisiywa for their participation in this study, the field assistants and David Koech for collecting study data, and Jackson Abuya and the late Livingstone Wanyama for performing microscopy for this study. This paper was published with the permission of the Director of the Kenya Medical Research Institute.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Financial support: This study was supported in part by National Institutes of Allergy and Infectious Diseases grants U01 AI056270, K08 AI01572, and D43 TW0080085 to Chandy C. John.
Authors' addresses: Melissa A. Rolfes and Matthew McCarra, Department of Pediatrics, University of Minnesota, Minneapolis, MN, E-mails: riede056@umn.edu and mcca0495@umn.edu. Ng'wena G. Magak, Department of Medical Physiology, School of Medicine, Moi University, Eldoret, Kenya, E-mail: gngwena@hotmail.com. Kacey C. Ernst, College of Public Health, University of Arizona, Tucson, AZ, E-mail: kernst@email.arizona.edu. Arlene E. Dent, Center for Global Health and Diseases, Case Western Reserve School of Medicine, Biomedical Research Building, West Administrative Offices #430, Cleveland, OH, E-mail: arlene.dent@case.edu. Kim A. Lindblade, Malaria Branch, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: kil2@cdc.gov. Chandy C. John, Center for Global Pediatrics, Department of Pediatrics, University of Minnesota, Minneapolis, MN, E-mail: ccj@umn.edu.
References
- 1.World Health Organization . Roll Back Malaria. Malaria Key Facts. 2011. http://www.rollbackmalaria.org/malariaMessages.html Available at. Accessed July 29, 2011. [Google Scholar]
- 2.Baird JK. Age-dependent characteristics of protection versus susceptibility to Plasmodium falciparum. Ann Trop Med Parasitol. 1998;92:367–390. doi: 10.1080/00034989859366. [DOI] [PubMed] [Google Scholar]
- 3.Bloland PB, Boriga DA, Ruebush TK, McCormick JB, Roberts JM, Oloo AJ, Hawley W, Lal A, Nahlen B, Campbell CC. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission II. Descriptive epidemiology of malaria infection and disease among children. Am J Trop Med Hyg. 1999;60:641–648. doi: 10.4269/ajtmh.1999.60.641. [DOI] [PubMed] [Google Scholar]
- 4.Smith T, Maire N, Dietz K, Killeen GF, Vounatsou P, Molineaux L, Tanner M. Relationship between the entomologic inoculation rate and the force of infection for Plasmodium falciparum malaria. Am J Trop Med Hyg. 2006;75:11–18. doi: 10.4269/ajtmh.2006.75.2_suppl.0750011. [DOI] [PubMed] [Google Scholar]
- 5.Carneiro I, Roca-Feltrer A, Griffin JT, Smith L, Tanner M, Schellenberg JA, Greenwood B, Schellenberg D. Age-patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: a systematic review and pooled analysis. PLoS ONE. 2010;5:e8988. doi: 10.1371/journal.pone.0008988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okiro EA, Al-Taiar A, Reyburn H, Idro R, Berkley JA, Snow RW. Age patterns of severe paediatric malaria and their relationship to Plasmodium falciparum transmission intensity. Malar J. 2009;8:4. doi: 10.1186/1475-2875-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reyburn H, Mbatia R, Drakeley C, Bruce J, Carneiro I, Olomi R, Cox J, Nkya WM, Lemnge M, Greenwood BM, Riley EM. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA. 2005;293:1461–1470. doi: 10.1001/jama.293.12.1461. [DOI] [PubMed] [Google Scholar]
- 8.Bodker R, Akida J, Shayo D, Kisinza W, Msangeni HA, Pedersen EM, Lindsay SW. Relationship between altitude and intensity of malaria transmission in the Usambara Mountains, Tanzania. J Med Entomol. 2003;40:706–717. doi: 10.1603/0022-2585-40.5.706. [DOI] [PubMed] [Google Scholar]
- 9.Garnham PC. Malaria epidemics at exceptionally high altitudes. BMJ. 1945;2:45–47. doi: 10.1136/bmj.2.4410.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerra CA, Gikandi PW, Tatem AJ, Noor AM, Smith DL, Hay SI, Snow RW. The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med. 2008;5:e38. doi: 10.1371/journal.pmed.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.John CC, McHugh MM, Moormann AM, Sumba PO, Ofulla AV. Low prevalence of Plasmodium falciparum infection among asymptomatic individuals in a highland area of Kenya. Trans R Soc Trop Med Hyg. 2005;99:780–786. doi: 10.1016/j.trstmh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Hay SI, Noor AM, Simba M, Busolo M, Guyatt HL, Ochola SA, Snow RW. Clinical epidemiology of malaria in the highlands of western Kenya. Emerg Infect Dis. 2002;8:543–548. doi: 10.3201/eid0806.010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindsay SW, Martens WJ. Malaria in the African highlands: past, present and future. Bull World Health Organ. 1998;76:33–45. [PMC free article] [PubMed] [Google Scholar]
- 14.de Zuleuta J, Kafuko GW, McCrae AW, Pedersen CK, Wasswa DF. A malaria eradication experiment in the highlands of Kigezi (Uganda) East Afr Med J. 1964;41:102–120. [PubMed] [Google Scholar]
- 15.Cohen JM, Ernst KC, Lindblade KA, Vulule JM, John CC, Wilson ML. Topography-derived wetness indices are associated with household-level malaria risk in two communities in the western Kenyan highlands. Malar J. 2008;7:40. doi: 10.1186/1475-2875-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noland GS, Hendel-Paterson B, Min XM, Moormann AM, Vulule JM, Narum DL, Lanar DE, Kazura JW, John CC. Low prevalence of antibodies to preerythrocytic but not blood-stage Plasmodium falciparum antigens in an area of unstable malaria transmission compared to prevalence in an area of stable malaria transmission. Infect Immun. 2008;76:5721–5728. doi: 10.1128/IAI.00591-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . Manual of Practical Entomology in Malaria. Part II: Methods and Techniques. Geneva: World Health Organization; 1975. [Google Scholar]
- 18.Shaukat AM, Breman JG, McKenzie FE. Using the entomological inoculation rate to assess the impact of vector control on malaria parasite transmission and elimination. Malar J. 2010;9:122. doi: 10.1186/1475-2875-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lines JD, Curtis CF, Wilkes TJ, Njunwa KJ. Monitoring human-biting mosquitoes (Diptera: Culicidae) in Tanzania with light-traps hung beside mosquito nets. Bull Entomol Res. 1991;81:77–84. [Google Scholar]
- 20.Drakeley C, Schellenberg D, Kihonda J, Sousa CA, Arez AP, Lopes D, Lines J, Mshinda H, Lengeler C, Armstrong Schellenberg J, Tanner M, Alonso P. An estimation of the entomological inoculation rate for Ifakara: a semi-urban area in a region of intense malaria transmission in Tanzania. Trop Med Int Health. 2003;8:767–774. doi: 10.1046/j.1365-3156.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith T, Schellenberg JA, Hayes R. Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med. 1994;13:2345–2358. doi: 10.1002/sim.4780132206. [DOI] [PubMed] [Google Scholar]
- 22.Menge DM, Ernst KC, Vulule JM, Zimmerman PA, Guo H, John CC. Microscopy underestimates the frequency of Plasmodium falciparum infection in symptomatic individuals in a low transmission highland area. Am J Trop Med Hyg. 2008;79:173–177. [PMC free article] [PubMed] [Google Scholar]
- 23.Gerritsen AA, Kruger P, van der Loeff MF, Grobusch MP. Malaria incidence in Limpopo Province, South Africa, 1998–2007. Malar J. 2008;7:162. doi: 10.1186/1475-2875-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giha HA, Rosthoj S, Dodoo D, Hviid L, Satti GM, Scheike T, Arnot DE, Theander TG. The epidemiology of febrile malaria episodes in an area of unstable and seasonal transmission. Trans R Soc Trop Med Hyg. 2000;94:645–651. doi: 10.1016/s0035-9203(00)90218-9. [DOI] [PubMed] [Google Scholar]
- 25.Dicko A, Sagara I, Diemert D, Sogoba M, Niambele MB, Dao A, Dolo G, Yalcouye D, Diallo DA, Saul A, Miller LH, Toure YT, Klion AD, Doumbo OK. Year-to-year variation in the age-specific incidence of clinical malaria in two potential vaccine testing sites in Mali with different levels of malaria transmission intensity. Am J Trop Med Hyg. 2007;77:1028–1033. [PubMed] [Google Scholar]
- 26.Kleinschmidt I, Sharp B. Patterns in age-specific malaria incidence in a population exposed to low levels of malaria transmission intensity. Trop Med Int Health. 2001;6:986–991. doi: 10.1046/j.1365-3156.2001.00817.x. [DOI] [PubMed] [Google Scholar]
- 27.Lindblade KA, Katungu I, Wilson ML. Fever and malaria in highland Uganda. Trans R Soc Trop Med Hyg. 2001;95:502–503. doi: 10.1016/s0035-9203(01)90018-5. [DOI] [PubMed] [Google Scholar]
- 28.Trape JF, Lefebvre-Zante E, Legros F, Druilhe P, Rogier C, Bouganali H, Salem G. Malaria morbidity among children exposed to low seasonal transmission in Dakar, Senegal and its implications for malaria control in tropical Africa. Am J Trop Med Hyg. 1993;48:748–756. doi: 10.4269/ajtmh.1993.48.748. [DOI] [PubMed] [Google Scholar]
- 29.John CC, Riedesel MA, Magak NG, Lindblade KA, Menge DM, Hodges JS, Vulule JM, Akhwale W. Possible interruption of malaria transmission, highland Kenya, 2007–2008. Emerg Infect Dis. 2009;15:1917–1924. doi: 10.3201/eid1512.090627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ernst KC, Lindblade KA, Koech D, Sumba PO, Kuwuor DO, John CC, Wilson ML. Environmental, socio-demographic and behavioural determinants of malaria risk in the western Kenyan highlands: a case-control study. Trop Med Int Health. 2009;14:1258–1265. doi: 10.1111/j.1365-3156.2009.02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.John CC, Moormann AM, Sumba PO, Ofulla AV, Pregibon DC, Kazura JW. Gamma interferon responses to Plasmodium falciparum liver-stage antigen 1 and thrombospondin-related adhesive protein and their relationship to age, transmission intensity, and protection against malaria. Infect Immun. 2004;72:5135–5142. doi: 10.1128/IAI.72.9.5135-5142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drakeley CJ, Carneiro I, Reyburn H, Malima R, Lusingu JP, Cox J, Theander TG, Nkya WM, Lemnge MM, Riley EM. Altitude-dependent and -independent variations in Plasmodium falciparum prevalence in northeastern Tanzania. J Infect Dis. 2005;191:1589–1598. doi: 10.1086/429669. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med. 1999;5:340–343. doi: 10.1038/6560. [DOI] [PubMed] [Google Scholar]
- 34.Sagara I, Sangare D, Dolo G, Guindo A, Sissoko M, Sogoba M, Niambele MB, Yalcoue D, Kaslow DC, Dicko A, Klion AD, Diallo D, Miller LH, Toure Y, Doumbo O. A high malaria reinfection rate in children and young adults living under a low entomological inoculation rate in a periurban area of Bamako, Mali. Am J Trop Med Hyg. 2002;66:310–313. doi: 10.4269/ajtmh.2002.66.310. [DOI] [PubMed] [Google Scholar]
- 35.Menegon M, Severini C, Sannella A, Paglia MG, Sangare D, Abdel-Wahab A, Abdel-Muhsin AA, Babiker H, Walliker D, Alano P. Genotyping of Plasmodium falciparum gametocytes by reverse transcriptase polymerase chain reaction. Mol Biochem Parasitol. 2000;111:153–161. doi: 10.1016/s0166-6851(00)00314-5. [DOI] [PubMed] [Google Scholar]
- 36.Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis. 2005;191:1932–1939. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandler CI, Drakeley CJ, Reyburn H, Carneiro I. The effect of altitude on parasite density case definitions for malaria in northeastern Tanzania. Trop Med Int Health. 2006;11:1178–1184. doi: 10.1111/j.1365-3156.2006.01672.x. [DOI] [PubMed] [Google Scholar]
- 38.Mmbando BP, Lusingu JP, Vestergaard LS, Lemnge MM, Theander TG, Scheike TH. Parasite threshold associated with clinical malaria in areas of different transmission intensities in north eastern Tanzania. BMC Med Res Methodol. 2009;9:75. doi: 10.1186/1471-2288-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farcas GA, Zhong KJ, Lovegrove FE, Graham CM, Kain KC. Evaluation of the Binax NOW ICT test versus polymerase chain reaction and microscopy for the detection of malaria in returned travelers. Am J Trop Med Hyg. 2003;69:589–592. [PubMed] [Google Scholar]
- 40.Mason DP, Kawamoto F, Lin K, Laoboonchai A, Wongsrichanalai C. A comparison of two rapid field immunochromatographic tests to expert microscopy in the diagnosis of malaria. Acta Trop. 2002;82:51–59. doi: 10.1016/s0001-706x(02)00031-1. [DOI] [PubMed] [Google Scholar]
- 41.Ratsimbasoa A, Randriamanantena A, Raherinjafy R, Rasoarilalao N, Menard D. Which malaria rapid test for Madagascar? Field and laboratory evaluation of three tests and expert microscopy of samples from suspected malaria patients in Madagascar. Am J Trop Med Hyg. 2007;76:481–485. [PubMed] [Google Scholar]
- 42.Baird JK. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol Today. 1995;11:105–111. doi: 10.1016/0169-4758(95)80167-7. [DOI] [PubMed] [Google Scholar]