Abstract
Visceral leishmaniasis (VL), caused by Leishmania infantum chagasi (L.i. chagasi syn. infantum) in northeastern Brazil, was responsible for 51,000 new VL cases from 1980 to 2003. Household presence of L. infantum-infected dogs is a major risk factor for human infection. Despite culling of dogs based on seropositivity, canine L. infantum seroprevalence remains near 20%, suggesting that dog culling is ineffective for preventing VL spread. We administered a cross-sectional survey to 224 households within 300 m of the homes of VL human patients diagnosed within the last year. The goal was to develop a model for voluntary preventative use based on characteristics and motivations of dog owners. We identified that owner knowledge deficiencies regarding canine transmission of L. infantum associated with increased risk of dog infection (odds ratio [OR] = 3.681, confidence interval [CI] = 1.223, 11.08). Higher owner education was associated with decreased levels of dog seropositivity (OR = 0.40, CI = 0.20, 0.81). Pet attachment (P = 0.036) and perception of risk/disease knowledge (P = 0.040) were significantly associated with willingness to voluntarily purchase canine VL prevention. These results highlight the importance of owner attachment to their pet in implementing reservoir-targeted zoonotic VL prevention.
Introduction
Rapid urbanization of metropolitan Brazil has resulted in public health challenges, including the re-emergence of visceral leishmaniasis (VL). The city and surrounding areas of Natal in Rio Grande do Norte State (RN) have increased in population from approximately 416,898 in 1980 to 1.3 million in 2007.1 This rapid population growth has resulted in periurban expansion that outpaces available government services and infrastructure.2 The protozoal parasite Leishmania infantum chagasi (L. infantum), the causative agent of VL in Brazil, has developed an urban endemic cycle caused by both the dense population of domestic dogs and acclimation of the vector species Lutzomyia longipalpis to the periurban environment.1,2
Cases of human VL (hVL) in Brazil have remained relatively constant since the year 2000, with an incidence of approximately 2.5–3 cases per 100,000 people.3 Although there have been recent increases in incidence in southern and western states, 60% of hVL cases are reported in the northeastern states of Brazil.1 Within these states are hyperendemic foci with much higher incidence.2 Domestic dogs are considered the predominant reservoir of L. infantum in hyperendemic foci, with canine seroprevalence between 8% and 40%.2,4–6 Ownership of a positive dog causes increased risk of hVL.7,8 Current public health policy in Brazil is aimed at early recognition and treatment of human cases and surveillance and removal of seropositive dogs.9 Euthanasia of positive dogs is considered to have limited efficacy for the following reasons: (1) the significant time between testing and removal of seropositive dogs, (2) a constant influx of naïve animals into the dog population with little reproductive management, and (3) vertical transmission between dam and pups as a means of maintaining canine VL (cVL) within the canine population.10 These factors increase the number of infectious dogs, creating a constant influx of susceptible animals and a means of maintaining infection, even in the absence of a robust vector. As a result, improved methods of VL management, both in terms of efficacy and cost, are needed to address the incidence of VL in humans and dogs in Brazil.
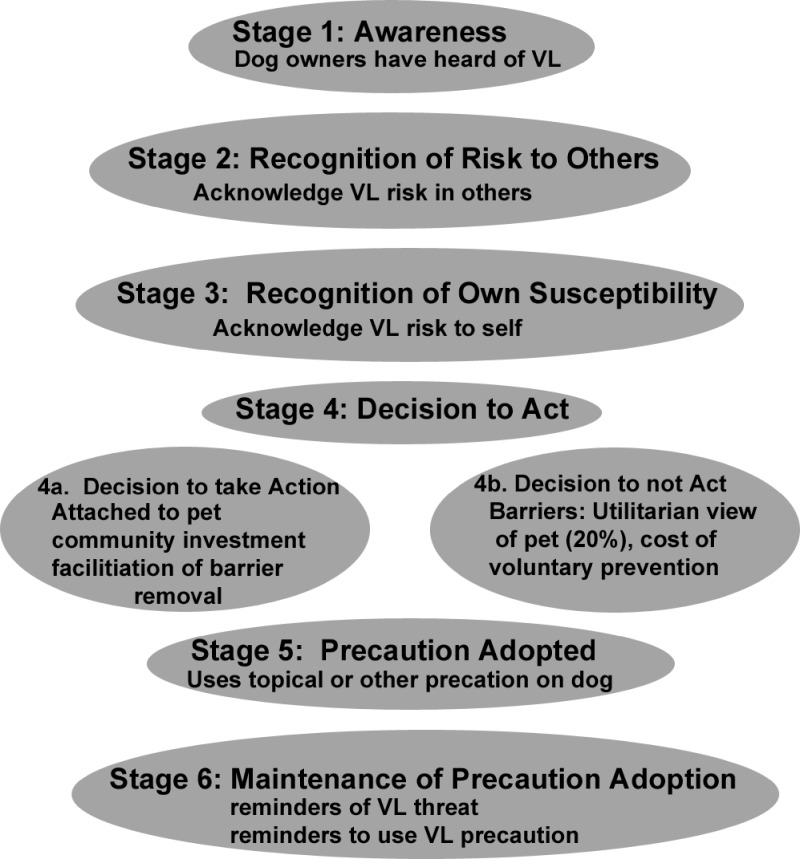
Multiple reports have evaluated the efficacy of topical insecticides in preventing transmission of VL in canine populations.11–14 Our study used precaution adoption process theory to evaluate perception of human risk caused by zoonotic cVL, current use of preventive measures, and likelihood of adopting a voluntary means of prevention. This theory defines stages of precaution adoption. (1) Individual is aware of the problem. (2) Individual recognizes the risk to others. (3) Individual acknowledges personal susceptibility. (4) Individual decides to adopt precaution. (5) Precaution is adopted. (6) Precaution adoption is maintained.15 Many factors impact a decision for precaution adoption, including the severity of risk, perceived likelihood of risk, effectiveness of prevention, and cost of prevention.15
Our working hypothesis was that dog owner attributes and/or risk factors associated with occurrence of cVL and that a model of precaution adoption would provide how these factors associate with owner decision to act on cVL prevention. This study used a cross-sectional household survey of demographic factors and social factors and novel assessment of Brazilian pet–owner attachment to determine the social role of pets in periurban Natal and the understanding of owners regarding dogs serving as a reservoir in hVL. We evaluated the motivational effect of these variables to voluntary purchase of prevention. Results of this survey will be immediately useful for health promotion efforts and zoonotic VL control in this area. This study is the first to apply the precaution adoption theory to zoonoses prevention.
Materials and Methods
Study area.
The geographic area for this study population was a 1.9 × 1.9-km area in Zona Norte, Natal, RN, Brazil. This area consists of three large neighborhoods, with a total of approximately 4,000 households based on previously conducted surveillance.16 There were approximately 1,000 dog-owning households within the study area. This area was selected, because it showed a high prevalence of both cVL and hVL.8 Enrollment and interviews were conducted concurrently over a 3-month period from May to August of 2010.
Sample size determination.
We targeted 225 households for the survey based on sample size calculation given an estimated baseline seroprevalence of 17.5% and a goal of detecting a relative risk of 2.0 (α = 0.05, power = 0.80) based on the effect of a single factor using the Fleiss sample size calculation with continuity correction. This power calculation suggested a sample size of at least 220 households.
Participants.
There were three eligibility criteria for study participants. (1) Individuals were the adult owner of a dog and the primary decision-maker for the dog. (2) The household in which the owner and dog resided was within 300 m of a recent human case (based on all known clinical human cases 12 months before study initiation). (3) Participates volunteered and gave informed consent. Households were identified through routine surveillance by the Center for Zoonoses Control in Natal. After collection of diagnostic samples from household dogs and before release of results, potential study participants were contacted in person by our university research team and informed about the content and goals of the survey. After informed consent was obtained, the survey was conducted over a 15- to 30-minute time period by trained, native Brazilian interviewers.
Survey instrument: Demographics.
The first section of the survey was directed at obtaining demographic data of both the human and dog residents of the household. This data allowed the general characterization of the pet-owning population based on factors including age, employment, sex, income, and education.
Survey instrument: Perception of risk.
This section contained three different parts, and each part was given equal (one-third) weight in the 10-point overall score: (1) perceived risk of activities and practices to acquire VL (maximum score contribution = 3.33), (2) knowledge of VL transmission and cVL clinical signs (maximum score contribution = 3.33), and (3) canine veterinary care and preventative use (maximum score contribution = 3.33). For the first part, a series of seven questions was posed regarding perceived risk of certain common activities or practices and rated as follows: I don't know, no risk, some risk, or high risk for hVL. The second part consisted of two open-ended questions: “what causes VL in people?” and “what does a dog with VL look like?” Answers to the open-ended question regarding human transmission were scored as follows: identification of the full parasite life cycle = 2, either the dog as the reservoir or the sandfly having a role in transmission = 1, and neither the dog nor the sandfly mentioned = 0. Answers to the open-ended question regarding signs of cVL were scored from zero to five, with one point awarded for each accurate clinical sign listed, including weight loss, anorexia, skin lesions, keratoconjunctivits, and excessive nail growth. For the third part, questions were asked regarding the pets' current level of veterinary care, if canine preventives were used, and the type of prevention. Veterinary care was scored as yes for any visit to a licensed veterinarian in the last year for any dog within the household. Any stated use of prevention was scored as yes, ranging from current use of permethrin to keeping animals away from other dogs on the street. These questions were scored as yes = 1 and no = 0. After equal weighting (33.33% contribution per section), scores were normalized to a 10-point risk and knowledge score, with 10 being the highest level of knowledge, accurately perceived risk, and usage of veterinary care and VL prevention and 0 being the lowest level, for statistical analysis.
Survey instrument: Lexington Attachment to Pets Scale.
We used a modified and translated version of the standardized Lexington Attachment to Pets Scale (LAPS).17 The LAPS questionnaire was reduced from 23 to 14 questions and translated to develop a culturally appropriate and easily comprehensible section. Answers were scored in a standardized manner as previously published.17 Self-reported health and self-reported happiness were scored with a five-point scale, with one being the worst and five being the best. These scores were compared with LAPS using univariate linear regression as described below.
Survey instrument: Willingness to pay for cVL preventative.
We asked one open-ended question regarding willingness to pay for VL preventative: “how much would you be willing to pay for cVL prevention per pet per month for the duration of your pet's life?” We arbitrarily broke this continuous data into quintiles for statistical analysis, because it was not normally distributed.
Survey translation was initially performed at the Iowa State University College of Veterinary Medicine by a native Brazilian and modified and translated by multiple individuals in Brazil fluent in both English and Portuguese. This iteration of the survey was then reverse-translated to ensure that the emphasis of the survey had not been altered because of translation, and it was approved by the Institutional Review Boards (IRBs) of Iowa State University and the Federal University of Rio Grande do Norte (UFRN). The survey was then tested in 50 households (one to three interviews at a time) to address any issues of comprehension, clarity, or sensitivity.
Interviewer training.
All surveys were conducted in spoken interview format because of the limited literacy of the target population, with the desire to obtain both a quantitative assessment and a qualitative understanding of the perception of the target population regarding cVL and the need to develop increased rapport within the target community. Interviewers were selected from the Department of Biochemistry at UFRN. All interviewers had extensive experience with VL and the dynamics of infectious disease in the community and had extensive field research and interview experience specific to VL. An initial 3-hour training session was conducted, during which time each question was evaluated thoroughly, interviewer questions were addressed, and methods and manner of interviewing were discussed. Each interviewer traveled with a second individual researcher to manage data collection. Two initial days of interviews were conducted with a discussion session after interviewing to ensure consistency in survey implementation and answer any additional questions by interviewers on full implementation.
Specimen collection and sample processing.
Samples were collected from dogs by veterinarians from either Iowa State University or Center for Zoonoses Prevention, Municipality of Natal, RN, Brazil. Between 3 and 6 mL whole blood were collected into (ethylenedinitrilo)tetraacetic acid (EDTA) collection tubes (BD, Franklin Lakes, NJ) from each dog. Whole blood was separated by centrifugation at 2,500 × g for 10 minutes. Serum was collected into one tube. Sera were transported within 24 hours from the Center for Zoonoses Control to the Immunogenetics laboratory at UFRN for serologic testing. Protection of experimental animals complies with the National Institutes of Health guidelines for the humane use of animals as approved by the Institutional Animal Care and Use Committee (IACUC) of Iowa State University and the UFRN Committee for the Ethical Use of Animals (CEUA).
Leishmania-specific enzyme-linked immunosorbent assay.
Soluble leishmania antigen (SLA) and K39 antigen enzyme-linked immunosorbent assay (ELISA) procedures were performed as previously described.18 Microassay plates (Costar, Cambridge, MA) were coated overnight at 4°C with L. infantum (MWHO/BR/00/L669) promastigote lysate at a concentration of 200 ng/well or K39 antigen (Infectious Disease Research Institute, Seattle, WA).18 Cutoff values were determined based on the mean absorbance of control sera plus 3 SDs. Each sample was analyzed in triplicate with negative (endemic healthy negative controls) and positive (known L. infantum-infected dog sera) controls on each plate. Mean of triplicate readings were compared against the established cutoff value for each plate to determine serostatus. Discordant samples were labeled as questionable and rerun with remaining serum. Comparisons with serostatus were based on a subcohort of tested dogs (N = 193).
Data analysis.
Data from this study were compiled using an EpiInfo database (Centers for Disease Control and Prevention, Atlanta, GA). For comparison of risk factors, SLA ELISA-positive and -negative households were compared using the household as the unit of analysis. Education levels were categorized into those individuals having a secondary education or higher and those individuals having a primary education or lower. Univariate analysis to determine association of dependent (household dog seropositivity) and independent variables was completed by a Fisher exact test at 95% confidence using GraphPad Prism version 5 for Windows (GraphPad Software, La Jolla, CA). Univariate logistic regression was used to determine associations between independent variables and LAPS score. Multivariable logistic regression analysis was completed using household canine seropositivity as the response variable and all major demographic variables and results from the risk and knowledge and LAPS survey sections as the explanatory variables (EpiInfo). The lowest income and education categories were used as referent variables in both logistic and linear regression models. Linear regression analysis was completed using 20 Reais ($11.25 USD) increments as the amount people were willing to pay monthly for cVL prevention as the response variable (EpiInfo). Willingness to pay analysis encompassed all independent variables within the survey. Odds ratios (ORs) were considered significant at a value of P < 0.05. Fit of the multivariate model was assessed by χ2 likelihood ratio analysis and graphic analysis of residual plots. Regression analysis was based on a subcohort of interviewees having results for every variable within the univariate and multivariate models (N = 144).
Results
This pet–owner cross-sectional observational study evaluated the relationship between VL knowledge, sociodemographic factors, attachment of owners to their pets, presence of cVL in the household, and how these factors impacted willingness of the community to voluntarily adopt prevention in addition to the current culling program. We constructed a survey tool from which a pragmatic understanding of this Brazilian pet-owning community could be gained, specifically regarding potential adoption of improved health intervention for cVL and hVL. Serologic status of dogs in the study was primarily determined by SLA and confirmed by K39 ELISA. The overall canine seropositivity rates were 26.2% for SLA and 11% for K39.
Demographic data.
Demographic data were collected on the household, the primary dog caregiver, and the dogs themselves. The sex of canine caretakers was primarily female (73.5%; 163) (Tables 1 and 2). Employment statistics for the study population indicated an unemployment rate of 16.3% (N = 36); 34.4% of participants were fully or partially employed (Tables 1 and 2), and 32.1% (N = 71) of participants indicated that they worked in the home (e.g., were stay at home parents) (Tables 1 and 2).
Table 1.
Univariate model of social variables associated with cVL
| Demographic variable | Study participants | Percent canine household seropositivity (n/total) | OR | 95% CI (low, high) | P value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 26.58 (59) | 32.10 (18/56) | 1.00 | ||
| Female | 73.42 (163) | 41.50 (54/130) | 1.50 | 0.78, 2.90 | 0.254 |
| Total | 222 | 186 | |||
| Age (years) | |||||
| < 30 | 26.85 (58) | 20.41 (10/49) | 1.00 | ||
| 30–45 | 38.43 (83) | 39.20 (29/74) | 2.51 | 1.01, 5.81 | 0.031* |
| 46–60 | 22.69 (49) | 54.29 (19/35) | 4.63 | 1.77, 12.12 | 0.002† |
| > 60 | 12.04 (26) | 40.91 (9/22) | 2.70 | 0.90, 8.10 | 0.087 |
| Total | 216 | 180 | |||
| Education | |||||
| Primary education or less | 47.27 (104) | 51.00 (42/83) | 1.00 | ||
| Secondary education or more | 52.73 (116) | 29.00 (30/102) | 0.407 | 0.22, 0.75 | 0.004† |
| Total | 220 | 185 | |||
| Employment | |||||
| Employed | 34.55 (76) | 38.02 (27/71) | 1.00 | ||
| Student | 6.82 (15) | 9.09 (1/11) | 0.16 | 0.02, 1.35 | 0.088 |
| Works in home | 32.27 (71) | 55.77 (29/52) | 2.05 | 0.99, 4.25 | 0.067 |
| Retired | 10.00 (22) | 26.32 (5/19) | 0.59 | 0.19, 1.78 | 0.425 |
| Unemployed | 16.36 (36) | 32.26 (10/31) | 0.78 | 0.32, 1.90 | 0.658 |
| Total | 220 | 184 | |||
| Income* (monthly minimum wages) | |||||
| < 2 | 53.30 (120) | 37.76 (37/98) | 1.00 | ||
| 2–3 | 37.80 (85) | 36.76 (25/68) | 0.96 | 0.51, 1.82 | 1.00 |
| > 4 | 8.80 (20) | 52.635 (10/19) | 1.83 | 0.68, 4.93 | 0.307 |
| Total | 225 | 185 | |||
Calculations were based on households with any seropositive dogs. ORs, CIs, and P values correspond to Fisher exact test calculations (GraphPad Software, La Jolla, CA) between categories. Answers to all questions were voluntary based on informed consent of the interviewee and approved by IRBs.
P < 0.05.
P < 0.01.
Table 2.
Univariate model of owner responses associated with cVL
| Demographic variable | Mean (SD) | Percent canine household seropositivity (n/total) | OR | 95% CI (low, high) | P value |
|---|---|---|---|---|---|
| Risk and knowledge score | 4.99 (1.57) | 38.91 (72/185) | 1.13 | 0.93, 1.37 | 0.217 |
| LAPS pet attachment score | 29.86 (6.27) | 38.71 (72/186) | 1.02 | 0.97, 1.08 | 0.416 |
| Monthly amount willing to pay for prevention | 38.06 (31.34) | 35.33 (53/150) | 1.01 | 0.99, 1.02 | 0.190 |
Calculations were based on households with any seropositive dogs. ORs, CIs, and P values correspond to univariate logistic regression for continuous variables. Answers to all questions were voluntary based on informed consent of the interviewee and approved by IRBs.
In the study population, 47% (N = 104) of respondents had less than a primary education, with 47.5% (N = 105) having some secondary education and only 5% (N = 11) having completed any college education (Tables 1 and 2). Compared with education, income was more evenly distributed. The minimum monthly salary in Brazil, equivalent to $287 (510 Reais [R]), is a commonly used measure of income, and it was the 2009 minimum monthly salary guaranteed to workers by law. This salary was equivalent to approximately $1.80/hour based on a 40-hour work week. There were 1.7% (N = 4) of households with income four times greater than the minimum monthly salary (2,142 R; $1,203), and 53.3% (N = 120) of respondents earned less than two minimum monthly salaries (1,120 R; $628) per month (Tables 1 and 2).
Univariate analysis of survey questions compared with the household canine seropositivity rate shows significantly increased likelihood of household canine seropositivity between age groups from 30–45 and 46–60 years (OR = 2.51, 95% confidence interval [CI] = 1.01, 5.81; OR = 4.63, 95% CI = 1.77, 12.12, respectively) (Table 1). We also evaluated the years of ownership of the dog and found no statistical difference in seroprevalence between groups (P = 0.226). The average number of years of dog ownership in this cohort was 3 years and 2 months, reflecting ongoing testing and dog culling and the overall short canine lifespan. Households of dog owners with a primary education or lower were significantly more likely to own a seropositive dog (OR = 0.407, 95% CI = 0.22, 0.75) (Table 1). A high percentage of the study population had low levels of formal education, which was significantly associated with canine seropositivity in the univariate analysis, indicating the impact of education and disease knowledge on VL dynamics.
Disease knowledge and perceived risk.
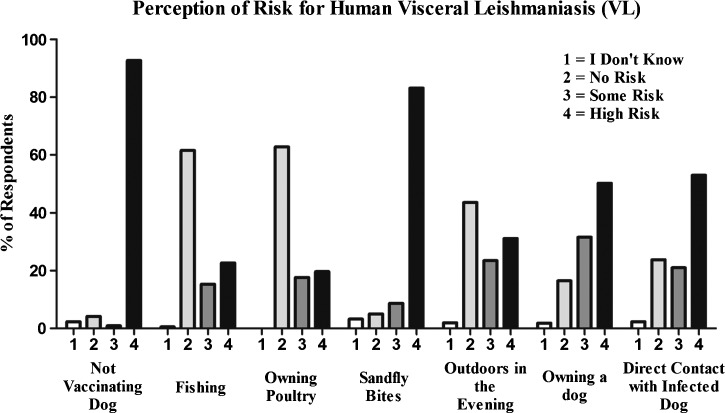
Targeting precaution adoption for VL prevention required a comprehensive understanding of the current knowledge, perceived risk, and prevention-seeking attributes of the study population. We first posed a series of questions to gauge the perception of multiple risk factors previously shown to be associated with hVL and some activities with potential but unknown risk (Figure 1)19–21; 224 respondents were asked to categorize typical activities and factors as having no risk, some risk, or high risk for the development of hVL. We found that members of the community possessed basic knowledge regarding VL transmission. In fact, 83.2% of participants stated that sandfly bites were high risk for acquiring hVL, indicating that in this regard, Ministry of Health health promotion efforts were successful. The risk of infected pet dogs in hVL was less well-known, with 50.2% of respondents claiming dog ownership as a high risk and 53% claiming contact with dogs as a high risk (Figure 1). Although no response in this section was significantly associated with canine seropositivity, respondents who indicated that they did not know if sandfly bites increased the risk of hVL were more likely to own an L. infantum-positive canine (OR = 3.829, CI = 0.82, 17.88, P = 0.088). Only 12 people did not know that sandflies transmit hVL, resulting in reduced statistical power. Knowledge of the role of sandflies in hVL transmission is high within the surveyed community; however, the role of the dog as a primary domestic reservoir for hVL and a means of transmission between dogs and humans is not well-understood within the community.
Figure 1.
Knowledge and risk perception related to the transmission of L. i. chagasi in periurban Brazil. Participants were asked to rate the risk of seven actions or occurrences as complete lack of knowledge (1; e.g., “I don't know”), no risk (2), some risk (3), or high risk (4). The y axis represents the percentage of respondent responses.
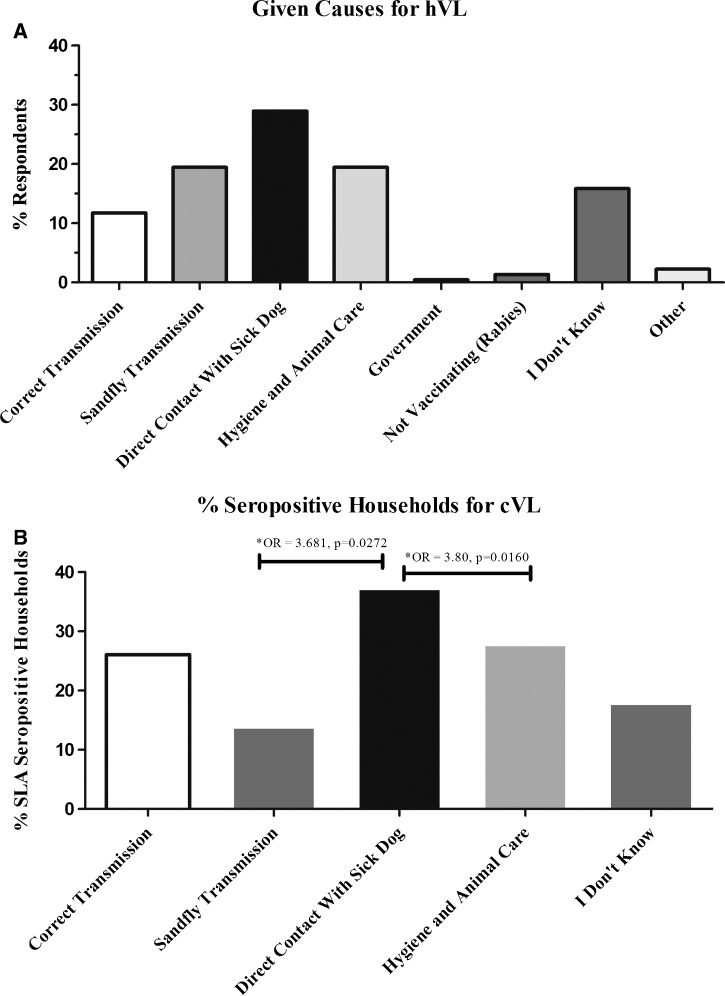
To better understand specific deficiencies in knowledge regarding hVL transmission, we asked open-ended questions regarding how humans become infected with L. infantum. When asked how people get hVL from dogs, the most common answers were (1) direct contact with a sick dog (28.96%), 2) being bitten by an infected sandfly (19.46%), and 3) care of your animals and hygiene (19.46%) (Figure 2A). Only 11.77% of respondents identified the complete endemic cycle of L. infantum with canine reservoir and sandfly transmission (Figure 2A). Many respondents (28.96%) indicated that transmission between dogs and humans was primarily through direct contact, which was significantly associated with increased risk for cVL compared with those respondents indicating the sandfly (OR = 3.681, CI = 1.223, 11.08, P = 0.0272) and those respondents indicating pet care and hygiene (OR = 3.800, CI = 1.264, 11.42, P = 0.0160) (Figure 2B). Respondents stating the major means of hVL transmission as the sandfly or hygiene were more likely to use some means of prevention for cVL (81% and 80%, respectively) compared with 56% of those respondents stating direct contact with dogs as the major means of human infection. Dog owners in general did recognize VL as a health threat and were willing to actively seek prevention, with 63.5% of respondents using some stated means of cVL prevention (data not shown). To understand precaution adoption within dog owners, we needed to evaluate other possible motivators in addition to knowledge of VL transmission and human risk. With the high rate of cVL in the study community as well as surveillance and euthanasia of positive dogs, we next considered the desire to protect household pets as a motivator for precaution adoption.
Figure 2.
Misperceptions about L. infantum transmission are associated with increased risk for cVL. (A) Percentage responding to “how do people get hVL?” (open-ended question). Responses were categorically sorted into groups (x axis) and are presented as the percentage of total responses. An answer of correct transmission was one indicating that sandfly feeding on infected canines and then on humans was a primary means of hVL (B). Categorical responses from A were compared with SLA ELISA seropositivity by contingency tables and Fisher exact tests for relative risk.
LAPS pet attachment scale of community pet owners.
LAPS was first developed and used in 1992, and it has since become the standard measurement of owner/pet attachment and animal welfare.17 We developed a modified and translated version of this survey tool. Based on this abbreviated survey tool, the mean community pet attachment score was 29.86 compared with the average LAPS score for equivalent questions in a randomized sample in the United States of 30.83.17 Multiple social variables were significantly associated with pet attachment, indicating the role of the household pet in owner social and emotional support. We designed a linear regression model of LAPS scores for comparison with multiple other continuous and categorical variables within the survey. We discovered that increasing pet attachment scores were significantly associated with increasing age (0.493 LAPs units/year of owner age, P = 0.0023), and increasing pet attachment was inversely associated with self-reported happiness (−0.015 LAPS units/category of self-reported happiness, P = 0.043). Pet attachment in the community was similar to the pet attachment found in the United States, and dogs play a significant social role of support and attachment in our survey area.
Social factors, cVL, and precaution adoption.
After the completion of all survey interviews, we had data in our three realms of interest, including (1) demographic data, (2) risk perception and current precaution adoption, and (3) pet attachment. We used a multivariate model to evaluate risk factors for VL positivity in an owner's pet based on interview data compared with SLA-based indirect ELISA and K39 indirect ELISA results (likelihood ratio statistic = 18.28, P < 0.01, degrees of freedom [df] = 6, N = 144) (Table 3). Households with a cVL-positive pet in the previous 2 years were classified as cVL-positive. Education level of the pet owner was significantly associated with reduced cVL seropositivity (OR = 0.40, CI = 0.198, 0.808, P = 0.0106). Owner income was not significantly associated with lower cVL seropositivity, and in fact, it was associated with increased likelihood of owning a seropositive canine. Education and income in this population were only weakly associated (r = 0.23, P < 0.001). Increased social networks of close friends and family were also associated with lower canine seropositivity (OR = 0.36, CI = 0.1429, 0.9063, P = 0.0301; data not shown). This demographic data, as well as data from previous sections, including an understanding of human risk, knowledge of VL transmission, and pet attachment levels, allowed us to evaluate these factors as motivators for the voluntary adoption of dog-targeted VL prevention.
Table 3.
Multivariate model of social variables associated with cVL
| Term | OR | 95% CI | Coefficient | SE | P value | |
|---|---|---|---|---|---|---|
| Low | High | |||||
| Owner age | 1.02 | 0.99 | 1.05 | 0.02 | 0.01 | 0.133 |
| Education category | 0.40 | 0.20 | 0.81 | −0.92 | 0.36 | 0.011* |
| Income (per monthly minimum wage) | 1.64 | 1.03 | 2.61 | 0.50 | 0.24 | 0.037* |
| Monthly amount willing to pay for cVL prevention (R/month) | 1.01 | 0.99 | 1.02 | 0.01 | 0.01 | 0.321 |
| LAPS (pet attachment units) | 0.99 | 0.93 | 1.06 | −0.01 | 0.03 | 0.813 |
| Risk and knowledge score (1–10) | 1.09 | 0.84 | 1.43 | 0.09 | 0.14 | 0.506 |
The unconditional multivariate logistic regression model incorporates all components from the univariate analysis and shows that education remains associated with lower canine seropositivity after adjustment for other model variables. All categorical variables use the lowest category as the referent (i.e., no education, less than one minimum wage). Multivariate model statistics: likelihood ratio test = 18.28, P = 0.0056, df = 6, N = 144.
P < 0.05.
To best assess the potential of a theoretical public health intervention, we assessed the willingness of the community to purchase an additional means of preventing cVL. We asked the following question: “if you had the option of purchasing an efficacious means to prevent VL in your pet that would be applied once a month, how much per month would you be willing to spend on this product per pet for the duration of the pet's life?” The mean amount per month that the individual pet owners were willing to pay was 37.28 R (median = 30 R; equivalent to $20.46 and $16.46, respectively). Only 13.6% of respondents indicated that they would not spend anything on prevention of cVL. To determine what factors motivated 84.4% of individuals to decide to adopt precaution (stage 4 of the precaution adoption model), we used a multivariate linear regression model with responses to the willingness to spend on prevention question as the dependent variable and all survey variables as the explanatory variables (Table 4) (R2 = 0.06, F(4, 146) = 2.272). Two variables were significantly positively associated with amount per month, grouped in 20 Reais increments, that individual pet owners were willing to pay: LAPS pet attachment score (β = 0.04 [0.003, 0.078], P = 0.036) and the risk and knowledge Score (β = 0.17 [0.005, 0.325], P = 0.043). This association indicated that increased pet attachment and knowledge of cVL transmission motivated owners to the adoption of prevention. It is likely, based on this data, that individual attachment to pets is a primary driving force in this community for adoption of an effective preventive in concert with the acknowledgment of risk and an understanding of L. infantum transmission.
Table 4.
Linear model of voluntary monthly expenditure (Reais) for cVL prevention
| Variable | Coefficient | 95% CI | F test | P value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Education | −0.11 | −0.347 | 0.119 | 0.37 | 0.0547 |
| Income (per monthly minimum wage) | −0.02 | −0.292 | 0.254 | 0.02 | 0.892 |
| LAPS (pet attachment units) | 0.04 | 0.003 | 0.078 | 4.931 | 0.036* |
| Risk and knowledge score (1–10) | 0.17 | 0.005 | 0.325 | 4.547 | 0.043* |
A multivariate linear regression model was constructed with monthly amount willing to pay for prevention as the dependent variable. A positive coefficient indicated a larger willingness to spend on preventative associated with increased explanatory variable (monetary units = 20 Reais increments). Model statistics: r2 = 0.06, df = 150, mean square = 4.366, F statistic = 2.272.
P < 0.05.
Discussion
VL is a zoonotic disease of significant importance in Brazil, with an estimated 500,000 cases worldwide annually.22 In Brazil, dogs are a main domestic reservoir for hVL.23,24 The VL Control Program (PCLV) was instituted in metropolitan areas of Brazil in 1994 after an epidemic of hVL in periurban favelas.9 The stated mission of the PCLV is to “reduce the case-fatality rates, degree of morbidity, and the transmission level of leishmaniasis.” To reduce reservoirs for human disease, the PCLV instituted a policy of canine serologic surveillance and culling.9 This approach has been minimally effective, because seropositivity within dogs remains over 20% in many areas of Brazil.9 High loss to follow-up, influx of naïve animals, and vertical transmission also complicate transmission dynamics of L. infantum.3,9,10 Additionally, owner participation with this voluntary testing is paramount for successful control. There is a need for effective means of VL control and improved adoption of this prevention by hyperendemic communities.
This study evaluates pet owners' social, demographic, and precaution adoption factors that influence canine infection with L. infantum and VL control and prevention. The Precaution Adoption Process, developed in the work by Weinstein15 in 1988, is a dynamic model staging precaution adoption into six stages from being unaware of a hazard to adopting and maintaining a prevention program (Figure 3). Our hypothesis was that use of the precaution adoption model to parse out pet owners' knowledge of VL and their approach to pet care and attachment indicates critical control points or intervention targets for reducing incidence of cVL and hVL. This study is the first to evaluate effects of perception and precaution adoption factors regarding a zoonotic disease (e.g., VL).
Figure 3.
The precaution adoption model created for canine-focused zoonotic VL control.
Communities and neighborhoods considered hyperendemic foci for cVL and hVL are often disadvantaged. Both income and education were low in the population surveyed for this study compared with national averages in Brazil. Previous research has characterized income, housing type, dog ownership, population growth index, and illiteracy rates as risk factors for cVL and hVL.8,19,20,25 In addition, comorbidities and increased likelihood of immune suppression within disadvantaged populations are significant risk factors for VL, including malnutrition, parasitism, aging, immune senescence, concurrent immunosuppressive therapy, and other immunosuppressive coinfections.8,19,25,26
Not surprisingly, misconceptions about transmission dynamics of L. infantum between dogs, sandflies, and people resulted in an increased risk for canine infection within the household; 83.2% of respondents indicated that sandfly bites led to increased risk for hVL. However, when asked how people are infected with VL from dogs, the most common answer was through direct contact with the dog (28.96%). Direct contact respondents were significantly more likely to own a positive dog than people who identified the sandfly as a primary means of transmission (Figure 2B). This data represent a basic understanding of sandfly transmission but a lack of understanding of the role of dogs as a domestic reservoir for hVL. Using the precaution adoption process, this finding indicates that a very large majority of the population had basic knowledge of VL and understood general risks to others (94.6%; precaution adoption stage 2) (Figure 3), and approximately 83.2% of the population understood individual risk (stage 3) (Figure 3); however, there was not a complete understanding of how this disease is maintained in the local environment, a factor particularly important to understand with zoonotic diseases. Targeted educational interventions directed to the importance of the sandfly in cVL and emphasizing the use of topical insecticides and management of the domestic environment could significantly impact the prevalence of VL in the target community. As with many communicable diseases with a behavioral component,27–30 improving literacy and educational development in disadvantaged communities will also benefit animal health, reducing the risk of zoonotic diseases.
Control of disease in reservoir animals is critical for control of zoonotic parasitic diseases.24 The Ministry of Health in Brazil has developed ongoing efforts to test and cull seropositive dogs in hyperendemic foci to prevent canine to human transmission of L. infantum.1,2,6 Our results indicated that dog owners in this area of Brazil harbored a similar attachment to their pets as dog owners in other areas of the developed world. Because of this attachment, testing and euthanasia may not be an optimal, let alone palatable, control strategy. Owner attachment to pet dogs negatively impacts voluntary participation in test and cull prevention programs. This study highlights the importance of disease knowledge in voluntary precaution adoption, including the use of precautions like insecticide baths, general pet care, and management of the household environment. Health promotion targeting gaps in knowledge and basic VL prevention based on a community model of precaution adoption could significantly reduce canine and human infections. Similar community-wide interventions have increased bed net usage for prevention of malaria in multiple communities.29,30
In this cross-sectional cohort, the level of pet owner education attained was significantly associated with reduced canine infection with L. infantum. This association very likely has a multifactorial effect, including maintenance of the peridomestic environment, knowledge about disease transmission, and prevention-seeking behavior. This finding is consistent with previous VL demographic studies.19 Contrary to multiple other studies, income did not show a significant association with reduced seropositivity for cVL.19,31 Although there were differences in education (between primary and secondary education levels), the economic status of our study population was largely homogenous. Additionally, this study was of limited size, and it may not have had enough power to detect more subtle economic effects among pet owners. A high percentage of interviewees were female in this study. Study participants indicated their primary role as the decision-maker regarding the pet. Thus, in a practical sense, the oversampling of females in the study accurately portrays the population making canine healthcare decisions and the target audience for potential canine-targeted VL intervention.
The human–animal bond has been recognized as beneficial to human health in multiple studies.17,32 Our study indicates that pet attachment levels in this community are very similar to the levels shown previously in the United States.17,32 This high level of pet attachment was not expected, because we expected a more utilitarian view of dogs in the household. We identified that, for our pet owner cohort, pets play a role of emotional and social support in the elderly population and those individuals without extensive support networks, because increased pet attachment was associated with increased owner age and lower self-reported happiness. Because our goal with this study was to obtain quantitative and qualitative understanding of the role of dogs in the community, interviewers asked open-ended questions about the role of pets in the home. These qualitative discussions supported the emotional importance of household dogs in the community. Together, these data indicate that pet owners in this community harbor significant attachment to their pets. This finding highlights a need for alternative means of prevention for cVL to reduce the necessity for dog culling, for the PCLV mission to be successful. Pet attachment may be a large factor in dog owners' decisions to act on precaution adoption (e.g., using a means to prevent cVL) but also keep their pets alive and well.
Multiple studies have evaluated the efficacy of monthly insecticide treatments and vaccination in preventing cVL.11–14,33–38 Although there is debate regarding their efficacy, it is likely that extensive community adoption of a topical pet insecticide would significantly reduce the incidence of cVL and therefore, hVL.11 We asked about possible voluntary purchase of a hypothetically effective topical preventive for cVL. We discovered that pet owners in this community would voluntarily purchase a monthly therapy for cVL and that a large majority (84.4%) would spend at least 20 R ($11.25 USD) per month. Willingness to adopt preventive therapy was significantly associated with high owner–pet attachment levels and high risk perception and knowledge score. This finding indicates that the desire to protect pet well-being and understanding the risk of cVL for human disease were significant motivators for stage 4 of precaution adoption (a decision to act). From a public health perspective, these associations are significant, because the goal of protecting human health is perhaps, in this case, most easily accomplished through the desire of owners to protect their pets.
The cross-sectional data contained in this study are a snapshot in time of the current population of this hyperendemic focus. Given the degree of dog movement, dog culling, owner movement, and funding constraints, we used this study to gain a cursory understanding of the study population with the goal of improving local VL prevention efforts. Specific data can be distinctly limited with this cross-sectional approach, and disease causation cannot be presumed. Our primary goal was to determine factors motivating individuals to adopt proposed precautions for VL, including the voluntary expenditure of money for topical pet insecticides.
The precaution adoption process model was created as a model to explain, predict, and perhaps, even encourage deliberate action to healthy behavior. The goal of using this type of model in disease prevention is to understand the decision-making process, how that process is conducted, how these decisions translate into deliberate action, and long-term maintenance of those actions. Our goal with this survey was to create a model of precaution adoption for this Brazilian community focused on dogs as the major domestic reservoir of L. infantum (Figure 3). Through an understanding of the decision-making process, such as the decision to adopt specific means of cVL prevention or the decision to consent for voluntary testing, the Center for Zoonoses Control will be able to address barriers to and promote facilitators of VL prevention. We found facilitating factors for the decision to adopt precaution (stage 4b) (Figure 3), including pet attachment, knowledge of VL, and perception of VL risk. Barriers to the adoption of precaution based on our study and interaction in the community include the cost of prevention, poor understanding of VL transmission or necessary effective prevention, low perception of risk (53% indicated dog as a risk for VL), and prioritizing individual efforts to more immediate health threats. Based on our data, the objectives of health prevention efforts should be to (1) identify effective means of prevention (something that has yet to be definitively determined), (2) target resources of health promotion to current misconceptions about household pets as the reservoir for hVL, (3) subsidize implementation of effective prevention, and (4) encourage and subsidize community programs to promote pet health, increasing the longevity of the household pet and further increasing pet attachment. The third objective could be accomplished with just individual participation, because a high percentage of the population is willing to bear some of the cost of VL prevention. Additional global objectives to target factors associated with reduced seroprevalence are to increase the level of general education, income, and community and individual social capital. These community investments gradually improve the microenvironment, including the quality of housing, quality of water storage, and cleanliness of the domestic environment.
This study serves as an example of the complex meshwork of community, including animals owned as pets, and how these components affect prevention of vector-borne zoonotic diseases. This study shows that alternative public health policies aimed at the prevention of hVL through the prevention of cVL, which target barriers to precaution adoption and facilitate adoption using subsidized preventatives in addition to continued surveillance, would be perceived more positively within the community, would be more effective in reducing hVL, and may be less costly than the current test and cull program.
ACKNOWLEDGMENTS
The authors thank the study community, including concerned pet owners. We thank the Center for Zoonoses Control and the Rio Grande do Norte Ministry of Health for their commitment and efforts in disease control and significant contributions to this study. We also thank the Immunogenetics laboratory staff at the Federal University of Rio Grande do Norte for assisting as trained interviewers and troubleshooting and survey development. The authors thank Doug E. Jones for conversations regarding the epidemiology of visceral leishmaniasis and its control.
Footnotes
Financial support: This project was funded through the Pfizer Animal Health/Morris Animal Foundation Veterinary Fellowship for Advanced Degrees, the NEWAID Foundation Fellowship for Research in Neglected Tropical Disease, and National Institutes of Health Grant 1 R21 AI088051-01.
Authors' addresses: Kevin J. Esch, Iowa State University, Veterinary Pathology, Ames, IA, E-mail: kjesch@iastate.edu. Nubia N. Pontes and Selma M. B. Jeronimo, Universidad Federal de Rio de Norte, Biochemistry, Natal, Brazil, E-mails: np12@ufrn.br and smbj@ufrn.br. Paulo Arruda and Annette O'Connor, Iowa State University, Veterinary Diagnostics and Preventative Medicine, Ames, IA, E-mails: paulohea@iastate.edu and oconnor@iastate.edu. Lorena Morais, Department of Public Health, Centro do Zoonoses, Natal, Brazil, E-mail: morrais.lorena@cz.rn.bz. Christine A. Petersen, Iowa State University, Ames, IA, E-mail: kalicat@iastate.edu.
References
- 1.Werneck GL. Forum: geographic spread and urbanization of visceral leishmaniasis in Brazil. Introduction. Cad Saude Publica. 2008;24:2937–2940. doi: 10.1590/s0102-311x2008001200023. [DOI] [PubMed] [Google Scholar]
- 2.Romero GA, Boelaert M. Control of visceral leishmaniasis in Latin America—a systematic review. PLoS Negl Trop Dis. 2010;4:e584. doi: 10.1371/journal.pntd.0000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barreto ML, Teixeira MG, Bastos FI, Ximenes RA, Barata RB, Rodrigues LC. Successes and failures in the control of infectious diseases in Brazil: social and environmental context, policies, interventions, and research needs. Lancet. 2011;377:1877–1889. doi: 10.1016/S0140-6736(11)60202-X. [DOI] [PubMed] [Google Scholar]
- 4.Tasca KI, Buzetti WA, Tenorio Mda S, Paulan Sde C, Lima FL, de Queiroz NM, Machado RZ, Oliveira TM, Neves MF, de Noronha AC, Jr, de Assis J. Parasitological, immunohistochemical and histopathological study for Leishmania chagasi detection in splenic tissues of dogs with visceral leishmaniasis. Rev Bras Parasitol Vet. 2009;18:27–33. doi: 10.4322/rbpv.01801005. [DOI] [PubMed] [Google Scholar]
- 5.Queiroz PV, Monteiro GR, Macedo VP, Rocha MA, Batista LM, Queiroz JW, Jeronimo SM, Ximenes MF. Canine visceral leishmaniasis in urban and rural areas of northeast Brazil. Res Vet Sci. 2009;86:267–273. doi: 10.1016/j.rvsc.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Nunes CM, Pires MM, da Silva KM, Assis FD, Goncalves Filho J, Perri SH. Relationship between dog culling and incidence of human visceral leishmaniasis in an endemic area. Vet Parasitol. 2010;170:131–133. doi: 10.1016/j.vetpar.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Lima ID, Queiroz JW, Lacerda HG, Queiroz PV, Pontes NN, Barbosa JD, Martins DR, Weirather JL, Pearson RD, Wilson ME, Jeronimo SM. Leishmania infantum chagasi in northeastern Brazil: asymptomatic infection at the urban perimeter. Am J Trop Med Hyg. 2012;86:99–107. doi: 10.4269/ajtmh.2012.10-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borges BK, Silva JA, Haddad JP, Moreira EC, Magalhaes DF, Ribeiro LM, Fiuza Vde O. Assessment of knowledge and preventive attitudes concerning visceral leishmaniasis in Belo Horizonte, Minas Gerais State, Brazil. Cad Saude Publica. 2008;24:777–784. doi: 10.1590/s0102-311x2008000400007. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira TM, Furuta PI, de Carvalho D, Machado RZ. A study of cross-reactivity in serum samples from dogs positive for Leishmania sp., Babesia canis and Ehrlichia canis in enzyme-linked immunosorbent assay and indirect fluorescent antibody test. Rev Bras Parasitol Vet. 2008;17:7–11. doi: 10.1590/s1984-29612008000100002. [DOI] [PubMed] [Google Scholar]
- 10.Boggiatto PM, Gibson-Corley KN, Metz K, Gallup JM, Hostetter JM, Mullin K, Petersen CA. Transplacental transmission of Leishmania infantum as a means for continued disease incidence in North America. PLoS Negl Trop Dis. 2011;5:e1019. doi: 10.1371/journal.pntd.0001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferroglio E, Poggi M, Trisciuoglio A. Evaluation of 65% permethrin spot-on and deltamethrin-impregnated collars for canine Leishmania infantum infection prevention. Zoonoses Public Health. 2008;55:145–148. doi: 10.1111/j.1863-2378.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- 12.Giffoni JH, de Almeida CE, dos Santos SO, Ortega VS, de Barros AT. Evaluation of 65% permethrin spot-on for prevention of canine visceral leishmaniasis: effect on disease prevalence and the vectors (Diptera: Psychodidae) in a hyperendemic area. Vet Ther. 2002;3:485–492. [PubMed] [Google Scholar]
- 13.Miro G, Galvez R, Mateo M, Montoya A, Descalzo MA, Molina R. Evaluation of the efficacy of a topically administered combination of imidacloprid and permethrin against Phlebotomus perniciosus in dog. Vet Parasitol. 2007;143:375–379. doi: 10.1016/j.vetpar.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Otranto D, Paradies P, Lia RP, Latrofa MS, Testini G, Cantacessi C, Mencke N, Galli G, Capelli G, Stanneck D. Efficacy of a combination of 10% imidacloprid/50% permethrin for the prevention of leishmaniasis in kenneled dogs in an endemic area. Vet Parasitol. 2007;144:270–278. doi: 10.1016/j.vetpar.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein ND. The precaution adoption process. Health Psychol. 1988;7:355–386. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- 16.Paz GF, Ribeiro MF, de Magalhaes DF, Sathler KP, Morais MH, Fiuza VO, Brandao ST, Werneck GL, Fortes-Dias CL, Dias ES. Association between the prevalence of infestation by Rhipicephalus sanguineus and Ctenocephalides felis felis and the presence of anti-Leishmania antibodies: a case-control study in dogs from a Brazilian endemic area. Prev Vet Med. 2010;97:131–133. doi: 10.1016/j.prevetmed.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Johnson TP, Garrity TF, Stallones L. Psychometric evaluation of the Lexington Attachment to Pets Scale (LAPS) Anthrozoos. 1992;5:160–175. [Google Scholar]
- 18.Braz RF, Nascimento ET, Martins DR, Wilson ME, Pearson RD, Reed SG, Jeronimo SM. The sensitivity and specificity of Leishmania chagasi recombinant K39 antigen in the diagnosis of American visceral leishmaniasis and in differentiating active from subclinical infection. Am J Trop Med Hyg. 2002;67:344–348. doi: 10.4269/ajtmh.2002.67.344. [DOI] [PubMed] [Google Scholar]
- 19.Coura-Vital W, Marques MJ, Veloso VM, Roatt BM, Aguiar-Soares RD, Reis LE, Braga SL, Morais MH, Reis AB, Carneiro M. Prevalence and factors associated with Leishmania infantum infection of dogs from an urban area of Brazil as identified by molecular methods. PLoS Negl Trop Dis. 2011;5:e1291. doi: 10.1371/journal.pntd.0001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouvea MV, Werneck GL, Costa CH, de Amorim Carvalho FA. Factors associated to Montenegro skin test positivity in Teresina, Brazil. Acta Trop. 2007;104:99–107. doi: 10.1016/j.actatropica.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Pedrosa Fde A, Ximenes RA. Sociodemographic and environmental risk factors for American cutaneous leishmaniasis (ACL) in the State of Alagoas, Brazil. Am J Trop Med Hyg. 2009;81:195–201. [PubMed] [Google Scholar]
- 22.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 23.Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. Canine leishmaniosis—new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 2008;24:324–330. doi: 10.1016/j.pt.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Day MJ. One health: the importance of companion animal vector-borne diseases. Parasit Vectors. 2011;4:49. doi: 10.1186/1756-3305-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa CH, Werneck GL, Rodrigues L, Jr, Santos MV, Araujo IB, Moura LS, Moreira S, Gomes RB, Lima SS. Household structure and urban services: neglected targets in the control of visceral leishmaniasis. Ann Trop Med Parasitol. 2005;99:229–236. doi: 10.1179/136485905X28018. [DOI] [PubMed] [Google Scholar]
- 26.Nascimento ET, Moura ML, Queiroz JW, Barroso AW, Araujo AF, Rego EF, Wilson ME, Pearson RD, Jeronimo SM. The emergence of concurrent HIV-1/AIDS and visceral leishmaniasis in northeast Brazil. Trans R Soc Trop Med Hyg. 2011;105:298–300. doi: 10.1016/j.trstmh.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman D, Smith JP. The increasing value of education to health. Soc Sci Med. 2011;72:1728–1737. doi: 10.1016/j.socscimed.2011.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chompook P, Todd J, Wheeler JG, von Seidlein L, Clemens J, Chaicumpa W. Risk factors for shigellosis in Thailand. Int J Infect Dis. 2006;10:425–433. doi: 10.1016/j.ijid.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Hargreaves JR, Glynn JR. Educational attainment and HIV-1 infection in developing countries: a systematic review. Trop Med Int Health. 2002;7:489–498. doi: 10.1046/j.1365-3156.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- 30.Gorter AC, Sandiford P, Pauw J, Morales P, Perez RM, Alberts H. Hygiene behaviour in rural Nicaragua in relation to diarrhoea. Int J Epidemiol. 1998;27:1090–1100. doi: 10.1093/ije/27.6.1090. [DOI] [PubMed] [Google Scholar]
- 31.de Almeida AS, Medronho Rde A, Werneck GL. Identification of risk areas for visceral leishmaniasis in Teresina, Piaui State, Brazil. Am J Trop Med Hyg. 2011;84:681–687. doi: 10.4269/ajtmh.2011.10-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raina P, Waltner-Toews D, Bonnett B, Woodward C, Abernathy T. Influence of companion animals on the physical and psychological health of older people: an analysis of a one-year longitudinal study. J Am Geriatr Soc. 1999;47:323–329. doi: 10.1111/j.1532-5415.1999.tb02996.x. [DOI] [PubMed] [Google Scholar]
- 33.Podaliri Vulpiani M, Iannetti L, Di Mattia T, Dalla Villa P. Leishmania infantum in a Central Italy dog shelter: retrospective study of serologic reactivity during a 4-year period in a confined dog population subjected to preventive and therapeutic treatment. Vet Parasitol. 2009;160:190–197. doi: 10.1016/j.vetpar.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Molina R, Miro G, Galvez R, Nieto J, Descalzo MA. Evaluation of a spray of permethrin and pyriproxyfen for the protection of dogs against Phlebotomus perniciosus. Vet Rec. 2006;159:206–209. doi: 10.1136/vr.159.7.206. [DOI] [PubMed] [Google Scholar]
- 35.Mencke N, Volf P, Volfova V, Stanneck D. Repellent efficacy of a combination containing imidacloprid and permethrin against sand flies (Phlebotomus papatasi) in dogs. Parasitol Res. 2003;90((Suppl 3)):S108–S111. doi: 10.1007/s00436-003-0905-7. [DOI] [PubMed] [Google Scholar]
- 36.Mercier P, Jasmin P, Sanquer A. Prevention of sand fly attack by topical application of a permethrin/pyriproxyfen combination on dogs. Vet Ther. 2003;4:309–316. [PubMed] [Google Scholar]
- 37.Alexander B, Barros VC, de Souza SF, Barros SS, Teodoro LP, Soares ZR, Gontijo NF, Reithinger R. Susceptibility to chemical insecticides of two Brazilian populations of the visceral leishmaniasis vector Lutzomyia longipalpis (Diptera: Psychodidae) Trop Med Int Health. 2009;14:1272–1277. doi: 10.1111/j.1365-3156.2009.02371.x. [DOI] [PubMed] [Google Scholar]
- 38.Reithinger R, Coleman PG, Alexander B, Vieira EP, Assis G, Davies CR. Are insecticide-impregnated dog collars a feasible alternative to dog culling as a strategy for controlling canine visceral leishmaniasis in Brazil? Int J Parasitol. 2004;34:55–62. doi: 10.1016/j.ijpara.2003.09.006. [DOI] [PubMed] [Google Scholar]