Abstract
Rapid urbanization in Brazil has meant that many persons from rural areas where Schistosoma mansoni is endemic have migrated to cities. Discovery of a focus of active transmission in the city of Salvador prompted a citywide survey for active and potential transmission sites. Cercariae shed from infected snails collected from four locations were used to determine how these samples were related and if they were representative of the parasite population infecting humans. Each cercarial collection was greatly differentiated from the others, and diversity was significantly lower when compared with eggs from natural human infections in one site. Egg samples collected 7 years apart in one neighborhood showed little differentiation (Jost's D = 0.01–0.03). Given the clonal nature of parasite reproduction in the snail host and the short-term acquisition of parasites, cercariae from collections at one time point are unlikely to be representative of the diversity in the human population.
Introduction
Infection with Schistosoma mansoni remains one of the most important public health problems in Brazil. In this country, the parasite infects 2–6 million persons in 9 states despite > 30 years of control programs.1 Since 2008, it has been responsible for 400–500 hospitalizations per year and a highly prevalent but poorly measured chronic burden of disease.2–4 In addition, it contributes to 500 deaths each year (http://portal.saude.gov.br/portal/arquivos/pdf/obitos_1990_2008_06_04_11.pdf). Across Brazil, the northeastern states are the most affected by schistosomiasis. In the northeastern state of Bahia, 146 of 417 municipalities are endemic and show widespread transmission, 144 have focal transmission, and 127 are unaffected. However, even those unaffected have a high potential for transmission, given the presence of the snail host and human and/or snail infections in neighboring areas and migration.5
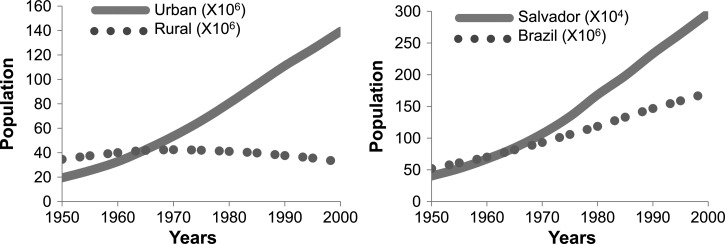
Although all of Brazil has experienced a demographic shift from a rural to an urban concentration of the population, the state of Bahia and its capital city of Salvador have seen much faster growth than the country as a whole (Figure 1). During 1980–2010, the greater metropolitan area of Salvador grew by more than 200% and now has more than 3 million persons. Although often considered primarily a rural problem, schistosomiasis today is found even in peri-urban and central regions of Salvador. Recently, autochthonous cases were identified around São Bartolomeu Park in the Ferroviária Suburban region of Salvador where in one neighborhood 30% of children surveyed were found to be infected, although most had never left the city.6
Figure 1.
Urban growth in Brazil and Salvador. A, Rural urban population growth in Brazil, 1950–2000. B, Salvador, Brazil population growth, 1950–2000. Source: United Nations Department of Economic and Social Affairs/Population Division World Urbanization Prospects: The 2005 Revision, available at: http://www.un.org/esa/population/publications/WUP2005/2005WUP_DataTables12.pdf.
In surveys conducted by the municipal Center for Control of Zoonosis (CCZ), Biomphalaria glabrata, the principal snail vector of schistosomiasis in Bahia, has been identified in water bodies throughout the city. Natural foci of S. mansoni infection in the city were already known in the 1950s and 1960s,7 and the urbanization of schistosomiasis in Brazil has been observed in other studies.6,8–13 Although the presence of urban schistosomiasis is not new, its increasing recognition indicates deterioration or inadequacy of the sanitation infrastructure combined with human migration as relevant factors in the transmission and maintenance of the disease in urban spaces.
The fundamental basis for population genetic analysis is that organisms that tend to breed together have characteristic allele frequency patterns when stable or show perturbations in the patterns when the population experiences forces that change its composition. We wished to compare the relatedness of cercariae released from several geographically separated populations of snails within the city of Salvador to each other and to eggs from the infected human population in one neighborhood of the city.
The CCZ in partnership with the Experimental Pathology Laboratory Research Center of the Oswaldo Cruz Institute Bahia conducted a malacologic survey of all major bodies of water in the city of Salvador for the presence of B. glabrata. The survey was designed to map and to determine the distribution and prevalence of schistosome infections with S. mansoni across the city. We used samples collected in this ongoing mapping effort to determine the relationship between cercariae in isolated urban water systems and those populations of parasites infecting humans.
Understanding the genetic distribution and parasite population dynamics has direct relevance for public health because it provides insights into how parasite populations recover, the nature of parasite persistence,14 geographic clustering, and movement among communities. It might contribute to public health by measuring the true impact of control efforts on parasite population reduction. This study concerns cercarial populations collected in newly identified areas of transmission in the state capital, Salvador, a modern metropolis of more than 3 million persons.
Materials And Methods
Study areas and sample collection.
Salvador is the most populous city in northeastern Brazil and has 3.6 million inhabitants in the greater metropolitan area.15 It is located on 706.8 km2 of hilly terrain in a humid tropical climate and is mostly composed of densely packed neighborhoods. In some areas, such as parks, there is dense vegetation typical of the coastal Atlantic forest. For public health purposes, the city is divided into 12 sanitary districts. A survey of snail populations was conducted district by district that included 158 major, permanent surface water sites consisting of dam and reservoir combinations, rivers, streams, drainage ditches/open sewers, streams, wetlands, ponds, springs, channels, wells and dikes. Agents of the CCZ responsible for the snail collections were given a one-week basic course on parasite and snail biology, snail identification, biosafety, use of personal protective equipment, and transport of biological material. For collection, each agent was equipped with scoops, forceps, rubber boots, and gloves.
The CCZ agents collected up to 50 snails within defined 10-meter segments along the targeted waterway or basin. Collections occurred at various times of the year. The coordinates of each snail collection were recorded with a handheld differential global positioning system unit, and the program ArcGis 9.3 (ESRI, Redlands, CA) was use for analysis of geographic positioning. These coordinates were then mapped to an outline of metropolitan Salvador at an initial scale of 1:2,000. The collected snails were speciated and placed in a covered, darkened tank with dechlorinated water for 48 hours. Pools of snails from each location were exposed to light from a 40-watt incandescent bulb in dechlorinated water to stimulate cercarial release for 20 minutes. After thorough washing, individual snails from positive pools were placed in 10 mL of dechlorinated water, exposed to light, and the water was examined for cercariae. The number of shedding snails was recorded. For each location, cercariae were pooled and fixed in 70% ethanol. Final cercarial numbers were estimated by microscopic examination of two 1-mL samples, except for the Lago de Urubu collection, for which numbers were estimated by extrapolation from results of a quantitative real-time polymerase chain reaction (PCR) and cercarial counts from collections from other sites (Dique do Cabrito = 6.5 × 10−5 pg of DNA/cercaria; Rio do Cobre = 5.6 × 10−5 pg of DNA/cercaria; and Pituaçu = 7.5 × 10−5 pg of DNA/cercaria).
To compare cercarial diversity with parasite egg diversity, we genotyped S. mansoni eggs collected in 2004 from 8 infected persons living in the São Bartolomeu neighborhood of Salvador and who had never visited rural schistosomiasis-endemic sites.16 Fecal samples were pooled in this instance before egg isolation. Egg isolation was performed as described17 by using selective sieving and sedimentation. In addition, in 2011, we genotyped individual infrapopulations collected from eggs found in fecal samples of 36 infected persons living in 3 of 6 defined microareas of São Bartolomeu. The microareas are administrative units used by the Family Health Program and each contains approximately 1800 persons. The microareas selected were not immediately adjacent to one another. These samples were not pooled, but genotyped as separate infrapopulations and analyzed together as component populations for each microarea. Cercariae from a laboratory strain maintained at the Oswaldo Cruz Foundation, Bahia (Feira de Santana strain) were used for comparison. DNA from 200 adult worms from a laboratory strain maintained at Case Western Reserve University (CWRU strain) was used for the PCR-positive control. Written consent was obtained from all human subjects. The Committee on Ethics in Research of the Oswaldo Cruz Foundation of Salvador, Bahia, the Brazilian National Committee on Ethics in Research and the Institutional Review Board for Human Investigation of University Hospitals Case Medical Center, Cleveland, Ohio approved the study design.
DNA extraction and S. mansoni DNA quantification.
Before DNA extraction, tubes with cercariae in 70% ethanol were centrifuged at 14,000 × g, the ethanol was drained, and the pellet was dried briefly. DNA was then extracted using the DNeasy Blood and Tissue DNA Isolation Kit (QIAGEN, Valencia, CA) according to the manufacturer's protocol. To quantify the S. mansoni DNA, a PCR was performed using primers specific to the S. mansoni small ribosomal RNA subunit18 as described.14
Genotyping and analysis.
All samples were genotyped in duplicate by PCR amplification of microsatellite loci using fluorescent-labeled primers to 14 microsatellite markers,14 followed by capillary electrophoresis for peak detection as described.17
For each site, the total number of alleles and the average effective allele number over all markers were calculated. The population effective allele number19 was calculated according to the equation 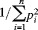 where pi is the frequency of the ith allele for each marker. Note that this is a simple transformation of expected heterozygosity.20 Differences in effective allele number were compared by using the Wilcoxon signed rank test.
where pi is the frequency of the ith allele for each marker. Note that this is a simple transformation of expected heterozygosity.20 Differences in effective allele number were compared by using the Wilcoxon signed rank test.
To measure genetic differentiation, Jost's D21 was calculated by using SPADE software.22 We used Jost's D as a measure of the degree of relatedness between groups. This index has been shown to perform best when the markers are highly polymorphic and in populations with high diversity.21,23,24 In previous studies, we found that Jost's D is proportionally similar to the F'ST. Jost's D for the São Bartolomeu infrapopulations in humans was determined by using the allele frequency from the six infections in each microarea weighted by the intensity of infection.14 Pairwise comparisons of allele frequencies between samples were performed with a bootstrap of 1,000 replicates. The allele counts used in SPADE were obtained by multiplying the allele frequencies for each locus by the number of cercariae that made up each sample.
Results
Of 158 sites investigated, 120 were positive for B. glabrata, which was the only S. mansoni intermediate host found. Snails produced cercariae at seven sites (Figure 2 and Table 1), and cercariae were available from five of these sites for DNA extraction. Infected snails were obtained from a variety of water body sizes and types (Table 2), but all were found to drain areas with dense human populations and a mixture of housing qualities. The prevalence of infection in snails ranged from 14.5% to 56%. The smallest number of cercariae shed from snails collected in the field (Table 1) was from the Itacaranhas sample (76) and the largest from the Rio do Cobre sample (14,800). Fourteen microsatellite markers were used to genotype the samples. DNA of cercariae shed from snails collected at the Lagoa do Urubú amplified poorly for all markers. Therefore, this sample was excluded from analysis of differentiation.
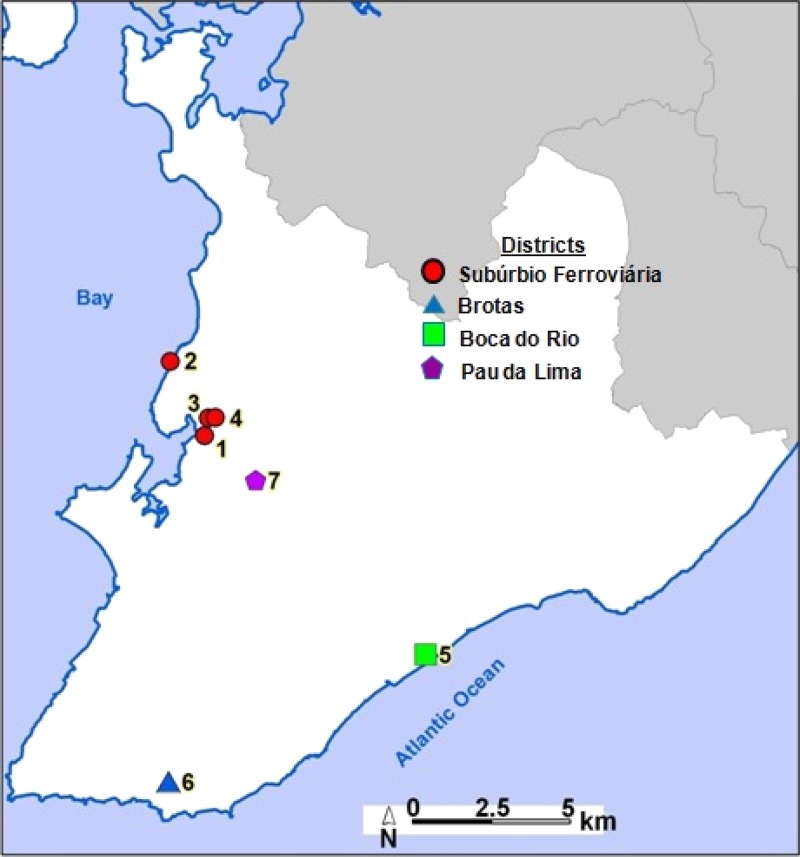
Figure 2.
Infected snail locations, Salvador, Bahia, Brazil. Schistosoma mansoni–infected snail sites, Salvador, Brazil. Greater metropolitan Salvador is show in white. 1 = Dique do Cabrito; 2 = Itacaranhas; 3 = Rio do Cobre; 4 = Parque São Bartolomeu; 5 = Parque Pituaçu; 6 = Avenida Vasco da Gama; 7 = Lagoa do Urubú.
Table 1 .
Schistosoma mansoni sample characteristics, Brazil
| Sample no. | Name | Source description | Parasite count (stage) | No. infected persons (%) |
|---|---|---|---|---|
| 1 | Dique do Cabrito | Snails from small neighborhood lake | 1,178* (cercariae) | 10 (22.0) |
| 2 | Itacaranhas | Snails from drainage ditch/sewer | 76 (cercariae) | 17 (23.0) |
| 3 | Rio do Cobre | Snails from shore of river outside São Bartolomeu Reserve | 14,800 (cercariae) | 20 (30.0) |
| 4 | Cachoeira | Snails from base of São Bartolomeu waterfall | Cercariae* | 12 (14.5) |
| 5 | Pituaçú | Snails from temporary water collections from a municipal park | 2,000 (cercariae) | 34 (56.6) |
| 6 | Av. Vasco da Gama | Snails from median strip drainage ditch/sewer | (cercariae)† | 4 (27.3) |
| 7 | Lagoa do Urubú | Snails from small neighborhood lake | 2,800 (cercariae) | 4 (40.0) |
| 8 | São Bartolomeu | Infected children from São Bartolomeu neighborhood (2004) | (eggs)† | 8 (30.2) |
| 9 | MA1 | Infected persons from São Bartolomeu neighborhood microarea 1 (2011) | 127,728 (eggs) | 12 (22.8) |
| 10 | MA3 | Infected persons from São Bartolomeu neighborhood microarea 3 (2011) | 30,616 (eggs) | 12 (23.1) |
| 11 | MA6 | Infected persons from São Bartolomeu neighborhood microarea 6 (2011) | 153,273 (eggs) | 12 (55.6) |
| 12 | Feira de Santana | Oswaldo Cruz Bahia laboratory strain | 29,600 (cercariae) | – |
| 13 | CWRU | Case Western Reserve University laboratory strain | ≈200 (adult worms) | – |
Estimated cercarial count by a quantitative polymerase chain reaction.
Samples no longer available for counting.
Table 2.
Schistosoma mansoni population genetic diversity, Brazil*
| Locus | Cercariae from field collections | Eggs from human infections | Cercariae | Worms | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||
| SMMS2 | 1 (1.00) | – | 3 (1.18) | 4 (1.89) | – | 3 (1.30) | 2 (1.77) | 2 (1.73) | 2 (1.58) | 2 (1.02) | 4 (1.99) | 4 (1.50) |
| SMMS16 | 3 (1.98) | 2 (1.88) | 5 (2.03) | 6 (2.69) | 3 (2.52) | 6 (4.24) | 6 (4.35) | 7 (3.87) | 7 (4.10) | 3 (2.02) | 6 (3.41) | 7 (3.01) |
| SMMS3 | 2 (1.36) | – | 6 (3.95) | 7 (4.80) | – | – | 12 (6.35) | 10 (5.41) | 12 (6.56) | 5 (1.90) | 7 (3.53) | 12 (4.23) |
| SMMS17 | 3 (1.10) | – | 4 (2.05) | 5 (3.05) | – | 4 (2.55) | 4 (2.45) | 4 (2.34) | 5 (2.39) | 2 (1.05) | 6 (2.51) | 5 (2.17) |
| SMMS18 | 5 (1.70) | 2 (1.60) | 8 (1.51) | 9 (3.20) | – | 7 (4.86) | 9 (3.99) | 11 (4.41) | 9 (4.23) | 11 (1.22) | 12 (3.85) | 12 (3.06) |
| SMMS21 | 3 (1.62) | 1 (1.00) | 3 (1.08) | 3 (1.33) | – | 4 (1.86) | 5 (1.75) | 5 (1.62) | 5 (1.79) | 4 (2.24) | 4 (1.97) | 5 (1.63) |
| SMDA23 | 8 (2.07) | 2 (1.01) | 9 (2.40) | 7 (1.52) | – | 9 (3.24) | 12 (3.88) | 11 (3.75) | 11 (3.18) | 8 (3.11) | 11 (4.71) | 12 (2.89) |
| 1F8A | 5 (1.11) | 3 (1.98) | 6 (2.92) | 7 (1.97) | – | 7 (3.84) | 8 (3.31) | 5 (4.02) | 8 (3.75) | 5 (1.38) | 7 (3.75) | 8 (2.80) |
| 13TAGA | 7 (2.84) | 5 (2.15) | 6 (2.44) | 5 (1.50) | – | 8 (8.46) | 9 (2.83) | 9 (3.24) | 9 (3.37) | 9 (3.03) | 11 (2.99) | 11 (3.29) |
| SM13-410 | 3 (2.03) | 3 (2.11) | 5 (2.78) | 5 (3.03) | – | 4 (2.35) | 5 (2.14) | 4 (2.04) | 5 (2.24) | 4 (2.10) | 4 (2.14) | 5 (2.30) |
| SMU31768 | 10 (2.00) | 12 (4.28) | 13 (3.64) | 12 (4.55) | – | 12 (5.25) | 13 (4.15) | 13 (4.04) | 13 (4.81) | 10 (3.01) | 12 (3.87) | 13 (3.96) |
| 15J15A | 8 (2.73) | 5 (2.22) | 7 (2.74) | 8 (4.72) | – | 8 (4.32) | 9 (3.93) | 9 (3.91) | 9 (3.78) | 8 (3.29) | 5 (2.42) | 9 (3.41) |
| 29E6A | 7 (1.73) | 5 (2.36) | 8 (2.63) | 6 (2.96) | 4 (2.83) | 6 (4.06) | 6 (3.90) | 6 (3.72) | 8 (3.90) | 7 (2.22) | 7 (3.77) | 9 (3.10) |
| SM13-478 | 9 (2.83) | 4 (2.45) | 7 (2.31) | 7 (2.55) | 2 (1.98) | 8 (5.17) | 12 (5.00) | 11 (4.66) | 12 (5.19) | 9 (2.69) | 5 (1.57) | 12 (3.31) |
| Total | 74 (1.86) | 44 (2.10) | 90 (2.41) | 91 (2.75) | 9 (2.44) | 86 (3.96) | 112 (3.56) | 107 (3.48) | 115 (3.63) | 87 (2.09) | 101 (2.95) | 124 (2.90) |
Values indicate observed number of alleles (effective number of alleles). Sample numbers are as in Table 1; samples 4 and 6 did not amplify by polymerase chain reaction. The total for each site indicates the sum of the number of observed alleles and the average of the effective allele number.
The total number of alleles observed in cercariae from all sites was 124, but the number of alleles found in cercariae sampled from any one site ranged from 44 in the district of Itacaranhas to 91 in the district of Pituaçu. The average effective allele number was similar across all cercarial samples. Eggs from infected persons in São Bartolomeu had a larger number of alleles compared with samples of cercariae, and a significantly greater effective allele number (3.48–3.96 versus 1.86–2.75) and therefore greater diversity (Table 2). DNA from cercariae of a laboratory strain in Brazil and more than 200 worms from the CWRU strain demonstrated 87 and 101 discrete alleles, respectively.
The genetic differentiation index Jost's D was calculated for the cercaria, egg, and worm populations (Table 3). Cercarial samples from the Cachoeira (São Bartolomeu), Avenida Vasco da Gama, and Lagoa do Urubú were excluded from analysis because of poor amplification. A pairwise comparison of the available cercarial collections around the city showed that each was highly differentiated from the other to a much greater extent than was observed for stool eggs between persons and communities in rural Bahia.17 We also observed that samples from humans in the same community (Table 3). São Bartolomeu; MA1, MA3, and MA6) showed the least differentiation despite sample collections separated by seven years. Cercariae in field collections were less diverse (total number of alleles = 102, weighted effective allele number = 2.72) than eggs from human infections (118 and 3.61). The difference between most field collections was as great as that between a field collection and laboratory specimens. The only two collections that suggested potential gene flow between them were the laboratory strain of Feira de Santana and Dique do Cabrito (mean Jost's D = 0.017). However, this result was likely spurious because these collections were reproductively isolated from each other for many years and no other samples from Brazil were similarly close. Therefore, there was no correlation geographic location and differentiation indices for cercariae from around the city.
Table 3.
Pairwise comparison of Jost's D differentiation index for Schistosoma mansoni, Brazil*
| Location | Cercariae from field collections | Eggs in human infections | Laboratory life cycle of worms | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dique do Cabrito | Itacaranhas | Rio do Cobre | Pituaçú | São Bartolomeu† | MA1 | MA3 | MA6 | Feira de Santana | CWRU | |
| Dique do Cabrito | – | 0.61 (0.60–0.63) | 0.50 (0.50–0.51) | 0.37 (0.36–0.37) | 0.34 (0.33–0.35) | 0.31 (0.31–0.32) | 0.37 (0.36–0.37) | 0.34 (0.34–0.34) | 0.02 (0.01–0.02) | 0.45 (0.44–0.46) |
| Itacaranhas | – | 0.52 (0.50–0.54) | 0.31 (0.28–0.33) | 0.35 (0.33–0.37) | 0.36 (0.35–0.38) | 0.33 (0.31–0.35) | 0.34 (0.32–0.36) | 0.60 (0.59–0.62) | 0.50 (0.47–0.52) | |
| Rio do Cobre | – | 0.30 (0.30–0.31) | 0.26 (0.25–0.26) | 0.26 (0.26–0.27) | 0.28 (0.27–0.28) | 0.25 (0.25–0.25) | 0.51 (0.51–0.51) | 0.36 (0.35–0.37) | ||
| Pituaçú | – | 0.16 (0.15–0.17) | 0.18 (0.17–0.18) | 0.17 (0.17–0.18) | 0.17 (0.17–0.18) | 0.37 (0.37–0.38) | 0.31 (0.30–0.32) | |||
| São Bartolomeu | – | 0.02 (0.02–0.02) | 0.03 (0.02–0.03) | 0.01 (0.01–0.01) | 0.33 (0.32–0.34) | 0.22 (0.21–0.23) | ||||
| MA1 | – | 0.02 (0.02–0.03) | 0.01 (0.01–0.01) | 0.30 (0.30–0.30) | 0.22 (0.21–0.22) | |||||
| MA3 | – | 0.02 (0.02–0.02) | 0.36 (0.35–0.36) | 0.23 (0.23–0.24) | ||||||
| MA6 | – | 0.33 (0.33–0.33) | 0.23 (0.22–0.24) | |||||||
| Feira de Santana | – | 0.44 (0.43–0.44) | ||||||||
| CWRU | – | |||||||||
Values in parentheses are 95% confidence intervals. Values in bold are statistically significant. CWRU = Case Western Reserve University.
Stools aggregated from 8 children in São Bartolomeu in 2004. MA1, 2, and 3 were component populations composed of genotyped infrapopulations from 12 persons. Markers SMMS 2, 3, and 17 were excluded from comparison for Itacaranhas because of poor amplification.
Discussion
Schistosomiasis mansoni is an endemic infectious disease with worldwide repercussions on the health of populations. The urbanization of schistosomiasis has come about through a process of human migration and settlement patterns that have left many cities of the developing world with areas as characteristic of the countryside as of the metropolis. Accordingly, the city of Salvador, Bahia, Brazil, has grown nearly 200% in the past 20 years and continues to show S. mansoni infections because of immigration from rural schistosomiasis-endemic areas. Identification of infected snails leaves little doubt that one component of urban schistosomiasis is local transmission, and this finding requires a different response from public health institutions than for imported cases alone. More than 75% of sites sampled in the city were positive for B. glabrata, and there was active shedding of cercariae detected at 4.4% of these sites. At sites in which infected snails were present, the prevalence among snails was high (> 20%). At almost all sites studied, children and adults used the water or nearby areas for leisure activities, making these sources risks for continued transmission. Under these circumstances, the identification and mapping of areas harboring snails infected by S. mansoni are aids to surveillance and intervention against this infection.25
Cercariae within the city had a high degree of genetic differentiation between sites. This finding was as great as between samples geographically isolated from Salvador and elsewhere in Brazil. This could be consistent with reproductive isolation for these samples either because of geographic isolation of infected persons or more precisely, isolation of their wastes. It may also represent immigration of infected humans from different schistosomiasis-endemic zones in which parasite populations would be reproductively isolated from one another. However, an additional consideration should also be the nature of how snails sample parasites from the human population. Reproduction in the snail is asexual, and individual snail infections represent only 1–4 parasites and thus a small number of genotypes.26 In addition, because the maximum life span of snails is estimated to be 18 months,27 there is a limited time for acquisition of new genotypes. This lower diversity in cercariae in field collections is reflected in the total allele number and weighted average effective allele number (102 and 2.72, respectively) compared with the higher number for eggs from human infections (118 and 3.61, respectively). The effective number of alleles represents the number of alleles of equal frequency necessary to reproduce the observed genetic diversity. However, comparisons of effective allele numbers need to be interpreted with caution because population size and reproductive mechanisms across developmental stages differ between samples. Cercariae from snail collections at a single point in time are not likely to represent the full genetic diversity of worms present in humans.
In contrast, in a study in Kenya, Steinhauer and others found little differentiation between cercariae shed from infected snails around Lake Victoria, but a higher diversity than in Salvador (calculated mean ± SD effective allele number = 5.3 ± 0.3).28 The intensity of transmission, geography of infection, genotyping techniques, numbers of infected snails, and underlying parasite population structure in humans may all contribute to these differences. Lake Victoria represents a single, unobstructed ecosystem in relation to snails and parasites, as shown by low differentiation indices. However, the landscape for snails in Salvador is fragmented and likely inhabited by parasite populations that were founded independently and with little exchange between them. Intensity of transmission is also a likely factor. For example, in a study in Damietta, Egypt, in which transmission was low because of intensive control efforts, the effective allele number (3.22, as calculated from expected heterozygosity) was also much lower than those for sites in Kenya.29
The methods used in these studies were also different from those used in our study. In the study of cercariae from Lake Victoria, genotypes were determined on pools of cercariae used to infect mice and obtain adult worms for DNA from discrete organisms. The study of cercariae in Egypt used total shed cercariae from individual snails for genotyping. In our study, we genotyped the pool of all cercariae from all snails at each site. Although the prevalence of infection was relatively high in the sites with infection in Salvador, we still are likely to have sampled fewer unique snails than the study in Kenya (where up to 5,000 snails were collected at a single site), resulting in our undersampling and greater apparent differentiation in the sample from Brazil.
Because the methods used to survey were not intended to be exhaustive, our results likely underestimated the number of infected sites. Infected snails were found at only 6% of sites with B. glabrata. Snail collections were not performed at the same time of year for all areas, and there is great variability in the snail population depending on seasonal climatic conditions. During the period of fieldwork, access to some locations was not possible because of flooding, and certain neighborhoods were occasionally inaccessible for other safety reasons. Methodologically, the snails collected tended to be larger, older individuals and these are notoriously more refractory to infection30 and may represent self-cured snails. Approximately 30 years ago, B. glabrata in some areas of Salvador was extensively studied by multiple groups and appeared particularly resistant to infection with allopatric strains of S. mansoni.7,30 However, the prevalence of infection for infected sites in this study was surprisingly high and likely contributes to maintenance of the parasite in many areas of the city. The active transmission of S. mansoni in the city implies that efforts must continue and expand to find and treat those infected and to prevent human fecal contamination of fresh water supplies.
In evaluating parasite population structure, different answers may be obtained depending on the developmental stage sampled. A comprehensive collection of S. mansoni eggs or miracidia can represent adult worm genotypes present in the human population.31 However, cercariae collected at one time point reflect the population in snails over approximately the past three months. Cross-sectional collection of snails at one time point combined with the limited capacity of a single snail to accept more than a few individual miracidia make it unlikely that this stage will represent the human population very well. In addition, Sturrock and others32 showed that snail infections probably occur in pulses and not as a continuous flow of miracidia, further intensifying an irregular temporal distribution of parasite genotypes. Despite heavy snail infections, our results show decreased parasite diversity in cross-sectional populations in snail hosts relative to human hosts.
The differentiation between snail infections in different regions of the same city contrasts strongly with the stability and similarity of human infections in one neighborhood. Jost's D was notably small between the São Bartolomeu microareas in 2011 and the pooled sample from children in São Bartolomeu collected seven years earlier. The small size of the sample, the combination of adults and children, the greater diversity in infrapopulations in humans, and the fact that these are urban infections only makes the similarity between these infrapopulations all the more striking. This finding suggests that most of these infections were locally acquired. Further sampling of these microareas will be important for confirming this observation.
The epidemiologic profile of schistosomiasis in Salvador is that of a chronic and potentially serious endemic disease because of the high prevalence of snails at water contact sites combined with the pollution of the environment with human waste where there is disorganized development. Assays of snail infections will have an important role in control measures and confirming interruption of transmission. The parasite population in snails also represents the subpopulation actively being transmitted. However, they might be less useful in cities such as Salvador for assessing parasite population structure and dynamics in the human host.
Footnotes
Financial support: This study was supported by National Institutes of Health grant R01 AI069195. Lúcio M. Barbosa and Samaly S. Souza and were supported by the Brazilian Federal Agency for the Support and Evaluation of Graduate Education and the Council for Scientific and Technological Development.
Authors' addresses: Samaly S. Souza, Lúcio M. Barbosa, Renato Barbosa Reis, Mitermayer G. Reis, and Zilton A. Andrade, Gonçalo Moniz Gonçalo Moniz Research Centre, Oswaldo Cruz Foundation, Salvador, Bahia, Brazil, E-mails: samalysouza@hotmail.com, lmacedo@aluno.bahia.fiocruz.br, georeis@gmail.com, miter@bahia.fiocruz.br, and zilton@cpqgm.fiocruz.br. Isabel C. Guimarães, Center for Control of Zoonoses, Municipal Secretariat of Health, Alto do Trobogy, Salvador, Bahia, Brazil, E-mail: belcguimaraes@gmail.com. Walter A. Blank and Ronald E. Blanton, Center for Global Health and Diseases, Case Western Reserve University, Biomedical Research Building, Cleveland, OH, E-mails: wab25@case.edu and reb6@case.edu.
References
- 1.Katz N. Schistosomiasis control in Brazil. Mem Inst Oswaldo Cruz. 1998;93((Suppl 1)):33–35. doi: 10.1590/s0074-02761998000700005. [DOI] [PubMed] [Google Scholar]
- 2.Assis AM, Prado MS, Barreto ML, Reis MG, Conceicao Pinheiro SM, Parraga IM, Blanton RE. Childhood stunting in northeast Brazil: the role of Schistosoma mansoni infection and inadequate dietary intake. Eur J Clin Nutr. 2004;58:1022–1029. doi: 10.1038/sj.ejcn.1601926. [DOI] [PubMed] [Google Scholar]
- 3.Brito LL, Barreto ML, Silva Rde C, Assis AM, Reis MG, Parraga IM, Blanton RE. Moderate- and low-intensity co-infections by intestinal helminths and Schistosoma mansoni, dietary iron intake, and anemia in Brazilian children. Am J Trop Med Hyg. 2006;75:939–944. [PubMed] [Google Scholar]
- 4.Stephenson L. The impact of schistosomiasis on human nutrition. Parasitology. 1993;107((Suppl)):S107–S123. doi: 10.1017/s0031182000075545. [DOI] [PubMed] [Google Scholar]
- 5.Ministério da Saúde . Vigilância e Controle de Moluscos de Importância Epidemiológica: Diretrizes Técnicas: Programa de Vigilância e Controle da Esquistossomose (PCE) Brasilia: Editora MS Documentação e Informação; 2007. [Google Scholar]
- 6.Guimaraes IC, Tavares-Neto J. Urban transmission of schistosomiasis in children from a neighborhood of Salvador, Bahia [in Portuguese] Rev Soc Bras Med Trop. 2006;39:451–455. doi: 10.1590/s0037-86822006000500006. [DOI] [PubMed] [Google Scholar]
- 7.Barbosa FS, Barreto AC. Differences in susceptibility of Brazilian strains of Australorbis glabratus to Schistosoma mansoni. Exp Parasitol. 1960;9:137–140. doi: 10.1016/0014-4894(60)90022-9. [DOI] [PubMed] [Google Scholar]
- 8.Barbosa CS, Araujo KC, Sevilla MA, Melo F, Gomes EC, Souza-Santos R. Current epidemiological status of schistosomiasis in the state of Pernambuco, Brazil. Mem Inst Oswaldo Cruz. 2010;105:549–554. doi: 10.1590/s0074-02762010000400034. [DOI] [PubMed] [Google Scholar]
- 9.Coura-Filho P. Schistosomiasis mansoni in urban territory. 2. A theoretical approach to the accumulation, concentration, and centralization of capital and the production of disease [in Portuguese] Cad Saude Publica. 1997;13:415–424. doi: 10.1590/s0102-311x1997000300017. [DOI] [PubMed] [Google Scholar]
- 10.Enk MJ, Amorim A, Schall VT. Acute schistosomiasis outbreak in the metropolitan area of Belo Horizonte, Minas Gerais: alert about the risk of unnoticed transmission increased by growing rural tourism. Mem Inst Oswaldo Cruz. 2003;98:745–750. doi: 10.1590/s0074-02762003000600006. [DOI] [PubMed] [Google Scholar]
- 11.Firmo JO, Lima Costa MF, Guerra HL, Rocha RS. Urban schistosomiasis: morbidity, sociodemographic characteristics and water contact patterns predictive of infection. Int J Epidemiol. 1996;25:1292–1300. doi: 10.1093/ije/25.6.1292. [DOI] [PubMed] [Google Scholar]
- 12.Kloos H, Correa-Oliveira R, dos Reis DC, Rodrigues EW, Monteiro LA, Gazzinelli A. The role of population movement in the epidemiology and control of schistosomiasis in Brazil: a preliminary typology of population movement. Mem Inst Oswaldo Cruz. 2010;105:578–586. doi: 10.1590/s0074-02762010000400038. [DOI] [PubMed] [Google Scholar]
- 13.Ximenes RA, Southgate B, Smith PG, Guimaraes Neto L. Migration and urban schistosomiasis. The case of Sao Lourenco da Mata, northeast of Brazil. Rev Inst Med Trop Sao Paulo. 2000;42:209–217. doi: 10.1590/s0036-46652000000400006. [DOI] [PubMed] [Google Scholar]
- 14.Blanton RE, Blank WA, Costa JM, Carmo TM, Reis EA, Silva LK, Barbosa LM, Test MR, Reis MG. Schistosoma mansoni population structure and persistence after praziquantel treatment in two villages of Bahia, Brazil. Int J Parasitol. 2011;41:1093–1099. doi: 10.1016/j.ijpara.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IGBE National Census: Total Population of Bahia (in Portuguese) 2010. http://www.ibge.gov.br/home/estatistica/populacao/censo2010/tabelas_pdf/total_populacao_bahia.pdf Accessed 2011. Available at.
- 16.Souza C, Lima L, Konovaloff L. Freshwater mollusks of the Belo Horizonte, MG microregion with emphasis on parasite vectors [in Portuguese] Rev Soc Bras Med Trop. 1997;3:449–456. [Google Scholar]
- 17.Blank WA, Reis EA, Thiong'o FW, Braghiroli JF, Santos JM, Melo PR, Guimaraes IC, Silva LK, Carmo TM, Reis MG, Blanton RE. Analysis of Schistosoma mansoni population structure using total fecal egg sampling. J Parasitol. 2009;95:881–889. doi: 10.1645/GE-1895.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomes AL, Melo FL, Werkhauser RP, Abath FG. Development of a real time polymerase chain reaction for quantitation of Schistosoma mansoni DNA. Mem Inst Oswaldo Cruz. 2006;101((Suppl 1)):133–136. doi: 10.1590/s0074-02762006000900021. [DOI] [PubMed] [Google Scholar]
- 19.Kimura M, Crow JF. The number of alleles that can be maintained in a finite population. Genetics. 1964;49:725–738. doi: 10.1093/genetics/49.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weir BS. Population Structure. Genetic Data Analysis II. Sunderland, MA: Sinauer Associates; 1996. pp. 161–198. [Google Scholar]
- 21.Jost L. GST and its relatives do not measure differentiation. Mol Ecol. 2008;17:4015–4026. doi: 10.1111/j.1365-294x.2008.03887.x. [DOI] [PubMed] [Google Scholar]
- 22.Chao A, Shen TJ. SPADE (Species Prediction And Diversity Estimation). Program and User's Guide. 2010. http://chao.stat.nthu.edu.tw Available at. [Google Scholar]
- 23.Gerlach G, Jueterbock A, Kraemer P, Deppermann J, Harmand P. Calculations of population differentiation based on GST and D: forget GST but not all of statistics! Mol Ecol. 2010;19:3845–3852. doi: 10.1111/j.1365-294X.2010.04784.x. [DOI] [PubMed] [Google Scholar]
- 24.Leng L, Zhang DE. Measuring population differentiation using GST or D? A simulation study with microsatellite DNA markers under a finite island model and nonequilibrium conditions. Mol Ecol. 2011;20:2494–2509. doi: 10.1111/j.1365-294X.2011.05108.x. [DOI] [PubMed] [Google Scholar]
- 25.Amaral RS, Tauil PL, Lima DD, Engels D. An analysis of the impact of the Schistosomiasis Control Programme in Brazil. Mem Inst Oswaldo Cruz. 2006;101((Suppl 1)):79–85. doi: 10.1590/s0074-02762006000900012. [DOI] [PubMed] [Google Scholar]
- 26.Steinauer ML, Mwangi IN, Maina GM, Kinuthia JM, Mutuku MW, Agola EL, Mungai B, Mkoji GM, Loker ES. Interactions between natural populations of human and rodent schistosomes in the Lake Victoria region of Kenya: a molecular epidemiological approach. PLoS Negl Trop Dis. 2008;2:e222. doi: 10.1371/journal.pntd.0000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritchie LS, Hemandes A, Rosa-Amador R. Biological potential of Australorbis glabrata, life span and reproduction. Am J Trop Med Hyg. 1966;15:614–617. doi: 10.4269/ajtmh.1966.15.614. [DOI] [PubMed] [Google Scholar]
- 28.Steinauer ML, Hanelt B, Agola LE, Mkoji GM, Loker ES. Genetic structure of Schistosoma mansoni in western Kenya: the effects of geography and host sharing. Int J Parasitol. 2009;39:1353–1362. doi: 10.1016/j.ijpara.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lotfy WM, Hanelt B, Mkoji GM, Loker ES. Genotyping natural infections of Schistosoma mansoni in Biomphalaria alexandrina from Damietta, Egypt, with comparisons to natural snail infections from Kenya. J Parasitol. 2011;97:156–159. doi: 10.1645/GE-2537.1. [DOI] [PubMed] [Google Scholar]
- 30.Michelson EH, DuBois L. Susceptibility of Bahian population of Biomphalaria glabrata to an allopatric strain of Schistosoma mansoni. Am J Trop Med Hyg. 1978;27:782–786. doi: 10.4269/ajtmh.1978.27.782. [DOI] [PubMed] [Google Scholar]
- 31.Blank WA, Test MR, Liu SF, Lewis FA, Blanton RE. Long-term genetic stability and population dynamics of laboratory strains of Schistosoma mansoni. J Parasitol. 2010;96:900–907. doi: 10.1645/GE-2463.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturrock RF, Karamsadkar SJ, Ouma J. Schistosome infection rates in field snails: Schistosoma mansoni in Biomphalaria pfeifferi from Kenya. Ann Trop Med Parasitol. 1979;73:369–375. doi: 10.1080/00034983.1979.11687272. [DOI] [PubMed] [Google Scholar]