Abstract
A better understanding of the mechanism of anemia associated with Schistosoma mansoni infection might provide useful information on how treatment programs are implemented to minimize schistosomiasis-associated morbidity and maximize treatment impact. We used a cross-sectional study with serum samples from 206 Kenyan school children to determine the mechanisms in S. mansoni-associated anemia. Serum ferritin and soluble transferrin receptor levels were measured by using an enzyme-linked immunosorbent assay. Results suggest that S. mansoni-infected persons are more likely (odds ratio = 3.68, 95% confidence interval = 1.33–10.1) to have levels of serum ferritin (> 100 ng/mL) that are associated with anemia of inflammation (AI) than S. mansoni-uninfected children. Our results suggest that AI is the most common form of anemia in S. mansoni infections. In contrast, the mechanism of anemia in S. mansoni-uninfected children was iron deficiency. Moreover, the prevalence of AI in the study participants demonstrated a significant trend with S. mansoni infection intensity (P < 0.001). Our results are consistent with those observed in S. japonicum-associated anemia.
Introduction
Schistosomiasis continues to burden 74 countries in the developing world, with estimates of 207 million individuals infected worldwide.1,2 Although approximately 20 million persons have severe disease and pathologic changes and 280,000 persons die of schistosomiasis every year, most infected persons have more subtle disease-associated morbidities.2,3 Anemia is a schistosomiasis-associated morbidity that can also contribute to fatigue, weakness, and reduced cognitive function.4–6 Because schistosomiasis prevalence and intensity usually peaks in children between the ages of 8 and 15 years, the height of schistosomiasis-related morbidity may coincide with a high need for optimal cognitive function, ability to focus, and energy and iron requirements in school age children.2,4–7 Moreover, school children in Kenya with heavy Schistosoma mansoni infections are 2.3 times more likely to be anemic than uninfected children.8 Meta-analysis of available data demonstrates that anemia is a major contributor to the disease-specific disability of schistosomiasis.3 Thus, schistosomiasis-associated anemia has the ability to impact quality of life of infected children and potentially contribute to the socioeconomic burden in disease-endemic areas.
A better understanding of the causal mechanisms underlying the relationship between anemia and schistosomiasis would be helpful to determine the frequency of anthelminthic treatment necessary to prevent schistosomiasis-associated anemia in this critical age group. Likewise, further insight into the mechanism of S. mansoni-associated anemia is important regarding interventions to improve iron status. Oral iron therapy may have decreased efficacy in the presence of anemia of inflammation (AI) caused by decreased intestinal absorption and sequestration of iron in macrophages.6 The mechanism of S. mansoni-associated anemia may provide useful information for schistosomiasis treatment program strategies. Recent cross-sectional studies on the three primary schistosome species (S. mansoni, S. haematobium, and S. japonicum) suggest that there are four possible mechanisms of schistosomiasis-associated anemia: iron deficiency anemia (IDA), splenic erythrocyte retention, autoimmune hemolysis, and AI.6,7,9,10 In S. japonicum-associated anemia, AI seems to contribute to anemia across all infection intensities, whereas iron loss in the stool (IDA) may also play a role, but only at the highest infection intensities.10–12 Our goal was to determine whether the mechanism of anemia in S. mansoni-associated anemia was similar.
The measurement of two serum biomarkers, serum ferritin (SF) and soluble transferrin receptor (sTfR), has been useful in determining whether IDA, AI, or both mechanisms are responsible for anemia caused by various infections.13–21 Serum ferritin directly correlates with total iron storage in the body and low levels are indicative of true IDA when hemoglobin (Hb) levels are reduced.13,18 When a person has reduced Hb levels and high SF levels, the patient is iron replete.13,22 If the person has adequate iron storage, a non-IDA, perhaps AI, may be the cause. Because SF levels can be increased during inflammation, they have limitations in accurately predicting the mechanism of anemia in anemic persons with intermediate ferritin levels.14,23 When the available iron for erythropoiesis is low (in the context of true iron deficiency), membrane transferrin receptors are released and sTfR levels are increased.19,23 Although sTfR levels are increased during iron deficiency, they are not appreciably affected by inflammation.14,23 Thus, with the additional measurement of sTfR levels and its ratio to SF in patients with intermediate serum ferritin values, it is possible to identify which mechanism(s) of anemia is in effect.13,15 Independently, the use of SF or sTfR to determine the mechanism of anemia is limited. However, the ratio of these biomarkers can help to distinguish the mechanism(s) in action. Our analyses of SF and sTfR suggest that AI is a contributing mechanism of S. mansoni-associated anemia and that the degree of AI is increased in persons with higher intensity infections.
Materials and Methods
Ethical clearance and informed consent.
This study was conducted in Asembo District of Nyanza Province in western Kenya. Informed consent was obtained from parents or legal guardians and assent was obtained from study participants. The study protocol was approved by the Scientific Steering Committee of the Kenya Medical Research Institute, the Ethics Review Committee of Kenya, and the Institutional Review Board of the Centers for Disease Control and Prevention.
Study participants.
This study was a subset of a larger anemia study that investigated the attributable risk of anemia caused by S. mansoni infection in 9–12-year-old children and had 2,745 participants (Montgomery SP and others, unpublished data). Children were eligible to participate if they attended public schools within 6 km of Lake Victoria. All children were screened for parasites and anemia. To reduce any effect that other infectious agents may have on the interpretation of our results, children who had malaria or hookworm infections were excluded from the mechanism of anemia study subset. Persons who met these criteria were grouped according to their S. mansoni infection status and randomly selected. Approximately half of the children in both groups were anemic according to the Kenya National Clinical Guidelines (Hb level < 12 g/dL).
Fecal examination.
Parasite burden was measured by single stool sample obtained from each person at their respective school. Each of the stool specimens was examined by using the Kato-Katz method in duplicate for S. mansoni, Ascaris lumbricoides, Trichuris trichiura, and hookworm eggs. The egg per gram (EPG) concentration was calculated for each fecal specimen. The geometric mean was determined for specimens with two positive slides. If there was only one positive slide in the sample, the single EPG value was used in the analysis. The intensity of S. mansoni infection was categorized using the World Health Organization criteria as follows: low (1–99 EPG), moderate (100–399 EPG) and heavy (≥ 400 EPG).
Blood collection and processing.
A finger prick sample of blood was collected by using Microvette CB 300 tubes (Sarstedt Inc., Newton, NC). Hemoglobin concentrations were determined for all blood specimens by using a portable, battery-operated hemoglobinometer (HemoCue, Angelholm, Sweden). Thin and thick blood smears were analyzed by microscopy to determine the malaria status of each participant.
Serum samples were separated within 12 hours of collection and stored at −20°C in separate cryovials until analyzed. Serum ferritin (Alpha Diagnostic International, San Antonio, TX) and sTfR (R&D Systems, Minneapolis, MN) levels were measured by using an enzyme-linked immunosorbent assay. All samples were run in duplicate, and the mean value was recorded for each sample. Schistosoma mansoni adult microsomal antigen immunoblot strips were made and used as described.24,25
Socioeconomic status, infection intensity, and statistical analyses.
Socioeconomic status (SES) scores were determined by using an asset questionnaire that addresses ownership of consumer items, drinking water source, toilet facilities, and other assets.26 Contingency analyses were performed with SAS software (SAS Institute, Cary, NC), including a multivariate model that adjusted for SES. The Mantel-Haenszel chi-square test was used to evaluate the role of SES as a potential confounder of the relationship between S. mansoni infection status and the mechanisms of anemia. The SES values were divided into quintiles and then stratified; the top two quintiles were compared with the lower three quintiles in the analysis. The chi-square test and chi-square analysis for trend were used to analyze the relationship between the mechanism of anemia and the intensity of S. mansoni infection. The Kruskal-Wallis test was used to compare SF and sTfR values across the intensities of infection, and groups. Hemoglobin values for each infection intensity were analyzed using one-way analysis of variance.
Definitions.
Schistosoma mansoni infection status was defined as having either one S. mansoni egg on one slide of a single fecal examination or displaying seropositivity with a S. mansoni adult microsomal antigen immunoblot. Iron deficiency anemia was defined as the presence of anemia (Hb level < 12 g/dL) and an SF level < 30 ng/mL. This cut-off value has been demonstrated to have a high sensitivity and specificity for detecting IDA.18 Anemic children with ferritin levels associated with being iron replete (SF level > 100 ng/mL) were defined as having AI.13,19,22 Children with anemia, an intermediate ferritin level (30–100 ng/mL), and an sTfR:log SF ratio > 2 were defined as having AI with true iron deficiency.13 Children with anemia, an intermediate ferritin level, and an sTfR:log SF ratio < 1 were defined as having AI.13 For study participants with a ratio between 1 and 2, and reference values for sTfR and SF levels, the result was indeterminate and no mechanism of anemia was defined.
Results
Samples from 206 children 9–12 years of age were analyzed. Of this group, 106 children were S. mansoni-infected and 100 were S. mansoni-uninfected. Of these children, SF and sTfR values were determined for 161 children. Because of limited serum volume, only serum ferritin levels were determined for 45 samples. Of the 206 children for whom samples were analyzed, 55% (113) were anemic. In the overall study, 51% (1,388 of 2,745) children were anemic on the basis of age-specific Hb levels determined in the Kenya National Clinical Guidelines.27
Iron deficiency anemia and S. mansoni infection.
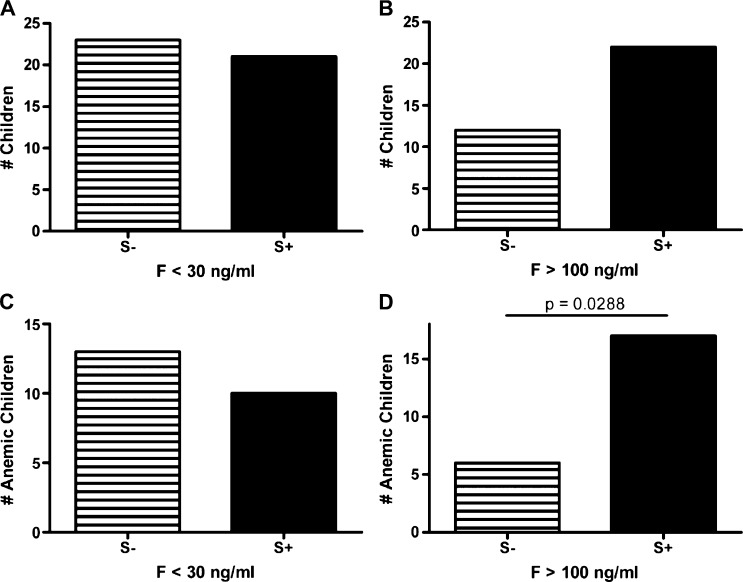
The overall prevalence of iron deficiency (SF level < 30 ng/mL) was high (44 of 206, 21%). Iron deficiency–associated anemia (SF level < 30 ng/mL and Hb < 12 g/dL) was prevalent in 11.2% (23 of 206) of the population. The frequency of participants with reduced serum ferritin levels was similar between S. mansoni-infected and uninfected groups, suggesting that S. mansoni infection did not influence the frequency of iron deficiency in these children (Figure 1). A similar result was obtained when these data were stratified by anemia status or SES as a proxy for access to food containing micronutrients such as iron. Schistosoma mansoni infection status did not influence the frequency of iron deficiency in anemic children. The prevalence of IDA decreased as the S. mansoni infection intensity increased (low = 23.1%, medium = 16.7%, high = 0%), but there was no significant negative correlation between S. mansoni intensity and IDA (Table 1).
Figure 1.
Distribution of ferritin levels in school children, western Kenya. The frequency of children with reduced ferritin levels were comparable between Schistosoma mansoni–infected (S+) and -uninfected (S−) children (1A). Although there was a difference between the frequency of children with increased ferritin levels, this difference was not significant (1B). When the frequency of reduced or elevated ferritin was analyzed for anemic children (1C and 1D), S. mansoni-infected children were 2.99 times more likely to have increased ferritin levels, consistent with anemia of inflammation, than uninfected children (95% confidence interval = 1.1–7.9) (1D).
Table 1.
Anemia, hemoglobin levels, and mechanisms of anemia according to Schistosoma mansoni intensity of infection in school children, western Kenya*
| Characteristic | S. mansoni infection status (EPG) | P | P (trend) | |||
|---|---|---|---|---|---|---|
| Infection intensity | Uninfected (0) | Low (1–99) | Moderate (100–399) | Heavy (≥ 400) | ||
| Anemia (%) | 55 (55) | 39 (55.7) | 7 (46.7) | 13 (61.9) | NS | NS |
| Hemoglobin (g/dL) | 11.6 (11.3–12) | 11.9 (11.5–12.3) | 11.3 (10.2–12.5) | 10.9 (9.9–11.9) | 0.068 | 0.027 |
| Ferritin (ng/mL) | 74.3 (52–96.5) | 78.2 (50.3–106) | 68.7 (35.7–101.8) | 86.4 (59.1–117.7) | NS | NS |
| sTfR (ng/mL) | 2.9 (2.7–3.2) | 2.8 (2.6–3.0) | 3.2 (2.8–3.7) | 2.7 (2.3–3.1) | NS | NS |
| % AI (A)† | 10.9 | 23.1 | 33.3 | 53.8 | 0.006 | 0.0005 |
| % IDA (B)† | 23.6 | 23.1 | 16.7 | 0 | NS | NS |
| % AI and IDA (C)† | 14.5 | 15.4 | 16.7 | 7.7 | NS | NS |
| % A + C‡ | 25.5 | 38.5 | 50 | 61.5 | NS | 0.008 |
| % B + C§ | 38 | 38.5 | 33.3 | 7.7 | NS | NS |
Values in parenthesis are 95% confidence intervals. EPG = eggs per gram (of feces); sTFR = soluble transferrin receptor; AI = anemia of inflammation; IDA = iron deficiency anemia.
Percentage of anemic children, by S. mansoni infection status, who had AI, IDA, or both.
Combined percentage of anemic children, by S. mansoni infection status, who had AI or AI and IDA.
Combined percentage of anemic children, by S. mansoni infection status, who had IDA or AI and IDA.
Anemia of inflammation and S. mansoni infection.
Serum ferritin levels consistent with AI (SF level > 100 ng/mL and Hb level < 12 g/dL) were identified for 24 (11.6%) of the 206 children in the subset. However, the participants with S. mansoni infections were significantly more likely to demonstrate AI than those without schistosomiasis (P = 0.01) (Table 2). Moreover, there was a significant difference in the frequency of AI between the S. mansoni infection groups (P = 0.006) (Table 1) that increased with intensity of infection (P = 0.0005). This finding remained significant even after the exclusion of uninfected subjects from the analysis (P < 0.05).
Table 2.
Comparison of anemia between Schistosoma mansoni–uninfected and –infected participants, western Kenya*
| Characteristic | Uninfected (n = 100) | Infected (n = 106) |
|---|---|---|
| Mechanism of anemia | ||
| Anemic (Hb level ≥ 12 g/dL) | 55 | 58 |
| IDA | 13 | 10 |
| AI† | 6 | 18 |
| IDA and AI | 8 | 8 |
| Undetermined | 28 | 22 |
Hb = hemoglobin; IDA = iron deficiency anemia; AI = anemia of inflammation.
Odds ratio = 3.68; 95% confidence interval = 1.33–10.1, P = 0.01.
Although high SF levels (> 100 ng/mL) tended to be more common in S. mansoni-infected persons who were anemic, the association of schistosomiasis with high ferritin levels did not reach statistical significance (P = 0.065) when we controlled for anemia status, which suggested that anemia is at least partially influenced by other factors. Similarly, for children in the top two SES quintiles, i.e., those children most likely to have adequate food access, the prevalence of elevated ferritin was greater in children with S. mansoni infection than for those without schistosomiasis. This finding provides further support that S. mansoni-associated anemia results from inflammation rather than an iron deficient diet. However, the association of schistosomiasis and high ferritin levels was not significant when we controlled for SES (P = 0.14). For children in the bottom three quintiles, there was no significant difference in the frequency of markers for AI in relation to S. mansoni infection. This finding is consistent with IDA as the predominate mechanism of anemia in persons with a lower SES status.
Serum transferrin receptor and S. mansoni infection.
Inflammation can result in increased SF levels, which when combined with IDA, gives the appearance of normal ferritin levels. To address this possibility in our study population, we analyzed serum ferritin values for children who had ferritin in the reference range (30–100 ng/mL) in conjunction with the respective sTfR values. When the ratio of sTfR to log ferritin concentration is < 1, AI is the projected mechanism; if the ratio is > 2, the anemia is likely associated with inflammation and iron deficiency.13 A similar frequency of anemic children in the S. mansoni-infected (13.8%, n = 8) and uninfected populations (14.5%, n = 8) had AI and IDA (Table 2). Only one S. mansoni-infected child with anemia had an sTfR:log ferritin ratio < 1, suggesting that the mechanism of anemia in this person was AI. There were 22 anemic, S. mansoni-infected children with normal ferritin and sTfR values. As a result, no mechanism of S. mansoni-associated anemia was elucidated for these children.
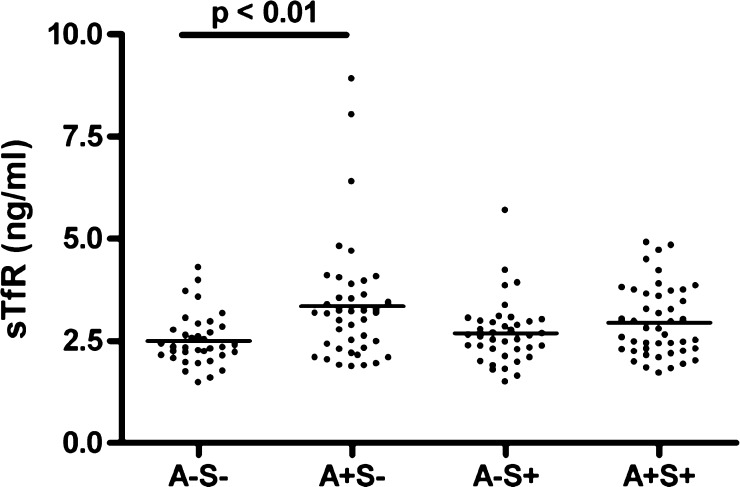
Analysis of sTfR values alone showed a significant difference between S. mansoni uninfected and infected, anemic and non-anemic groups (P = 0.009, by Kruskal-Wallis analysis of variance) (Figure 2). Dunn's post test demonstrated that the serum sTfR values of S. mansoni-negative anemic children are significantly higher than those of children with normal Hb values, suggesting that the mechanism of anemia in this group is IDA (P < 0.01) (Figure 2). In contrast, this difference was not observed between anemic and nonanemic subgroups of S. mansoni-infected persons, which suggests that inflammation is a primary cause of anemia in this group; in the presence of S. mansoni infection, the anemic and non-anemic children had similar mean sTfR levels (P > 0.05) (Figure 2).
Figure 2.
Soluble serum transferrin receptor (sTfR) levels in anemic (A+) and nonanemic (A−) children with (S+) or without (S−) schistosomiasis, western Kenya. The four groups differed significantly (P = 0.009, by Kruskal-Wallis test). sTfR was significantly increased in uninfected, anemic children compared with children who had no anemia or schistosomiasis (P < 0.01, by Dunn's post test).
Infection intensity.
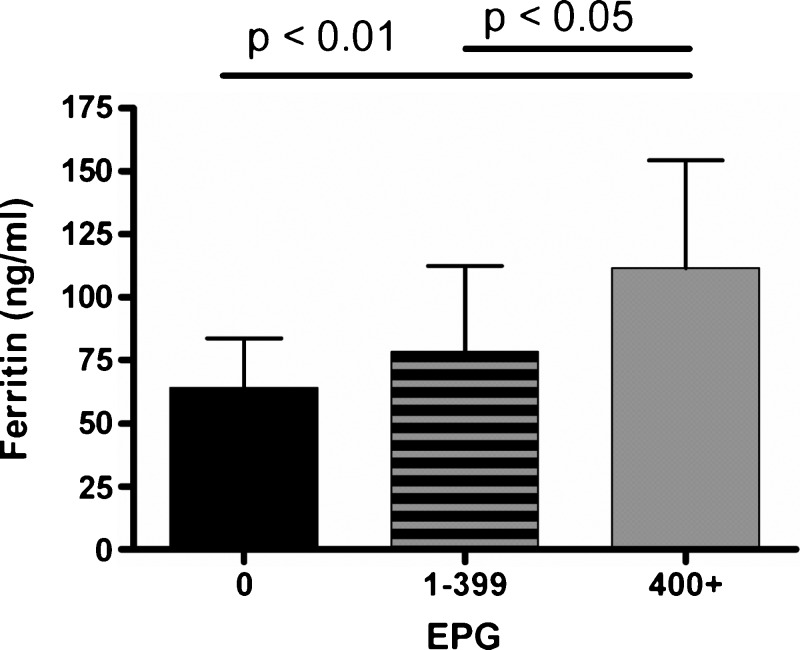
When Hb values were analyzed by S. mansoni infection intensity for all 206 children, the differences between categories of intensity were not significant (P = 0.068) (Table 1), but mean Hb values decreased with increasing infection intensity (P = 0.027, by chi-square test for trend). When the median ferritin values were compared with infection intensity, anemic children with high infection intensities had a median ferritin level that differed significantly from that of uninfected anemic children (P < 0.01) (Figure 3). Because there were only six children with anemia and a moderate infection intensity, we combined the low and moderate infection intensity groups. The median ferritin level for low and medium infection intensity groups did not significantly differ from the uninfected anemic persons. However, when the median ferritin levels for low and medium S. mansoni intensities was compared with that of the high intensity group, there was a significant difference (P < 0.05) (Figure 3). It is noteworthy that the median ferritin value for the high infection intensity, anemic group was 103 ng/mL (range = 30–303 ng/mL), which was slightly above the AI cutoff value.
Figure 3.
Ferritin levels by Schistosoma mansoni infection intensity in school children, western Kenya. Data represent means and upper 95% confidence intervals. Kruskal-Wallis analysis indicated a significant difference in the median ferritin values for the three eggs per gram (of feces) (EPG) categories (P = 0.004). Dunn's post test analysis indicated a significant difference between the uninfected and highly infected groups (P < 0.01) and between the low/medium intensity infection group and the high intensity group (P < 0.05).
Discussion
Several studies have shown an association between S. mansoni infection and anemia. However, this relationship is poorly understood and the mechanisms contributing to anemia are complex.3,6 Although IDA has been a proposed mechanism of schistosomiasis-associated anemia because of extra-corporal blood loss from egg movement through the intestinal or bladder wall, the mechanism of S. mansoni-associated anemia has not been studied. To elucidate the contributing mechanisms in S. mansoni-associated anemia, we excluded participants with concomitant hookworm or malaria infections because these parasites are independently associated with anemia. This initial investigation of the mechanism of S. mansoni-associated anemia suggests that AI is the primary contributing mechanism of anemia.
To investigate the contribution of IDA to schistosomiasis-associated anemia, we evaluated the prevalence of IDA across all S. mansoni infection intensities. We observed that the prevalence of IDA (Hb level < 12 g/dL and SF level < 30 ng/mL) decreased as S. mansoni infection intensity increased. In addition, we did not detect a difference in the prevalence of IDA between S. mansoni-infected and -uninfected children (Figure 1C). Decreased iron stores linked with IDA did not seem to be the predominate mechanism of S. mansoni-associated anemia, and iron deficiency was not sufficient to explain S. mansoni-associated anemia in this population.
We also evaluated the possible contributing role of AI to S. mansoni-associated anemia by measurement of SF and sTfR levels. We found the same frequency of children with evidence of AI and IDA in the S. mansoni-infected and -uninfected groups (Table 2). We analyzed the difference in the prevalence of AI (SF level > 100 ng/mL or 30–100 ng/mL with an sTfR:log SF ratio < 1) across all infection intensities. In this cross-sectional study, the intensity of S. mansoni infection was significantly associated with AI (P = 0.0005) (Table 1). When the median ferritin values were analyzed across all S. mansoni infection intensities, anemic children with high intensity infections differed significantly from uninfected anemic children (P < 0.01) (Figure 3). Furthermore, the median ferritin value for the high intensity infection anemic group was 103 ng/mL and the range of values was 30–303 ng/mL, which excluded IDA alone as a mechanism of anemia in this group. The results suggested that the mechanism of anemia in all children with high intensity infections was either AI alone or a combination of IDA and AI (Table 1). Finally, the odds of a child having AI were much more likely in S. mansoni-infected persons than uninfected persons (odds ratio = 3.68, 95% confidence interval = 1.33–10.1). Thus, our analysis of the mechanism of S. mansoni-associated anemia from measurements of SF and sTfR levels supports an inflammatory mechanism more than one involving iron deficiency.
Soluble transferrin receptor is not appreciably affected by inflammation, making it a valuable iron status marker when inflammation is present. However, the concentration of sTfR in serum is increased during iron deficiency. When sTfR levels were analyzed independently, significantly higher levels of sTfR were observed in the anemic S. mansoni-uninfected children when compared with nonanemic S. mansoni-uninfected children (Figure 2). Conversely, the mean sTfR level in anemic S. mansoni-infected children compared with that in nonanemic S. mansoni-infected children was not statistically different. The absence of an increase in sTfR levels suggests that IDA is not a primary contributing mechanism of anemia in the context of S. mansoni infection but that AI predominates.
There were several limitations to this study. The finger prick blood volume restricted the serum volume considerably. The serum volume for most samples was, at best, adequate to run the ferritin and sTfR enzyme-linked immunosorbent assays in duplicate. The number of persons included was reduced because of a greater malaria prevalence in the overall study than expected, and all positive blood smear patients were omitted from the analysis because of the association between malaria infection and anemia. In addition, S. mansoni prevalence in this study area was less than what had been observed in previous studies in this area, resulting in fewer participants with S. mansoni infection and anemia. With a low number of patients in the moderate-intensity and high-intensity S. mansoni infection groups, we were limited in our statistical analyses. Although it is unlikely that children in this age group were positive for human immunodeficiency virus (HIV), we did not determine HIV status for the study participants. Thus, HIV positivity in the children could have contributed to AI and confounded our results.
Because of reduced sample volumes and number of eligible participants, we had to limit the number of biomarkers we investigated. Although we did not have an independent measure of inflammation for this study, we did have the controlled variable of chronic disease, S. mansoni infection, in which we assessed anemia. Additional measurement of markers of inflammation, such as pro-inflammatory cytokines, hepcidin and C-reactive protein, could have provided additional support for the mechanism of AI in schistosomiasis-associated anemia.6,11 Although pro-inflammatory cytokine markers and C-reactive protein are less specific for diagnosing AI, there is great promise for future use of hepcidin, a putative mediator of anemia of inflammation.28 The results from the first human hepcidin immunoassay were reported in 2008, but a commercial kit is not yet available.29 Further studies in S. mansoni-infected school children with larger sample sizes are needed to confirm whether these results are generalizable.
Our findings suggest that anemic study participants who were not infected with S. mansoni had reduced serum ferritin levels and increased sTfR levels, consistent with IDA. In S. mansoni-infected anemic persons, we found that patients were significantly more likely to have increased ferritin levels, which correlates with patients who have storage forms of iron rather than bioavailable forms, likely the result of AI. Given the etiologic differences of reduced bioavailable iron during IDA and AI, treatment strategies also differ substantially. Iron supplementation is recommended in the context of true IDA. However, it has limited utility in the context AI when iron is sequestered from iron-dependent host tissues.30–34 In malaria-endemic areas such as western Kenya, there is growing concern that iron therapy or iron replete state may increase the risk of malaria morbidity, which further supports weighing the costs and benefits of iron provision, particularly in the presence of AI.35,36 The current therapeutic recommendation for AI is treatment of the underlying infection or condition.37
This study showed that AI is associated with S. mansoni-infection in children, which is consistent with studies of anemia in S. japonicum-infected persons.10,11 Recently, cognitive deficits were found to be associated with non-IDA (primarily AI) as a result of S. japonicum infection.38 It will be important to determine if AI plays a role in cognitive deficits in children with S. mansoni. This information will contribute to the design of treatment programs that address the underlying disease, S. mansoni infection, and ameliorate schistosomiasis-associated morbidities.
ACKNOWLEDGMENTS
We thank the Ministry of Public Health and Sanitation and all study participants for their cooperation. This study is published with the permission of the Director, Kenya Medical Research Institute.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Financial support: This study was supported in part by an appointment to the Emerging Infectious Diseases Fellowship Program administered by the Association of Public Health Laboratories and funded by a grant from the Schistosomiasis Research Program at the DBL – Institute for Health Research and Development and the Centers for Disease Control and Prevention.
Authors' addresses: Sara E. Butler, Susan P. Montgomery, and W. Evan Secor, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: saebutler@gmail.com, zqu6@cdc.gov, and was4@cdc.gov. Erick M. Muok, Kezia Odhiambo, Pauline M. N. Mwinzi, and Diana M. S. Karanja, Center for Global Health Research, Kenya Medical Research Institute, Kisumu, Kenya, E-mails: EMuok@kemricdc.org, kezahinyi@yahoo.com, PMwinzi@kemricdc.org, and DKaranja@kemricdc.org.
References
- 1.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.Global Network Schistosomiasis Fact Sheet. 2009. http://globalnetwork.org/about-ntds/factsheets Available at. Accessed August 20, 2009.
- 3.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson L. The impact of schistosomiasis on human nutrition. Parasitology. 1993;107((Suppl)):S107–S123. doi: 10.1017/s0031182000075545. [DOI] [PubMed] [Google Scholar]
- 5.Nelson M. Anaemia in adolescent girls: effects on cognitive function and activity. Proc Nutr Soc. 1996;55:359–367. doi: 10.1079/pns19960035. [DOI] [PubMed] [Google Scholar]
- 6.Friedman JF, Kanzaria HK, McGarvey ST. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol. 2005;21:386–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 7.United Nations Administrative Committee on Coordination, Standing Committee on Nutrition Fourth Report on the World Nutrition Situation: Nutrition throughout the Life Cycle. 2000. http://www.unscn.org/layout/modules/resources/files/rwns4.pdf Available at. Accessed July 20, 2010.
- 8.Koukounari A, Estambale BB, Njagi JK, Cundill B, Ajanga A, Crudder C, Otido J, Jukes MC, Clarke SE, Brooker S. Relationships between anaemia and parasitic infections in Kenyan schoolchildren: a Bayesian hierarchical modelling approach. Int J Parasitol. 2008;38:1663–1671. doi: 10.1016/j.ijpara.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolentino K, Friedman JF. An update on anemia in less developed countries. Am J Trop Med Hyg. 2007;77:44–51. [PubMed] [Google Scholar]
- 10.Friedman JF, Kanzaria HK, Acosta LP, Langdon GC, Manalo DL, Wu H, Olveda RM, McGarvey ST, Kurtis JD. Relationship between Schistosoma japonicum and nutritional status among children and young adults in Leyte, the Philippines. Am J Trop Med Hyg. 2005;72:527–533. [PubMed] [Google Scholar]
- 11.Leenstra T, Acosta LP, Langdon GC, Manalo DL, Su L, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF. Schistosomiasis japonica, anemia, and iron status in children, adolescents, and young adults in Leyte, Philippines. Am J Clin Nutr. 2006;83:371–379. doi: 10.1093/ajcn/83.2.371. [DOI] [PubMed] [Google Scholar]
- 12.Kanzaria HK, Acosta LP, Langdon GC, Manalo DL, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF. Schistosoma japonicum and occult blood loss in endemic villages in Leyte, the Philippines. Am J Trop Med Hyg. 2005;72:115–118. [PubMed] [Google Scholar]
- 13.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 14.Malope BI, MacPhail AP, Alberts M, Hiss DC. The ratio of serum transferrin receptor and serum ferritin in the diagnosis of iron status. Br J Haematol. 2001;115:84–89. doi: 10.1046/j.1365-2141.2001.03063.x. [DOI] [PubMed] [Google Scholar]
- 15.Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052–1057. [PubMed] [Google Scholar]
- 16.Lee EJ, Oh E, Park Y, Lee HK, Kim BK. Soluble transferrin receptor (sTfR), ferritin, and sTfR/log ferritin index in anemic patients with nonhematologic malignancy and chronic inflammation. Clin Chem. 2002;48:1118–1121. [PubMed] [Google Scholar]
- 17.Suominen P, Punnonen K, Rajamaki A, Irjala K. Serum transferrin receptor and transferrin receptor-ferritin index identify healthy subjects with subclinical iron deficits. Blood. 1998;92:2934–2939. [PubMed] [Google Scholar]
- 18.Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG. Clinical utility of soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44:45–51. [PubMed] [Google Scholar]
- 19.Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1((Suppl 1)):S4–S8. doi: 10.2215/CJN.01490506. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson BJ, Skikne BS, Simpson KM, Baynes RD, Cook JD. Serum transferrin receptor distinguishes the anemia of chronic disease from iron deficiency anemia. J Lab Clin Med. 1992;119:385–390. [PubMed] [Google Scholar]
- 21.Ioannou G, Spector J, Scott K, Rockey DC. Prospective evaluation of a clinical guideline for the diagnosis and management of iron deficiency anemia. Am J Med. 2002;113:281–287. doi: 10.1016/s0002-9343(02)01226-3. [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation Kidney Dialysis Outcome Quality Initiative (KDOQI) Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47((Suppl 3)):S11–S145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Brugnara C. Iron deficiency and erythropoiesis: new diagnostic approaches. Clin Chem. 2003;49:1573–1578. doi: 10.1373/49.10.1573. [DOI] [PubMed] [Google Scholar]
- 24.Tsang VC, Tsang KR, Hancock K, Kelly MA, Wilson BC, Maddison SE. Schistosoma mansoni adult microsomal antigens, a serologic reagent. I. Systematic fractionation, quantitation, and characterization of antigenic components. J Immunol. 1983;130:1359–1365. [PubMed] [Google Scholar]
- 25.Tsang VC, Hancock K, Maddison SE, Beatty AL, Moss DM. Demonstration of species-specific and cross-reactive components of the adult microsomal antigens from Schistosoma mansoni and S. japonicum (MAMA and JAMA) J Immunol. 1984;132:2607–2613. [PubMed] [Google Scholar]
- 26.Gwatkin DR, Rustein S, Johnson K, Pande R, Wagstaff A. Socio-Economic Differences in Health, Nutrition and Population in Kenya. Washington, DC: HNP/Poverty Thematic Group, The World Bank; 2000. [Google Scholar]
- 27.Government of Kenya Ministry of Health . Nutritional and haematologic conditions. In: Kimathi NA, Micheni JN, Muriithi A, editors. Clinical Guidelines for Diagnosis and Treatment of Common Conditions in Kenya. Nairobi, Kenya: The Regal Press Kenya Ltd.; 2002. pp. 190–202. [Google Scholar]
- 28.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 29.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 30.Weiss G. Pathogenesis and treatment of anaemia of chronic disease. Blood Rev. 2002;16:87–96. doi: 10.1054/blre.2002.0193. [DOI] [PubMed] [Google Scholar]
- 31.Weiss G. Iron and immunity: a double-edged sword. Eur J Clin Invest. 2002;32((Suppl 1)):70–78. doi: 10.1046/j.1365-2362.2002.0320s1070.x. [DOI] [PubMed] [Google Scholar]
- 32.Means RT., Jr Recent developments in the anemia of chronic disease. Curr Hematol Rep. 2003;2:116–121. [PubMed] [Google Scholar]
- 33.Cartwright GE. The anemia of chronic disorders. Semin Hematol. 1966;3:351–375. [PubMed] [Google Scholar]
- 34.Matzner Y, Levy S, Grossowicz N, Izak G, Hershko C. Prevalence and causes of anemia in elderly hospitalized patients. Gerontology. 1979;25:113–119. doi: 10.1159/000212328. [DOI] [PubMed] [Google Scholar]
- 35.Oppenheimer SJ, Gibson FD, MacFarlane SB, Moody JB, Harrison C, Spencer A, Bunari O. Iron supplementation increases prevalence and effects of malaria: report on clinical studies in Papua New Guinea. Trans R Soc Trop Med Hyg. 1986;80:603–612. doi: 10.1016/0035-9203(86)90154-9. [DOI] [PubMed] [Google Scholar]
- 36.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, Kabole FM. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 37.Leenstra T, Coutinho HM, Acosta LP, Langdon GC, Su L, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF. Schistosoma japonicum reinfection after praziquantel treatment causes anemia associated with inflammation. Infect Immun. 2006;74:6398–6407. doi: 10.1128/IAI.00757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson CL, Acosta LP, Hochberg NS, Olveda RM, Jiz M, McGarvey ST, Kurtis JD, Bellinger DC, Friedman JF. Anemia of inflammation is related to cognitive impairment among children in Leyte, the Philippines. PLoS Negl Trop Dis. 2009;10:e533. doi: 10.1371/journal.pntd.0000533. [DOI] [PMC free article] [PubMed] [Google Scholar]