Abstract
Animal and human studies suggest that Schistosoma mansoni infection may increase risk of human immunodeficiency virus (HIV) acquisition. Therefore, we tested 345 reproductive age women in rural Tanzanian villages near Lake Victoria, where S. mansoni is hyperendemic, for sexually transmitted infections (STIs) and schistosomiasis by circulating anodic antigen (CAA) serum assay. Over one-half (54%) had an active schistosome infection; 6% were HIV-seropositive. By univariate analysis, only schistosome infection predicted HIV infection (odds ratio [OR] = 3.9, 95% confidence interval = [1.3–12.0], P = 0.015) and remained significant using multivariate analysis to control for age, STIs, and distance from the lake (OR = 6.2 [1.7–22.9], P = 0.006). HIV prevalence was higher among women with more intense schistosome infections (P = 0.005), and the median schistosome intensity was higher in HIV-infected than -uninfected women (400 versus 15 pg CAA/mL, P = 0.01). This finding suggests that S. mansoni infection may be a modifiable HIV risk factor that places millions of people worldwide at increased risk of HIV acquisition.
Introduction
Schistosomiasis is caused by a parasitic infection that affects over 200 million people worldwide, with approximately 85% of cases in Africa.1,2 Previously, in a cross-sectional study of Tanzanian women, we found that the odds of being infected with human immunodeficiency virus (HIV) were fourfold higher for subjects with Schistosoma haematobium infection than subjects without S. haematobium,3 and we postulated that the chronic genital inflammation caused by S. haematobium eggs pre-disposes to HIV infection. We observed a similar trend in women with S. mansoni infection, although the numbers were small, and the association did not reach statistical significance (unpublished data). Primate models support the hypothesis that S. mansoni infection predisposes to HIV infection. Rhesus macaques with active S. mansoni infection were 17 times more susceptible to simian HIV (SHIV) acquisition after rectal inoculation than macaques without S. mansoni.4 Although a variety of interactions between S. mansoni and HIV infection in humans have been described,5–10 a direct association between active S. mansoni and HIV has not been documented in humans. If S. mansoni is a risk factor for HIV acquisition, this finding could have major implications for HIV prevention work in much of the world.
We, therefore, performed a cross-sectional study to explore the relationship between S. mansoni and HIV infections in women living on the shores of Lake Victoria, including screening for other genital tract infections, which are well-known HIV risk factors, to adjust our analysis for any possible confounders. The screening of large numbers of women in S. mansoni-endemic villages was facilitated by the use of a sensitive and specific serum test for the diagnosis of Schistosoma infection. The circulating anodic antigen (CAA) test detects a Schistosoma worm antigen in the serum, and it is 80–95% sensitive and 98–100% specific for diagnosis of schistosomiasis.11–13 CAA is a highly glycosylated excretory antigen originating from the parasite gut and released into the host blood circulation when the worm regurgitates the undigested compounds of the gut.14 Schistosome circulating antigen detection performs well in both HIV-negative and -positive individuals,9 and it is recognized by the World Health Organization as an alternative diagnostic method to parasitologic examination of multiple stool or urine samples.15,16
Materials And Methods
Study sites and subjects.
This study was conducted in seven rural villages with high rates of both S. mansoni and HIV, chosen for their locations at a distance of 1–25 km from Lake Victoria in western Tanzania (Figure 1). Lake Victoria is infested with the Biomphalaria snail, the intermediate host of S. mansoni, and inhabitants of the study sites have among the highest prevalence of S. mansoni infection in the world. Repeat surveys conducted by our group between 1999 and 2011 have documented a high prevalence of S. mansoni in these villages; in contrast, the prevalence of S. haematobium is < 3% in adults in the same villages.3,17–20 HIV is also prevalent in these villages; a 2004 study showed that 8% of adults greater than 15 years of age were HIV-infected.21 The major mode of HIV transmission is heterosexual intercourse, and there are more females than males infected, with a female to male ratio of HIV infection of 1.2:1.0.21
Figure 1.
Lake Victoria, surrounding countries, and the Mwanza study area (shaded).
We recruited women ages 18–50 years who were seeking routine pre-natal, post-natal, or pediatric care for themselves or their newborn children in the seven study villages. The study team visited each of the health posts for 1 week between January of 2010 and August of 2011 and invited consecutive women at the posts to participate in the study.
Study procedures and sample collection.
The study included an oral questionnaire, a gynecologic exam, and phlebotomy. Gynecologic examinations included wet preparations, which were examined on-site for diagnoses of Trichomonas vaginalis, Candida species, and bacterial vaginosis by the criteria in the work by Amsel and others.22 Endocervical swabs were collected for Chlamydia trachomatis and Neisseria gonorrhoeae testing at the laboratory of the National Institute for Medical Research (NIMR) in Mwanza, Tanzania. Venous blood was also collected, and serum was separated and stored at –20°C at the NIMR laboratory. Testing for syphilis and preparation of a portion of CAA test strips were performed at NIMR. Additional test strip preparation and all test strip reading were performed at the Leiden University Medical Center.
Women were offered on-site voluntary HIV counseling and testing in Kiswahili by a trained nurse counselor. Rapid tests (SD Bioline; Standard Diagnostics, Inc., Kyonggi-do, South Korea) were used with confirmatory testing by a second test (Alere Determine; Inverness Medical, Princeton, NJ) as per the national testing algorithm, and patients received their results and post-test counseling on the same day.
Patients diagnosed with HIV were referred to the local HIV clinic for free care and treatment. Women with trichomoniasis, candidiasis, or bacterial vaginosis were provided treatment on the same day. Women who tested positive for gonorrhea, Chlamydia, syphilis, or schistosomiasis were treated at a follow-up visit as soon as laboratory results were available.
Laboratory analyses.
Schistosoma.
CAA is a glycoprotein that is produced by gut epithelial cells of schistosomal worms14 and secreted in large quantities into the host bloodstream during active infection.23 The CAA test does not distinguish S. haematobium from S. mansoni infection. The test usually becomes negative within 1 week of successful antischistosomal therapy.24
CAA testing was performed using the upconverting phosphor (UCP) technology lateral flow assay as previously described.12 Serum was treated with 4% (wt/vol) trichloroacetic acid to remove proteins and antibody complexes. After centrifugation, the supernatant was mixed with an assay buffer containing an anti-CAA mouse monoclonal antibody conjugated to UCP reporter particles and incubated for 1 hour at 37°C. The mixture was applied to a lateral flow test strip with a capture line of the same antibody, and chromatography was permitted to continue until strips were dry. Strips were read using a modified Packard Fluorocount meter, and test line results were normalized to the control line results for each test strip. A CAA value ≥ 10 pg/mL was considered positive based on a series of negative controls (highest value plus 2 SDs). CAA values were stratified by intensity as greater or less than 3,000 pg/mL, which represents approximately 100 eggs per 1 gram of stool.25
C. trachomatis and N. gonorrhoeae.
DNA was extracted from endocervical swab specimens and tested for C. trachomatis and N. gonorrhoeae using Amplicor CT/NG specimen preparation, amplification, internal control, and detection kits (Roche Molecular Systems, Branchburg, NJ). Gonorrhea results were confirmed using 16S rRNA PCR testing.
Syphilis serology.
Serum was tested for syphilis using the Rapid Plasma Reagin (RPR) test, with confirmation of positive RPR results by the Treponema pallidum Particle Agglutination assay (TPPA).
Ethics.
The study was approved by the Institutional Review Boards at Bugando Medical Center and Weill Cornell Medical College and the National Institute for Medical Research in Tanzania. All women were informed about the study by a nurse fluent in Kiswahili and provided written informed consent before participation.
Statistical methods.
Data were entered into a REDCap database (Vanderbilt University, Nashville, TN) and analyzed using Stata version 11 (Stata Corporation, College Station, TX). Continuous variables were summarized by median and interquartile range (IQR), and categorical variables were summarized by frequency and percentage. Simple logistic regression (for univariate analysis) followed by multiple logistic regression (for multivariate analysis) were used to examine factors associated with HIV. In multiple logistic regression models, comprehensive adjustment was not pursued, because it yielded failure in convergence. Associations between factors and the endpoint were summarized using odds ratios (ORs) with 95% confidence intervals (CIs) and associated P values. In regression analyses, age and distance from the lake were analyzed as continuous variables, whereas other variables were analyzed as binary variables.
We also compared the intensity of schistosome infection between the HIV-positive and -negative groups using the non-parametric Wilcoxon test (because of severe non-normality of this data) and the non-parametric Jonckheere–Terpstra (JT) trend test.26 Two-sided hypotheses/tests were assumed for calculation of all CIs and P values.
Results
Patient characteristics.
We invited 432 eligible women living in seven villages within 25 km of Lake Victoria to participate. Of these women, 345 (80%) women provided informed consent and completed all study procedures.
The median age in this population was 30 years (IQR = 24–36) (Table 1). All but one woman reported contact with potentially contaminated water at least daily. Over three-fourths of women had not been treated for schistosomiasis in the past 5 years, and more than one-half of women had never been treated.
Table 1.
Baseline characteristics of 345 women in S. mansoni-endemic villages near Lake Victoria in Tanzania
| Characteristic | Value |
|---|---|
| Age in years | |
| Median | 30 |
| IQR | 24–36 |
| Number of living children | |
| Median | 4 |
| IQR | 2–6 |
| Reports more than one sexual partner in the past 6 months | 12 (5%) |
| Reports no current sexual partner | 23 (9%) |
| History of miscarriage | 116 (39%) |
| Distance of village from Lake Victoria (km) | |
| 0–8 | 123 (36%) |
| 9–17 | 125 (36%) |
| 18–25 | 97 (28%) |
| Daily contact with potentially infectious water | 344 (99%) |
| Never previously treated for schistosomiasis | 178 (52%) |
| Never previously tested for HIV | 114 (42%) |
Non-missing data were included in each calculation.
Prevalence of schistosomiasis, genital tract infections, and HIV.
We diagnosed active schistosomiasis in 185 (54%) women in this study population (Table 2). Among those women harboring a schistosome infection, the median CAA value was 446 pg/mL (IQR = 86–2,338); 21 of 345 (6%) women were HIV-infected. The prevalence of other gynecologic infections ranged from gonorrhea in 1 (< 1%) woman to reactive syphilis serology in 26 (8%) women (Table 2).
Table 2.
Prevalence of infections in 345 rural Tanzanian women
| Infection | Prevalence |
|---|---|
| Schistosomiasis | 185 (54%) |
| HIV-1 | 21 (6%) |
| Syphilis | 26 (8%) |
| Gonorrhea | 1 (0.3%) |
| Chlamydia | 15 (4%) |
| Trichomoniasis | 4 (1%) |
| Bacterial vaginosis | 19 (6%) |
| Candidiasis | 15 (4%) |
Factors associated with schistosome infection.
In both univariate and multivariate analysis, distance from Lake Victoria was the only factor that was significantly associated with schistosome infection, with an OR of 0.95 (0.92–0.97) for each increasing kilometer away from the lake (P < 0.001) (Table 3). The highest prevalence was 81% in a village situated < 1 km from the shore of Lake Victoria, whereas the lowest prevalence (38–42%) was observed in villages that were 12–22 km inland. Age, prior receipt of praziquantel, and genital tract infections did not significantly predict whether women were currently infected with schistosomes.
Table 3.
Associations of potential risk factors with schistosome infection (univariate analysis)
| Potential risk factor | Schistosomiasis-positive (N = 185) | Schistosomiasis-negative (N = 160) | OR for association with schistosome infection (95% CI) | P value |
|---|---|---|---|---|
| Median age (IQR) | 30 (25–36) | 29 (23–37) | 1.02 (0.99–1.05) | 0.15 |
| Received praziquantel in past 5 years | 21 (19%) | 16 (15%) | 1.4 (0.7–2.9) | 0.35 |
| Gonorrhea and/or Chlamydia cervicitis | 7 (4%) | 9 (6%) | 0.7 (0.2–1.8) | 0.42 |
| Syphilis | 17 (10%) | 9 (6%) | 1.8 (0.8–4.0) | 0.19 |
| Median kilometers from Lake Victoria (IQR) | 10 (1–14) | 12 (8–22) | 0.95 (0.92–0.97) | < 0.001 |
Non-missing data were included in each calculation.
Factors associated with HIV infection.
Schistosome infection was significantly associated with HIV infection, whereas other factors were not. Of 185 women with positive CAA levels, 17 (9%) women were HIV-infected, whereas of 160 women without detectable CAA, 4 (3%) women were HIV-infected (OR = 3.9 [95% CI = 1.3–12.0], P = 0.015) (Table 4). The ORs for Chlamydia and syphilis were both 2.4, although neither was statistically significant. The OR for each increasing 1 year of age was 1.00 (0.95–1.06; P = 0.90). The OR for each increasing 1 km away from Lake Victoria, which correlates with living closer to major roads, was 1.01 (0.95–1.07; P = 0.71). In multivariate analysis, schistosome infection was the single best predictor of HIV infection. Other variables were not significant and did not affect the relationship between Schistosoma and HIV infection. Specifically, when multiple regression models were used to control for age, sexually transmitted infections (STIs), and distance from the lake, the association between Schistosoma and HIV remained statistically significant with an OR of 6.2 (1.7–22.9; P = 0.006).
Table 4.
Associations of potential risk factors with HIV infection (univariate analysis)
| Potential risk factor | HIV-positive (N = 21) | HIV-negative (N = 324) | OR for association with HIV infection (95% CI) | P value |
|---|---|---|---|---|
| Median age (IQR) | 32 (25–36) | 30 (24–36) | 1.00 (0.95–1.06) | 0.90 |
| Median kilometers from Lake Victoria (IQR) | 12 (10–22) | 10 (8–22) | 1.01 (0.95–1.07) | 0.71 |
| Chlamydia infection | 2 (10%) | 13 (4%) | 2.4 (0.5–11.4) | 0.27 |
| Gonorrhea infection | 0 | 1 (3%) | – | – |
| Positive syphilis serology | 3 (16%) | 23 (7%) | 2.4 (0.6–8.7) | 0.19 |
| Trichomonas infection | 0 | 4 (1%) | – | – |
| Bacterial vaginosis | 0 | 19 (6%) | – | – |
| Candidal infection | 0 | 15 (5%) | – | – |
| Schistosome infection | 17 (81%) | 168 (52%) | 3.9 (1.3–12.0) | 0.015 |
Non-missing data were included in each calculation. For age and kilometers from the lake, the OR corresponds to the OR estimate for one unit increase in the exposure (i.e., per 1-year increase in age and 1-km increase in distance).
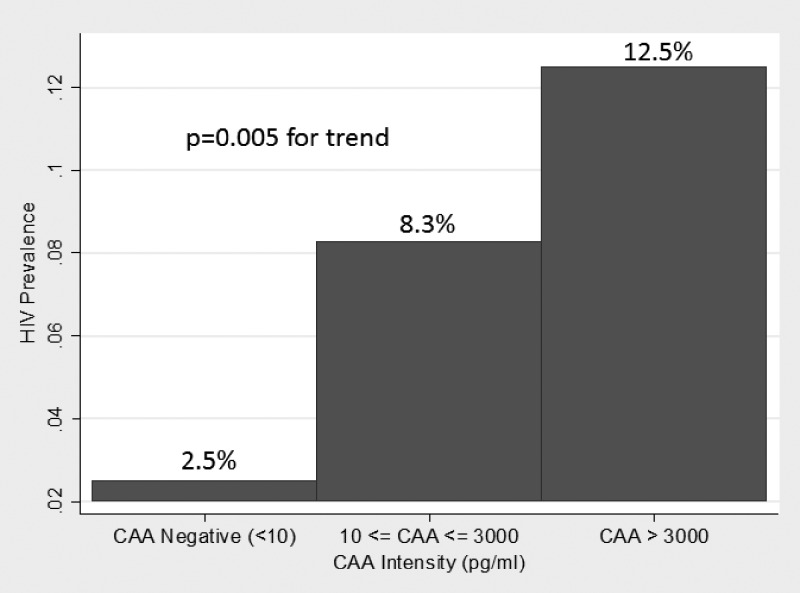
The median intensity of schistosome infection was significantly higher among HIV-infected than -uninfected women (400 versus 15 pg/mL, P = 0.01). When women were stratified by CAA intensity, the prevalence of HIV infection significantly increased among groups (P = 0.005 by JT test) (Figure 2).
Figure 2.
Prevalence of HIV infection by intensity of schistosome infection as determined by serum CAA concentration.
Factors associated with other STIs.
Cervicitis caused by gonorrhea and/or Chlamydia was significantly associated with younger age (OR = 0.87 [0.79–0.96] for each increasing year of age, P = 0.006). No other significant associations were observed.
Discussion
In women living in S. mansoni-endemic areas near Lake Victoria, more than one-half were schistosome-infected, and Schistosoma infection was strongly associated with HIV. This finding suggests that S. mansoni infection and the chronic inflammation from the gut caused by S. mansoni eggs may be a risk factor for HIV acquisition. Schistosomiasis may be placing millions of women throughout sub-Saharan Africa at increased risk of becoming HIV-infected, and mass therapy of women of reproductive age for schistosomiasis may be an effective HIV prevention strategy.
Animal models support the hypothesis that S. mansoni infection increases host susceptibility to HIV infection. Studies in macaques showed that the rectal inoculum of SHIV required for SHIV acquisition was 17 times lower in macaques with than without S. mansoni infection.4 In contrast, prior S. mansoni infection did not significantly change the required infectious dose when SHIV was inoculated intravenously.4,27 Researchers postulated that S. mansoni infection increased the number of activated CD4+ T cells in the gut-associated lymphoid tissue (GALT) and thereby increased the optimal targets for SHIV infection. Mouse models of schistosomiasis show that S. mansoni eggs induce a Th17 CD4+ T-cell response in the gut mucosa.28,29 A growing body of literature suggests that HIV preferentially infects Th17 CD4+ T cells in gut and genital tissue mucosa and that increased numbers of these cells may increase susceptibility to HIV infection.30,31 Additional human studies are needed to determine if S. mansoni eggs induce a Th17 cell immune response in the GALT and if these Th17 cells express HIV susceptibility factors, such as CC Chemokine Receptor 5 (CCR5) and integrin α4β7.
Our work is also supported by recent findings from the Rakai region of Uganda, where S. mansoni is endemic.32 As noted perceptively in the work by Secor,33 HIV-infected individuals more often had antibodies to schistosome antigens than HIV-uninfected individuals. Unlike in our study, in which CAA positivity indicates live worm infestation, the detection of antibodies to schistosome antigens does not prove active schistosomiasis. Coupled with our findings, this population-based study lends additional clinical support to our finding that S. mansoni infection and not only S. haematobium infection (as previously reported) is associated with HIV.
In addition, S. mansoni-infected individuals displayed higher densities of the HIV chemokine receptors CCR5 and CXCR4 on their CD4+ T cells and monocytes than individuals with schistosomiasis that had been previously treated.34 S. mansoni infection was also shown to increase the HIV RNA viral load in HIV-positive patients with untreated S. mansoni infection compared with patients with S. mansoni infections that had been treated.35 Several earlier non-randomized studies did not find an effect of praziquantel treatment on viral load,5,6,36,37 but this result has been postulated to be caused by transient increases in the schistosomiasis-conducive Th2 environment immediately after treatment.5,38 S. mansoni-infected individuals are also reported to excrete fewer ova than individuals without HIV.7,10 Our results support these findings that show complex interactions between Schistosoma and HIV infections as well as the growing consensus that schistosome infection, including S. mansoni, may be a risk factor for HIV acquisition.1,33
Our work suggests that, among rural African women in whom the prevalence of genital tract infections is low, schistosome infection may be a major contributor of risk for HIV acquisition. Over one-half of our population was infected with schistosomes, leading to an estimated population-attributable fraction for HIV acquisition caused by schistosome infection of 69% (36–81%) using previously described methods.39 In contrast, the population-attributable fractions for genital tract infections in our population were 7% and below. The direction of our findings supports the well-described association between HIV and STIs, but the inability of our study to show statistical significance may be caused by the low prevalence of STIs in this rural population. We postulate that, in our population and other rural populations with few traditional HIV risk factors, such as multiple sexual partners and high rates of genital tract infections, schistosome infection may play a role as a key driver of HIV transmission. Of note, this study addresses the risk only in women aged 18–50 years who were seen at rural health clinics, and it does not address whether men or younger adolescent girls are also at increased risk.
The CAA test is a valuable diagnostic test for schistosome infections. The antigen becomes detectable in serum approximately 5 weeks after infection.40 CAA levels fall rapidly post-treatment, with a serum half-life of 2 days,24 and they rise with reinfection.41 The test is, therefore, highly time-specific for active schistosome infections. CAA assays have been recommended for serologic screening programs, in which repeated parasitologic examinations of urine and stool are logistically complicated.42 The CAA test has recently been developed into a very sensitive, robust lateral flow strip test, making test performance in rural settings increasingly feasible.12 The test is unable to distinguish between species of schistosomal infections. Therefore, projects relying on CAA testing either will only allow species-specific conclusions when performed in regions with sharp geographic demarcation of schistosome species, like in our study, or will not be able to differentiate between schistosome species.
Schistosomiasis is usually acquired in childhood, infecting 50–90% of children living in endemic areas by early adolescence.2 Thus, acquisition of schistosome worms typically pre-dates sexual activity and concomitant exposure to HIV. The World Health Organization recommends that children in endemic areas receive targeted, periodic school-based praziquantel treatment.1 Women do not often receive mass treatment. In our population, more than one-half of women had never received antischistosomal treatment, despite living in a hyperendemic area and coming into daily contact with unclean water. A policy of routine periodic praziquantel administration for women seeking reproductive healthcare services (including family planning, cervical cancer screening, and pre-natal/post-natal care) would be a safe,1 efficient, and inexpensive way to control schistosomiasis and, moreover, potentially to prevent HIV acquisition in this vulnerable group.
This cross-sectional study shows a strong association but does not prove a causal role of schistosomiasis in HIV acquisition. An interventional trial would be necessary to show causality. However, we feel that the best explanation of our findings is that pre-existing schistosome infection modifies mucosal immunity and predisposes to HIV infection. The highest incidence of schistosome infection typically occurs in childhood between the ages of 5 and 15 years, and in individuals with ongoing exposure, it produces a chronic infection over decades. More than 80% of our study participants denied receiving praziquantel in the past 5 years, and more than one-half reported never being treated. Therefore, it seems most likely that the large proportion of our study participants had chronic, untreated schistosome infection that pre-dated their exposure to HIV. Although it is conversely possible that HIV infection increases susceptibility to schistosomiasis,43 the former explanation for the association most aptly combines both natural history and biological plausibility data. Also, it should be noted that the use of ORs in this study could possibly overestimate the relationship between HIV and schistosome infection compared with relative risk because of the fact that schistosome infection was common in our population.44
In conclusion, more than one-half of rural Tanzanian women seen at health posts in S. mansoni-endemic areas of the Lake Victoria region had evidence of active schistosome infection, and the prevalence of HIV among these women was markedly higher than among those women without schistosome infection. Active S. mansoni infection may be a modifiable HIV risk factor that is contributing to high rates of new HIV infections among millions of women living in sub-Saharan Africa.
ACKNOWLEDGMENTS
We greatly appreciate the assistance of Dr. Jaco J. Verweij in transporting materials for circulating anodic antigen testing to the National Institute for Medical Research laboratory and instructing laboratory technicians in Mwanza in the performance of the test. We also thank Bugando Medical Centre, the National Institute for Medical Research, Mwanza Research Centre, the Mwanza Interventional Trials Unit, Leiden University Medical Center, and Sengerema District Hospital for their support. Most of all, the authors thank the Tanzanian women for their willing participation in this study.
Footnotes
Financial support: This work was supported by the 2009 Merle A. Sande/Pfizer Fellowship Award in International Infectious Diseases, which is awarded annually by the Infectious Diseases Society of America Education & Research Foundation and the National Foundation for Infectious Diseases. This work was also supported by Agency for Healthcare Research and Quality Grant T32HS000066, National Institute for Allergy and Infectious Diseases Grant T32AI007613, and Clinical and Translational Science Center at Weill Cornell Medical College Grant UL1-RR024996 (to J.A.D.). Various improvements of the upconverting phosphor-LF assay for circulating anodic antigen detection, involving the implementation of dry reagents (allowing worldwide shipping), increased sensitivity, and handheld upconverting phosphor readers, were developed, in part, with financial support from US National Institutes of Health Grant UO1DE017855 and the University of Georgia Research Foundation, Inc. (Schistosomiasis Consortium for Operational Research and Evaluation [SCORE] project).
Authors' addresses: Jennifer A. Downs, Robert N. Peck, Warren D. Johnson Jr., and Daniel W. Fitzgerald, Center for Global Health, Weill Cornell Medical College, New York, NY, E-mails: jna2002@med.cornell.edu, rnp2002@med.cornell.edu, wdjohnso@med.cornell.edu, and dfitzgerald@gheskio.org. Govert J. van Dam and Lisette van Lieshout, Department of Parasitology, Leiden University Medical Center, Leiden, The Netherlands, E-mails: G.J.van_Dam@lumc.nl and E.A.van_Lieshout@lumc.nl. John M. Changalucha, National Institute for Medical Research, Mwanza Research Centre, Mwanza, Tanzania, E-mail: jchangalucha@yahoo.com. Paul L. A. M. Corstjens and Claudia J. de Dood, Department of Molecular Cell Biology, Leiden University Medical Center, Leiden, The Netherlands, E-mails: P.Corstjens@lumc.nl and C.J.de_Dood@lumc.nl. Heejung Bang, Division of Biostatistics, Department of Public Health Sciences, University of California, Davis, CA, E-mail: hbang@phs.ucdavis.edu. Aura Andreasen, Department of Clinical Research, London School of Hygiene and Tropical Medicine, London, United Kingdom; and Mwanza Interventional Trials Unit, Mwanza, Tanzania, E-mail: aura.andreasen@mitu.or.tz. Samuel E. Kalluvya, Department of Internal Medicine, Bugando Medical Centre, Mwanza, Tanzania, E-mail: samuelkalluvya@yahoo.com.
References
- 1.World Health Organization . Schistosomiasis, Fact Sheet No. 115. Geneva: World Health Organization; 2012. [Google Scholar]
- 2.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 3.Downs JA, Mguta C, Kaatano GM, Mitchell KB, Bang H, Simplice H, Kalluvya SE, Changalucha JM, Johnson WD, Jr, Fitzgerald DW. Urogenital schistosomiasis in women of reproductive age in Tanzania's Lake Victoria region. Am J Trop Med Hyg. 2011;84:364–369. doi: 10.4269/ajtmh.2011.10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chenine AL, Shai-Kobiler E, Steele LN, Ong H, Augostini P, Song R, Lee SJ, Autissier P, Ruprecht RM, Secor WE. Acute Schistosoma mansoni infection increases susceptibility to systemic SHIV clade C infection in rhesus macaques after mucosal virus exposure. PLoS Negl Trop Dis. 2008;2:e265. doi: 10.1371/journal.pntd.0000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown M, Mawa PA, Joseph S, Bukusuba J, Watera C, Whitworth JA, Dunne DW, Elliott AM. Treatment of Schistosoma mansoni infection increases helminth-specific type 2 cytokine responses and HIV-1 loads in coinfected Ugandan adults. J Infect Dis. 2005;191:1648–1657. doi: 10.1086/429668. [DOI] [PubMed] [Google Scholar]
- 6.Elliott AM, Mawa PA, Joseph S, Namujju PB, Kizza M, Nakiyingi JS, Watera C, Dunne DW, Whitworth JA. Associations between helminth infection and CD4+ T cell count, viral load and cytokine responses in HIV-1-infected Ugandan adults. Trans R Soc Trop Med Hyg. 2003;97:103–108. doi: 10.1016/s0035-9203(03)90040-x. [DOI] [PubMed] [Google Scholar]
- 7.Fontanet AL, Woldemichael T, Sahlu T, van Dam GJ, Messele T, Rinke de Wit T, Masho W, Yeneneh H, Coutinho RA, van Lieshout L. Epidemiology of HIV and Schistosoma mansoni infections among sugar-estate residents in Ethiopia. Ann Trop Med Parasitol. 2000;94:145–155. [PubMed] [Google Scholar]
- 8.Ganley-Leal LM, Mwinzi PN, Cetre-Sossah CB, Andove J, Hightower AW, Karanja DM, Colley DG, Secor WE. Correlation between eosinophils and protection against reinfection with Schistosoma mansoni and the effect of human immunodeficiency virus type 1 coinfection in humans. Infect Immun. 2006;74:2169–2176. doi: 10.1128/IAI.74.4.2169-2176.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kallestrup P, Zinyama R, Gomo E, Butterworth AE, van Dam GJ, Erikstrup C, Ullum H. Schistosomiasis and HIV-1 infection in rural Zimbabwe: implications of coinfection for excretion of eggs. J Infect Dis. 2005;191:1311–1320. doi: 10.1086/428907. [DOI] [PubMed] [Google Scholar]
- 10.Karanja DM, Colley DG, Nahlen BL, Ouma JH, Secor WE. Studies on schistosomiasis in western Kenya: I. evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am J Trop Med Hyg. 1997;56:515–521. doi: 10.4269/ajtmh.1997.56.515. [DOI] [PubMed] [Google Scholar]
- 11.Leutscher PD, van Dam GT, Reimert CM, Ramarakoto CE, Deelder AM, Ornbjerg N. Eosinophil cationic protein, soluble egg antigen, circulating anodic antigen, and egg excretion in male urogenital schistosomiasis. Am J Trop Med Hyg. 2008;79:422–426. [PubMed] [Google Scholar]
- 12.Corstjens PL, van Lieshout L, Zuiderwijk M, Kornelis D, Tanke HJ, Deelder AM, van Dam GJ. Up-converting phosphor technology-based lateral flow assay for detection of Schistosoma circulating anodic antigen in serum. J Clin Microbiol. 2008;46:171–176. doi: 10.1128/JCM.00877-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polman K, Diakhate MM, Engels D, Nahimana S, Van Dam GJ, Falcao Ferreira ST, Deelder AM, Gryseels B. Specificity of circulating antigen detection for schistosomiasis mansoni in Senegal and Burundi. Trop Med Int Health. 2000;5:534–537. doi: 10.1046/j.1365-3156.2000.00600.x. [DOI] [PubMed] [Google Scholar]
- 14.de Water R, Fransen JA, Deelder AM. Ultrastructural localization of the circulating anodic antigen in the digestive tract of Schistosoma mansoni using monoclonal antibodies in an immunogold labeling procedure. Am J Trop Med Hyg. 1986;35:549–558. doi: 10.4269/ajtmh.1986.35.549. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . Report of the Scientific Working Group Meeting on Schistosomiasis. Geneva: World Health Organization; 2006. [Google Scholar]
- 16.World Health Organization . Report of an Informal Working Group on Urogenital Schistosomiasis and HIV Transmission. Geneva: World Health Organization; 2010. [Google Scholar]
- 17.Clements AC, Lwambo NJ, Blair L, Nyandindi U, Kaatano G, Kinung'hi S, Webster JP, Fenwick A, Brooker S. Bayesian spatial analysis and disease mapping: tools to enhance planning and implementation of a schistosomiasis control programme in Tanzania. Trop Med Int Health. 2006;11:490–503. doi: 10.1111/j.1365-3156.2006.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lwambo NJ, Siza JE, Brooker S, Bundy DA, Guyatt H. Patterns of concurrent hookworm infection and schistosomiasis in schoolchildren in Tanzania. Trans R Soc Trop Med Hyg. 1999;93:497–502. doi: 10.1016/s0035-9203(99)90349-8. [DOI] [PubMed] [Google Scholar]
- 19.Malenganisho WL, Magnussen P, Friis H, Siza J, Kaatano G, Temu M, Vennervald BJ. Schistosoma mansoni morbidity among adults in two villages along Lake Victoria shores in Mwanza district, Tanzania. Trans R Soc Trop Med Hyg. 2008;102:532–541. doi: 10.1016/j.trstmh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 20.van Dam GJ, Wichers JH, Ferreira TM, Ghati D, van Amerongen A, Deelder AM. Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen. J Clin Microbiol. 2004;42:5458–5461. doi: 10.1128/JCM.42.12.5458-5461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wambura M, Urassa M, Isingo R, Ndege M, Marston M, Slaymaker E, Mngara J, Changalucha J, Boerma TJ, Zaba B. HIV prevalence and incidence in rural Tanzania: results from 10 years of follow-up in an open-cohort study. J Acquir Immune Defic Syndr. 2007;46:616–623. doi: 10.1097/QAI.0b013e31815a571a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 23.De Jonge N, Gryseels B, Hilberath GW, Polderman AM, Deelder AM. Detection of circulating anodic antigen by ELISA for seroepidemiology of schistosomiasis mansoni. Trans R Soc Trop Med Hyg. 1988;82:591–594. doi: 10.1016/0035-9203(88)90523-8. [DOI] [PubMed] [Google Scholar]
- 24.de Jonge N, De Caluwe P, Hilberath GW, Krijger FW, Polderman AM, Deelder AM. Circulating anodic antigen levels in serum before and after chemotherapy with praziquantel in schistosomiasis mansoni. Trans R Soc Trop Med Hyg. 1989;83:368–372. doi: 10.1016/0035-9203(89)90507-5. [DOI] [PubMed] [Google Scholar]
- 25.Polman K, de Vlas SJ, Gryseels B, Deelder AM. Relating serum circulating anodic antigens to faecal egg counts in Schistosoma mansoni infections: a modelling approach. Parasitology. 2000;121:601–610. doi: 10.1017/s0031182000006843. [DOI] [PubMed] [Google Scholar]
- 26.Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- 27.Siddappa NB, Hemashettar G, Shanmuganathan V, Semenya AA, Sweeney ED, Paul KS, Lee SJ, Secor WE, Ruprecht RM. Schistosoma mansoni enhances host susceptibility to mucosal but not intravenous challenge by R5 clade C SHIV. PLoS Negl Trop Dis. 2011;5:e1270. doi: 10.1371/journal.pntd.0001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shainheit MG, Smith PM, Bazzone LE, Wang AC, Rutitzky LI, Stadecker MJ. Dendritic cell IL-23 and IL-1 production in response to schistosome eggs induces Th17 cells in a mouse strain prone to severe immunopathology. J Immunol. 2008;181:8559–8567. doi: 10.4049/jimmunol.181.12.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutitzky LI, Bazzone L, Shainheit MG, Joyce-Shaikh B, Cua DJ, Stadecker MJ. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL-17. J Immunol. 2008;180:2486–2495. doi: 10.4049/jimmunol.180.4.2486. [DOI] [PubMed] [Google Scholar]
- 30.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, Khoruts A, Frank I, Else J, Schacker T, Silvestri G, Douek DC. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinnon LR, Nyanga B, Chege D, Izulla P, Kimani M, Huibner S, Gelmon L, Block KE, Cicala C, Anzala AO, Arthos J, Kimani J, Kaul R. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011;187:6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 32.Stabinski L, Reynolds SJ, Ocama P, Laeyendecker O, Ndyanabo A, Kiggundu V, Boaz I, Gray RH, Wawer M, Thio C, Thomas DL, Quinn TC, Kirk GD. Rakai Health Sciences Program High prevalence of liver fibrosis associated with HIV infection: a study in rural Rakai, Uganda. Antivir Ther. 2011;16:405–411. doi: 10.3851/IMP1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Secor WE. The effects of schistosomiasis on HIV/AIDS infection, progression and transmission. Curr Opin HIV AIDS. 2012;7:254–259. doi: 10.1097/COH.0b013e328351b9e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Secor WE, Shah A, Mwinzi PM, Ndenga BA, Watta CO, Karanja DM. Increased density of human immunodeficiency virus type 1 coreceptors CCR5 and CXCR4 on the surfaces of CD4(+) T cells and monocytes of patients with Schistosoma mansoni infection. Infect Immun. 2003;71:6668–6671. doi: 10.1128/IAI.71.11.6668-6671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallestrup P, Zinyama R, Gomo E, Butterworth AE, Mudenge B, van Dam GJ, Gerstoft J, Erikstrup C, Ullum H. Schistosomiasis and HIV-1 infection in rural Zimbabwe: effect of treatment of schistosomiasis on CD4 cell count and plasma HIV-1 RNA load. J Infect Dis. 2005;192:1956–1961. doi: 10.1086/497696. [DOI] [PubMed] [Google Scholar]
- 36.Lawn SD, Karanja DM, Mwinzia P, Andove J, Colley DG, Folks TM, Secor WE. The effect of treatment of schistosomiasis on blood plasma HIV-1 RNA concentration in coinfected individuals. AIDS. 2000;14:2437–2443. doi: 10.1097/00002030-200011100-00004. [DOI] [PubMed] [Google Scholar]
- 37.Modjarrad K, Zulu I, Redden DT, Njobvu L, Lane HC, Bentwich Z, Vermund SH. Treatment of intestinal helminths does not reduce plasma concentrations of HIV-1 RNA in coinfected Zambian adults. J Infect Dis. 2005;192:1277–1283. doi: 10.1086/444543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Secor WE. Immunology of human schistosomiasis: off the beaten path. Parasite Immunol. 2005;27:309–316. doi: 10.1111/j.1365-3024.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- 39.Todd J, Grosskurth H, Changalucha J, Obasi A, Mosha F, Balira R, Orroth K, Hugonnet S, Pujades M, Ross D, Gavyole A, Mabey D, Hayes R. Risk factors influencing HIV infection incidence in a rural African population: a nested case-control study. J Infect Dis. 2006;193:458–466. doi: 10.1086/499313. [DOI] [PubMed] [Google Scholar]
- 40.van Dam GJ, Bogitsh BJ, van Zeyl RJ, Rotmans JP, Deelder AM. Schistosoma mansoni: in vitro and in vivo excretion of CAA and CCA by developing schistosomula and adult worms. J Parasitol. 1996;82:557–564. [PubMed] [Google Scholar]
- 41.Kremsner PG, Enyong P, Krijger FW, De Jonge N, Zotter GM, Thalhammer F, Muhlschlegel F, Bienzle U, Feldmeier H, Deelder AM. Circulating anodic and cathodic antigen in serum and urine from Schistosoma haematobium-infected cameroonian children receiving praziquantel: a longitudinal study. Clin Infect Dis. 1994;18:408–413. doi: 10.1093/clinids/18.3.408. [DOI] [PubMed] [Google Scholar]
- 42.van Lieshout L, Polderman AM, Deelder AM. Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop. 2000;77:69–80. doi: 10.1016/s0001-706x(00)00115-7. [DOI] [PubMed] [Google Scholar]
- 43.Karanja DM, Hightower AW, Colley DG, Mwinzi PN, Galil K, Andove J, Secor WE. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet. 2002;360:592–596. doi: 10.1016/S0140-6736(02)09781-7. [DOI] [PubMed] [Google Scholar]
- 44.Trushin P, Bang H. The role of epidemiology and biostatistics in health news reporting. In: Finkel M, editor. Public Health in the 21st Century. Santa Barbara, CA: ABC-CLIO, LLC; 2010. [Google Scholar]